- Investigation of the ceramic tiles that contains boron frit
Sinem Aksana,* and Nezehat Edizb
aKütahya Dumlupınar University, Metallurgical and Materials Engineering Department, Kutahya Türkiye
bKütahya Dumlupınar University, Mining Engineering Department, Kutahya TürkiyeThis article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
The floor tiles contain porosity depending on the production process. Therefore, water absorption is higher for the floor tiles. The water absorption permeability leads to problems such as visible spots on the surface of the products after laying. This situation returns to firms in the customer complaint. Impervious engobe has been investigated as a possible solution to this problem. In this study, a more sintered engobe was tried to be obtained. The amount of glassy phase increased due to increased boron frit, so water permeability decreases were observed. The permeability problem has been occurred in the samples which have prescription rates below 20% boron frit. XRD,SEM,DTA,Thermal Microscope and expansion analysis was performed. According to the results obtained from SEM images of impervious engobe samples were found to be more compact. This shows that spaces have been closed due to increasing amount of the boron frit. The lower sphere temparature have observed in the thermal microscope analysis for the engobes which contain more boron frit. So the sintering temperature has been reduced and thus the floor tiles permeability has been reduced.
Keywords: Engobe, Boron frit, Permeability.
Engobe is using to concealed the ceramic body colour by covering the product between body and the glaze with the required colour and optimum density. Also it is using to fit the thermal expansion between the body and the glaze. The other role of the engobe is reducing water absorption [1]. The engobe layer is situated between the tile body and glaze and acts as an obscuration layer. It has been shown Fig. 1.
Boron, as boric oxide is an important chemical component of many raw materials and a variety of frits used as intermediate products by the ceramic industry [2].
Boron is an important component of glaze and engobe compositions. By the development of the technology, boron had been used in glazes and engobes by inclusion in a frit to render it insoluble. Boron had been many beneficial functions in ceramic glazes by using as a frit. Boron is a flux that never increases thermal expansion and also it improves chemical durability. We can eliminate hazardous lead oxide in engobes and glazes by using boron oxide. Also feldspars are included in ceramic body as a fluxing agent [3]. Fluxes are the materials which lower the melting point of a glaze. They can be called melters. Silica melts by itself but at a very high temperature. Therefore it needs additions of flux to make a practical glaze. The most common flux for temperatures below 1100 °C is lead oxide (PbO), but since it is poisonous it is no longer used in modem crockery glazes. Another powerful flux is boron or boric oxide, B203, which is not poisonous and is used in glazes in the form of borax or boric acid [4].
In general, the higher the boric oxide content, the lower the firing temperature of the glaze. In ceramic tiles, boric oxide remains an essential component in almost all types of tile, whether wall, floor, porous. Frits are used in engobes to render the desired soluble elements sodium, potassium and boron insoluble in water [4]. They need to be insoluble since soluble elements will migrate during drying processes that give rise to glaze and engobes defects as well as the possibility of effluent problems as they would be present in wastewaters. The frit also ensures that the melting process commences at an early stage, that is before the glaze firing process itself. This ensures that high gloss in the engobes and the glaze firing process is easily obtained.
Floor tiles contain porosity depending on their production process, raw materials and fields of application in their structures. Consequently, water absorption of floor tiles is high. This water absorption causes stain problems on the surface of the product because of water permeability after covering. This situation gets back as customer complaints to the companies. For the solution of the problem impervious engobe studies have been carried out [5].
It was observed in E. Günay’s research that, the sintering time was less effective in the sintering behavior of the system compared to the sintering temperature and B2O3 additions [6].
ZrSiO4 is widely used as a glaze opacifier in the ceramic glaze industry, and many studies have reported the effect of frit composition on the solubility of zirconium compounds [7, 8]. Opacity results from the difference between the refractive index of an opacifier and a glassy matrix [9]. Thus, the greater the difference in refractive indices between these two, the higher the light scattering and hence the more satisfactory the opacity [10]. The emitted light intensity also depends on the particle size of the crystalline phases, as long as it is larger than the wavelength of the light. The finer the particle size, the larger the surface area, the greater the number of reflective surfaces, and the more frequently the light’s path is disrupted [11]. This produces a greater reflection and higher opacity. Another factor that affects the opacity of a material is the amount of opacifying phase present. Increasing the number of crystallites also increases the number of reflective surfaces, which increases the opacity of the material. The opacity of the glaze may result from the nature of the glaze or the presence of opacifying agents in the glaze. 23 Among commercial frits, those containing zircon (ZrSiO4) and zirconia (ZrO2) attract great attention. ZrSiO4 causes the formation of opaque frites often referred to as “zirconium white” [12]. Also frit systems were developed for fast single firing floor tiles [13].
The purpose of this study is developing new floor tile engobe compositions that have maximum whiteness and maximum opacity level in the course of improving floor tile engobe characteristics. In this sense, it is aimed to decrease porosities by increasing the amount of boron frit. So that vitreous phase has been increased and water absorption has been decreased. For that purpose imperviousness test has been performed by applying different engobes which have been prepared by using boron frit on floor tile structures to pull down sintering temperature by increasing melting phase.
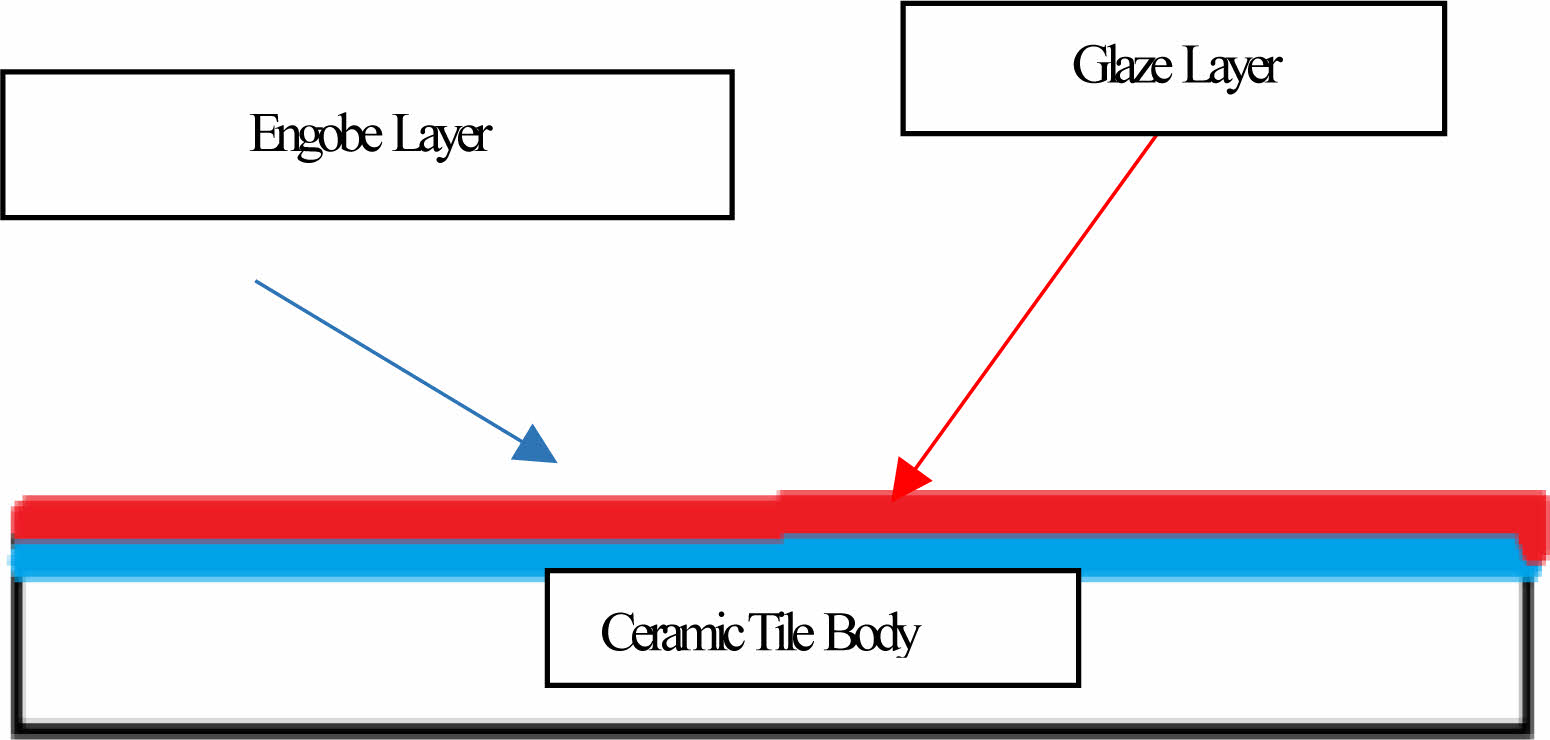
|
Fig. 1 The cross-section of the engobe layer |
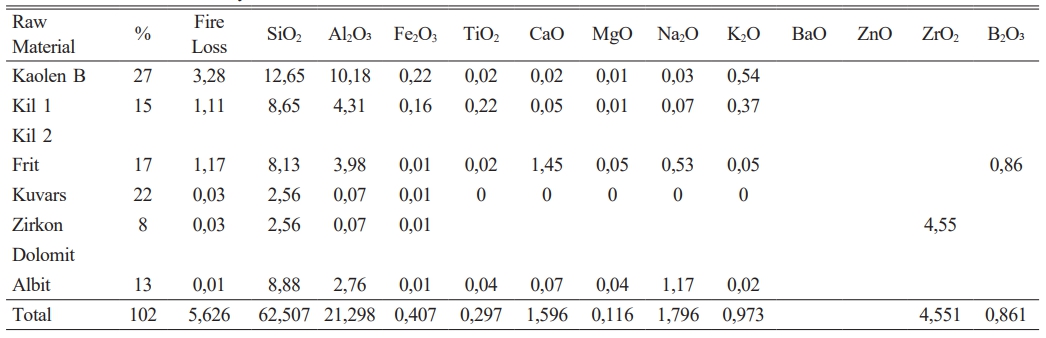
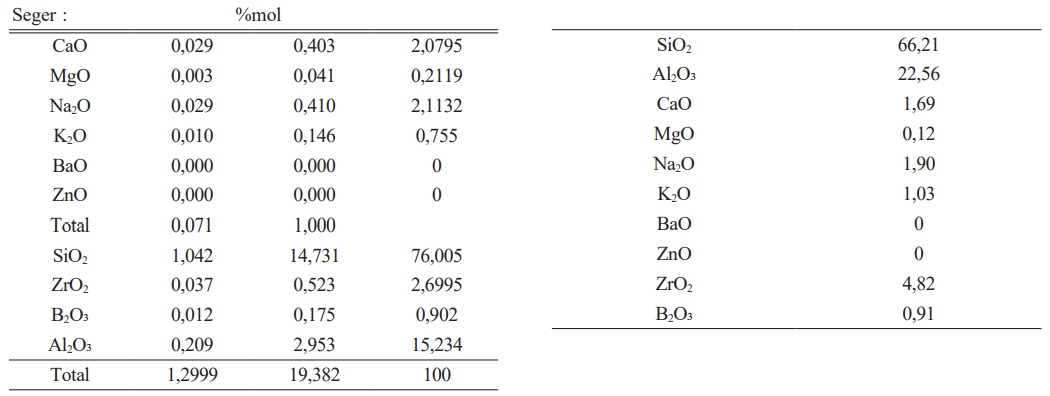
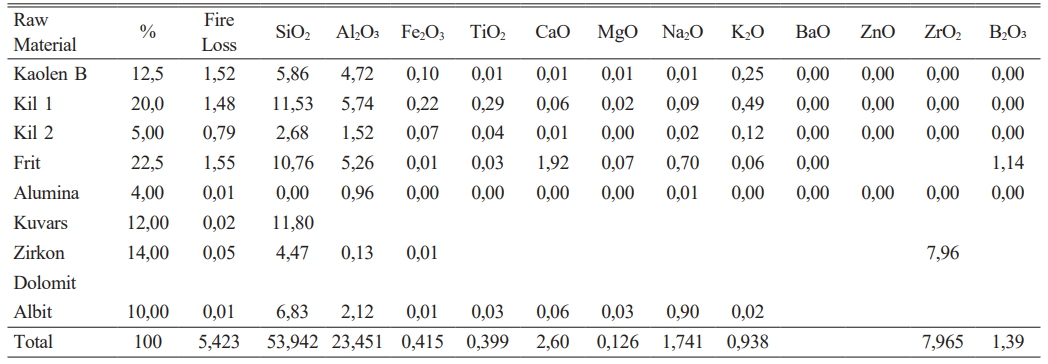
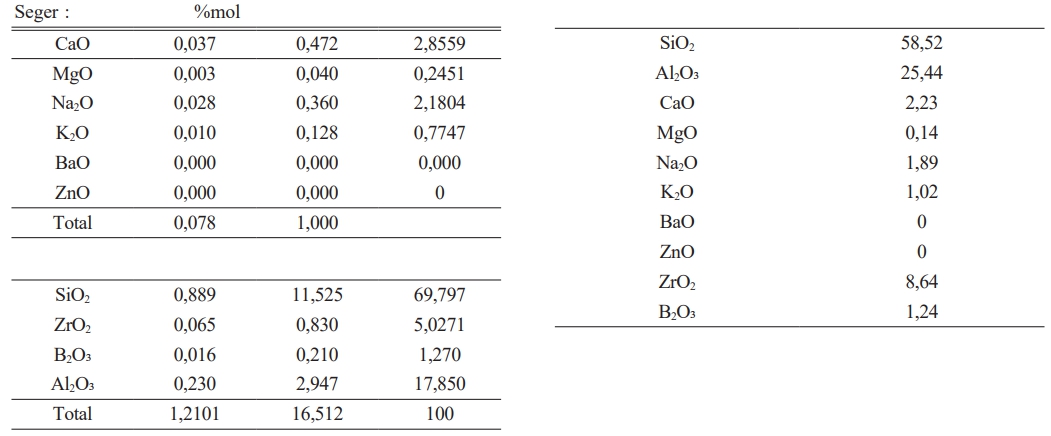
The experimental studies for the purpose which is indicated above have been completed in three stages. In the first stage, it has been tried to decrease melting phase for the highly opaque engobe compositions. In the second stage perviousness tests after sintering and L, a, b measurements of the same engobes have been performed. In the third stage characterization processes of the engobes have been completed. Analysis has been made by receiving the images of XRD, thermal microscope, dilatometer analysis and SEM. By this means,the sintering mechanism and the arising phases have been scrutinized. 20 different types of engobe compositions which were prepared in the first stage were grinded in 100 gr. capacity of dry matter, lab-type alumina ball porcelain mill in 10 minutes. Litre weight measurement was made at the end of grinding for all the engobe compositions. After determining the tare of the pyknometer, the engobe is loaded and weighed. The value which was seen while weighing is multiplied by 10 and the result gives the litre weight in gr/lt. approximately 1430 g/lt weighed engobes which are used in operating conditions were prepared for the floor tile engobes. Application has been performed with airbrush. 35 gr engobe was applied for a 33×33 cm tile.
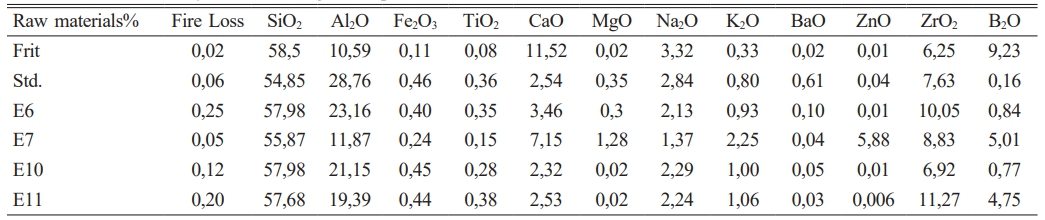
Chemical analysis of fired engob samples was performed using the Spectro Brand X-lab 2000 device.
They were sintered in the kiln for 30 minutes on 1190’ C after drying 10 minutes by using the standart glaze. It has been given at Fig. 2.
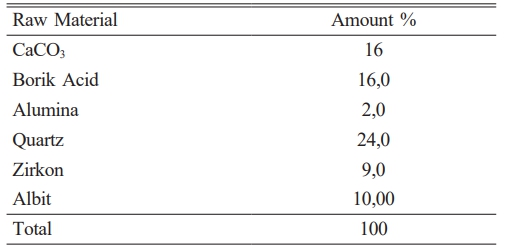
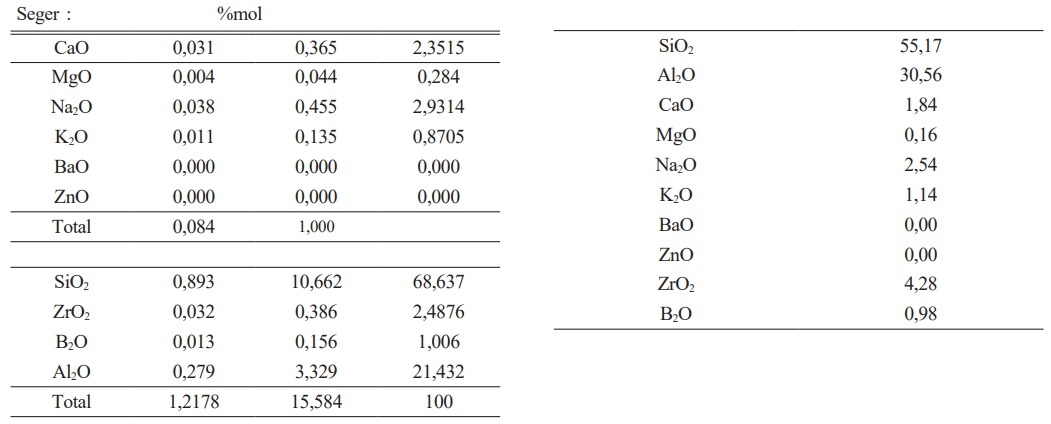
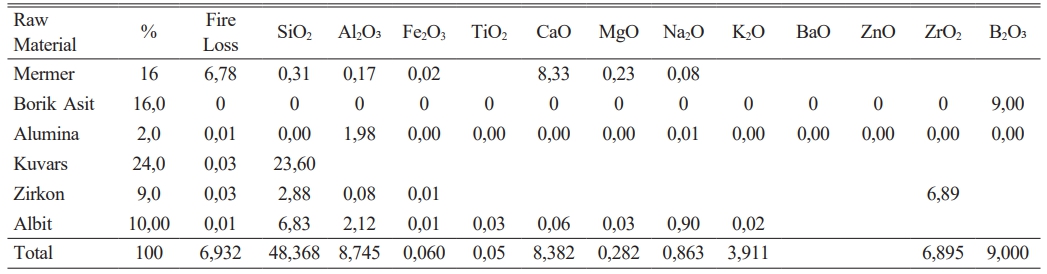
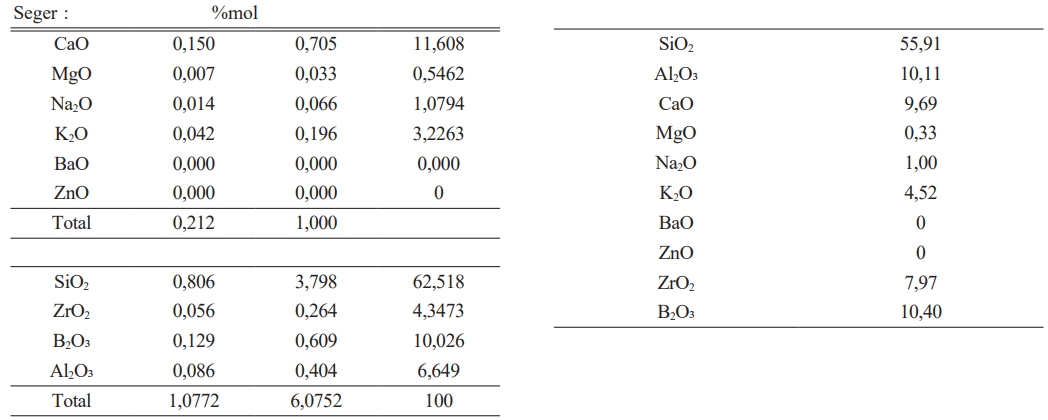
It has been given in Table 2 amount of raw materials which belong to frit prescription and in Table 1 comparative amount of raw materials which belog to each engobe prescription has been given. It is very important for this study the amount of B2O3.
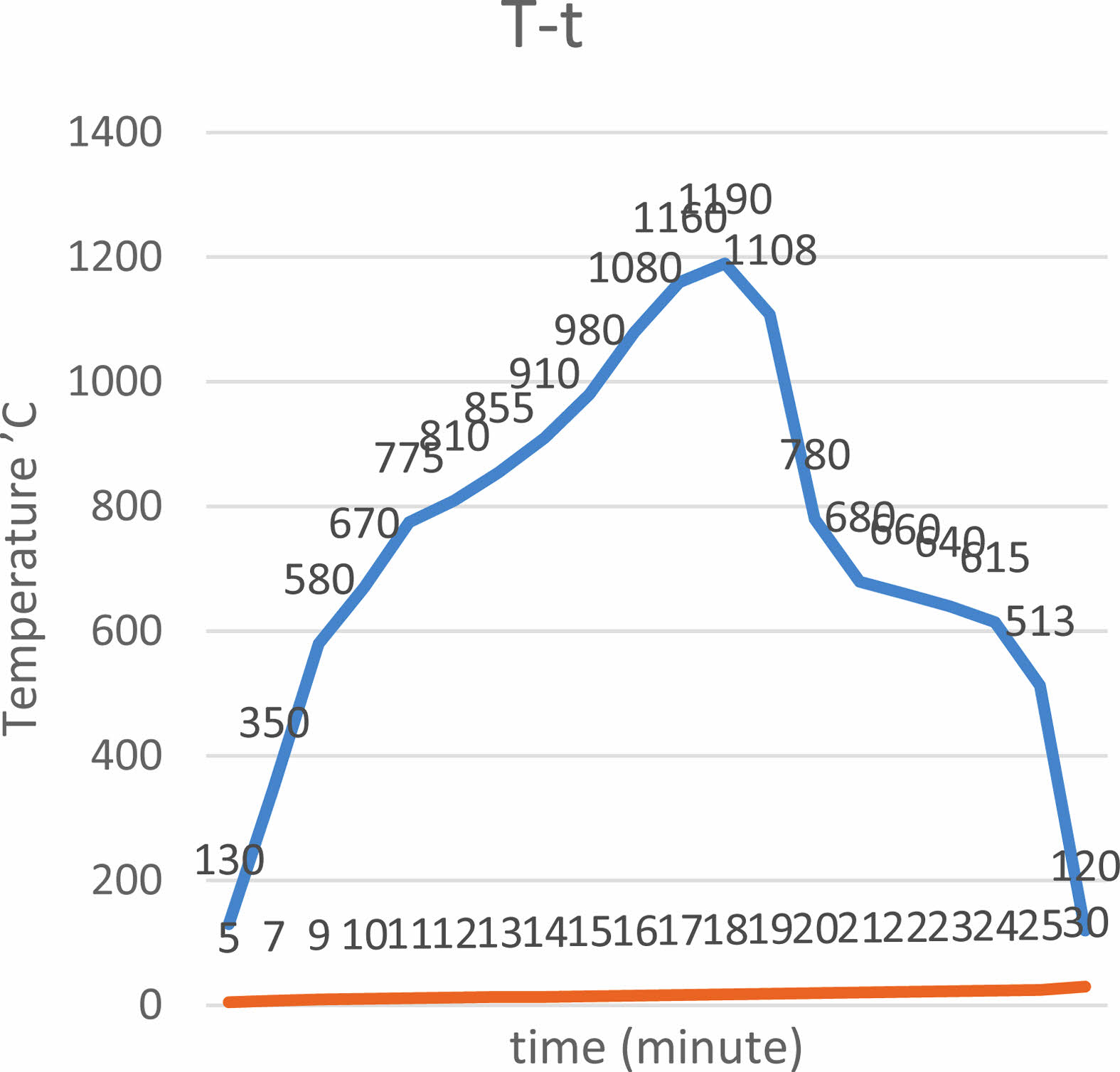
|
Fig. 2 The firing cycle of the engobes. |
Permeability Test Measurements
The floor tiles engobe perviousness tests were done in business laboratory of Kütahya Ceramic Factory. After being clinged to a grouted plate, the floor tiles waited for 1 day. Than it is as certain that whether they left stain or not by inspection.
According to the test results it is determined that E7, E8 and E9 engobes are impervious. In Table 3. But whitness of E9 engobe which was developed without zircon is very low Fig. 3. E7 and E8 engobes are greyer than the standard engobe. The studies continue in order to approximate the whiteness of the engobes to the standard engobe. Whiteness value of E10 engobe is very close to the standard engobe but perviousness has been observed. The perviousness E11 engobe is zero. However, greyness is a matter as it can be understood by looking at L, a, b values. Although E12 engobe does not have a perviousness problem, it has got grayness issue. Despite the whiteness value of E5 engobe is quite good,it has got a perviousness problem.
E13, E14, E15 engobes do not have perviousness problems but it is understood that they are hard engobes. Although E16, E17, E20 engobes do not have perviousness problem, greyness issue has been observed by inspection
Colour Measurements
Colour measurement had been performed with Minolta CR 300 measuring device. A colour value-detector which detects colour value of the device was located on the surfaces of scoured engobes. Colour values were observed from the indicator of the device after being pushed the button. The results has been shown at Fig. 3.
In previous studies, various attempts were made to determine a relationship between engob opacity and zirconium silicate. For this purpose, an industrial engob containing standard zirconium silicate (A) and another engobe without zircon (B) were tested. After these two engobes were applied to the body, they were fired at different temperatures.It was observed that the engob layer without zircon had a lower whiteness index at all tested temperatures. For example, at 1140 °C, engob A behaved especially at L and Ib (whiteness index) values of 90.05 and 77.5. However, engob B without zircon gave whiteness values of 78.02 and 72.2 in the same parameters [14].
According to this result, it is seen that between the zircon ratio and opacity, the effect of zircon on the mechanism increases. As a general view, engob opacity with additional zircon may cause different mechanisms. If excess zirconium silicate particles are added to the system, the particles may become insoluble in the glassy phase during firing and then begin to separate into the glassy phase. As the zirconium particles begin to solidify again during the opacification in the cooling cycle, the zirconium silicate becomes insoluble in the glassy phase [14].
All the colours can be desciribed in 3 coordinate system.
Coordinate X, L = white - black
Coordinate Y, a = red - green
Coordinate Z, b = yellow - blue
The degree of opacity depends on other engob components such as aluminum and zinc. X-ray studies have shown that the main opacifying phase in cooked engobes is zircon. When used with frit, the opacifying phase was determined to be zircon as a result of the heat treatment, as seen in Fig. 4 [15].
ZrSiO4 (Zircon) has been widely used as glaze opacifier in the ceramic glaze industry and several researches have reported the influence of frit composition on the solubility of zirconium compounds. Zircon gives rise to opaque frits which are glossy, viscous and with a low fusibility [13, 15].
X-Ray Diffraction Analysis
This analysis was performed with X-ray diffraction device by using the Rigaku Miniflex series. CuKα radiation which was acquired by applying 40 kV voltage and 30 mA current to the Cu tube was performed by using (λ = l,54046 Å).
Qualitative stage analysis was performed with XRD to locate the kind of crystal stages that consisted on engobes after sintering, investigate crystal progress that occurred as a result of sintering at the same heat and compare the crystal amount of the engobes relatively. That has been shown in Fig. 4 and Fig. 5.
According to the XRD analysis of scoured engobe zircon stage has developed as whitening stage (Fig. 4) According to the XRD analysis of the fired samples, it was observed that the zircon phase was formed as the whitening phase.
Scanning Electron Microscopy
SEM images have been performed in the SAM laboratory.
E11 engobe is more compact than the standart engobe. It has been shown in Fig. 6 and Fig. 7. Thus, we can understand that the permeability problem can be solved with more compact engobe layer.
Also it has been shown more detailed in Fig. 8 and Fig. 9 with 11000X magnification.
In accordance with the results which have been obtained from SEM images,it is observed that E11 engobe is intenser than the standard engobe. This shows that the spaces among crystals close better with increasing of vitreous phase. As a result, water absorption has decreased and the perviousness problem has been removed in E11 engobe. Primary mullite formation has been observed in performed in SEM images. SEM images support XRD results. Primary mullite formation has maintained high chemical resistance, low thermal expansion and good thermal shock resistance to the structure (Figs. 7-10).
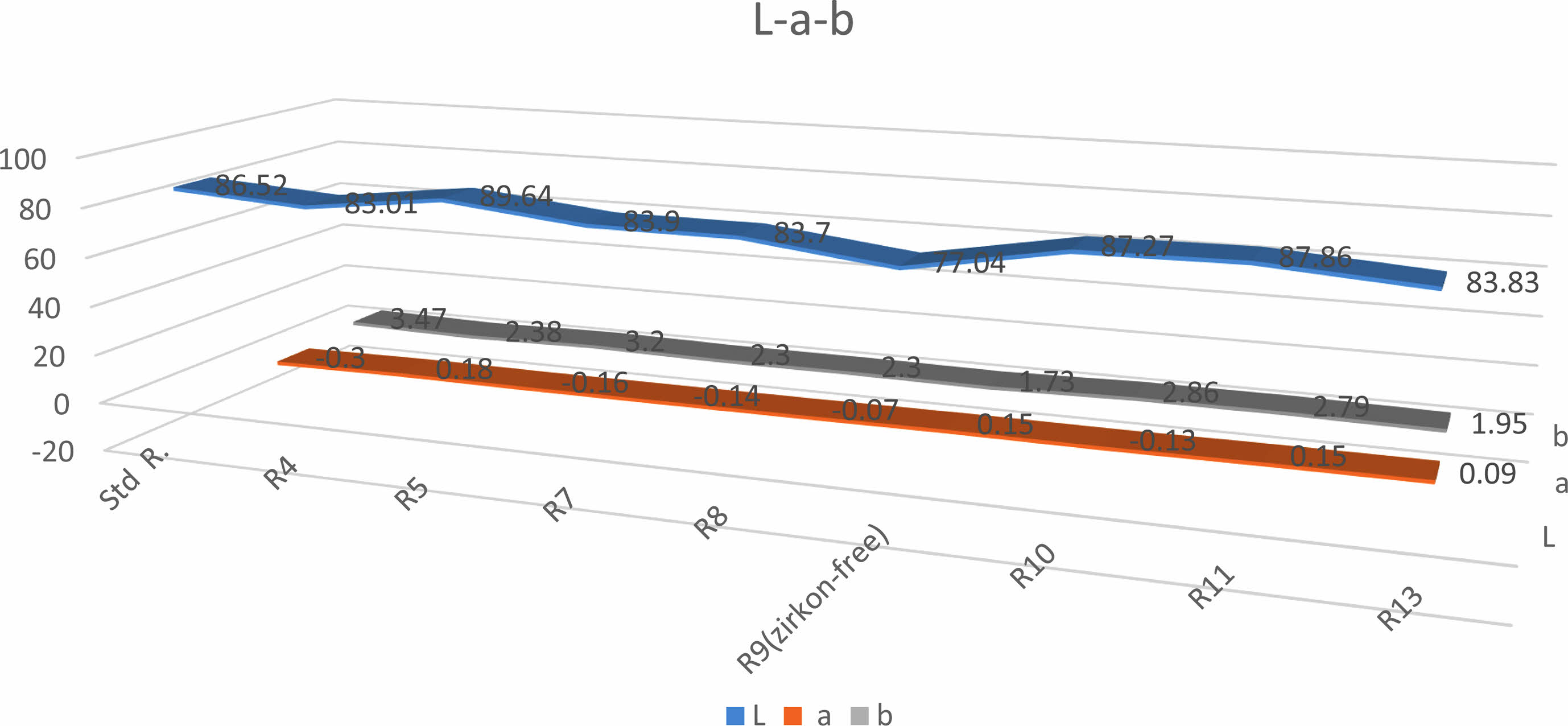
|
Fig. 3 The results of the colour measurements |
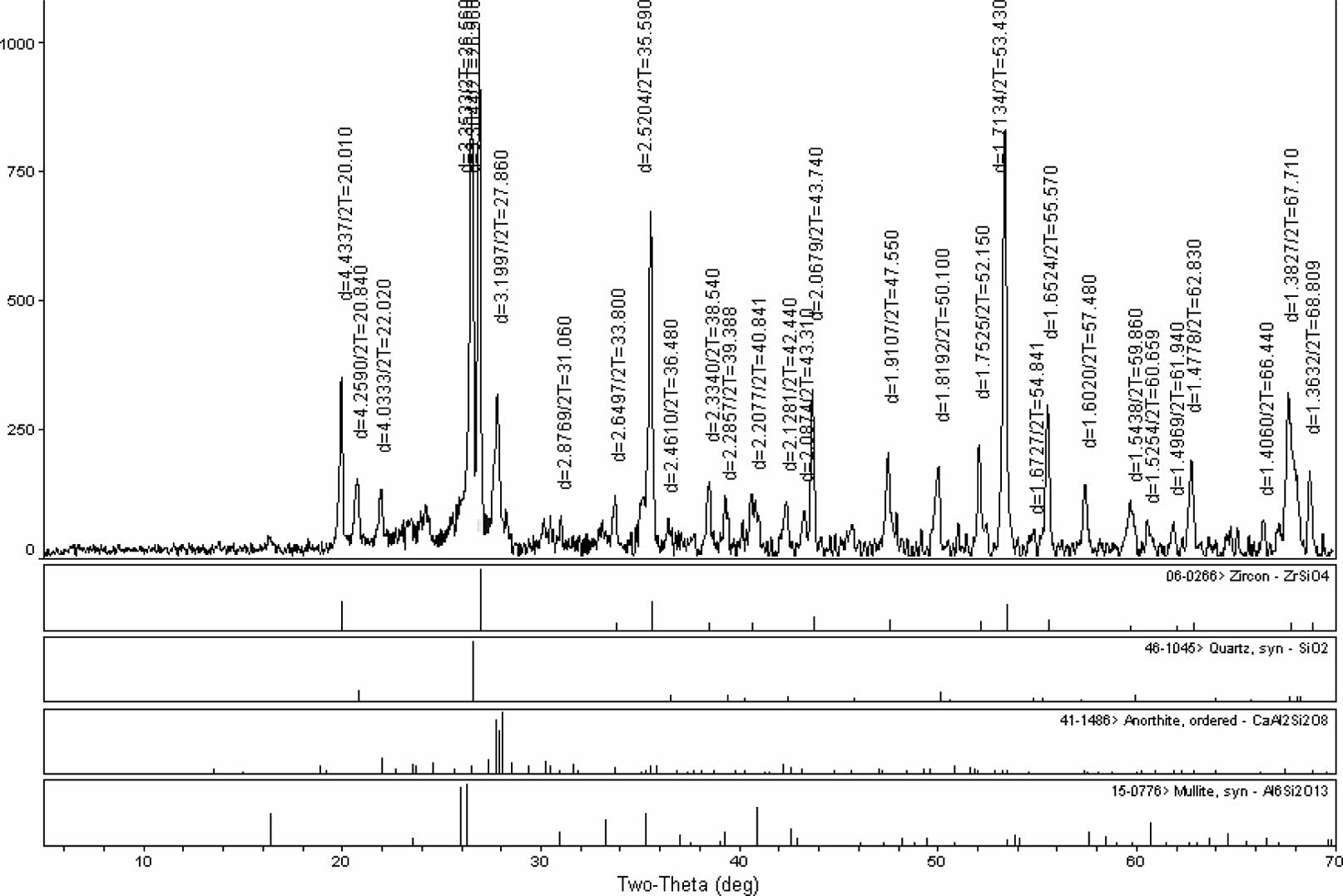
|
Fig. 4 Xrd pattern of the fired standart engobe. |
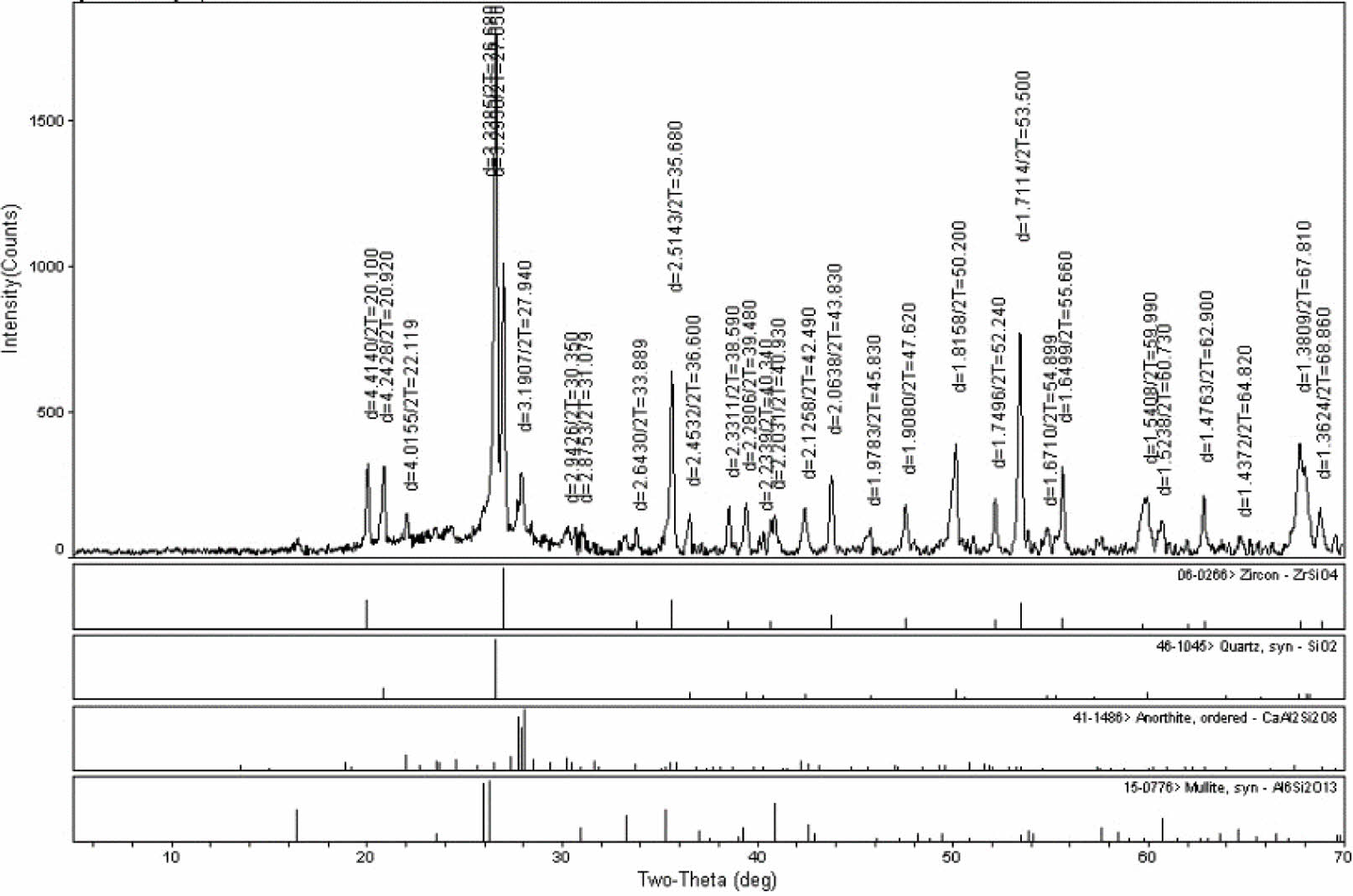
|
Fig. 5 Xrd pattern of the fired E11 engobe. |
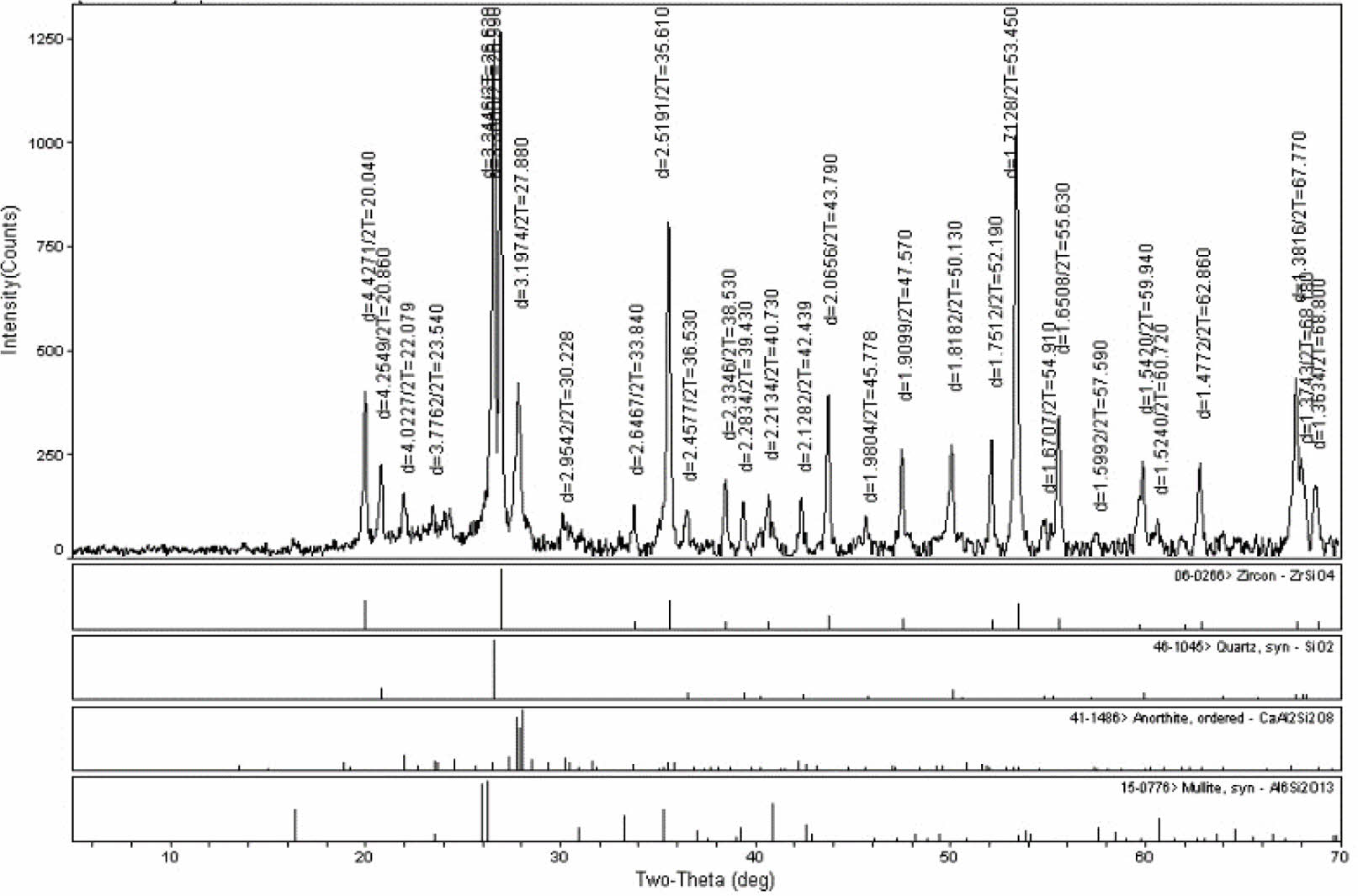
|
Fig. 6 Xrd pattern of the fired E6 engobe. |

|
Fig. 7 SEM image of E11 Engobe. |

|
Fig. 8 SEM image of standart Engobe (5000 K). |

|
Fig. 9 SEM image of standart engobe (11000X). |

|
Fig. 10 SEM image of E11 engobe (11000X). |
Differential Thermal Analysis is performed by Netzch STA 409EP model device in Dumlupınar University.
According to Fig. 12
• Body water; Separated at 68 ⁰C-100 ⁰C
• Organic degradation; at 315 ⁰C
• Removal of crystal water from kaolin; at 506 ⁰C
• Quartz transformation; at 574 ⁰C
• 1. mullite formation; at 1006 ⁰C
Thermal Microscopy Analysis and Thermal Expansion Analysis
Thermal microscopy analysis has been performed in Çanakkale Seramik Factory. Thermal expansion analysis of the acquired engobes has been performed with NETZSCH DIL 402 PC dilatometre device to indicate their thermal coefficient and in consequence of controlling the cohesion with the structure to use.
For dilatometre analysis; the engobes which were prepared with wet grinding was dewatered in plaster moulds and sintered in accordance with tile scouring cycle.
An appropriate dimension of dilatometer bar was prepared from sintered engobe mass and placed into the specimen holder which is parallel with measuring direction. This device keeps the specimen of which piston measurement will be performed suspended by applying adjustable stable force continuously to the edge of the specimen. Through this movement of the piston the change of the specimen size is measured. Dilatometer analysis of the acquired engobe specimen was performed at 550 K’ with 25/10.0 (K/min) heating rate. After the analysis of the engobes has been showed that the thermal expansion value is suitable for using with the standart glaze. Fig. 13, 14, 15, 16, 17, 19
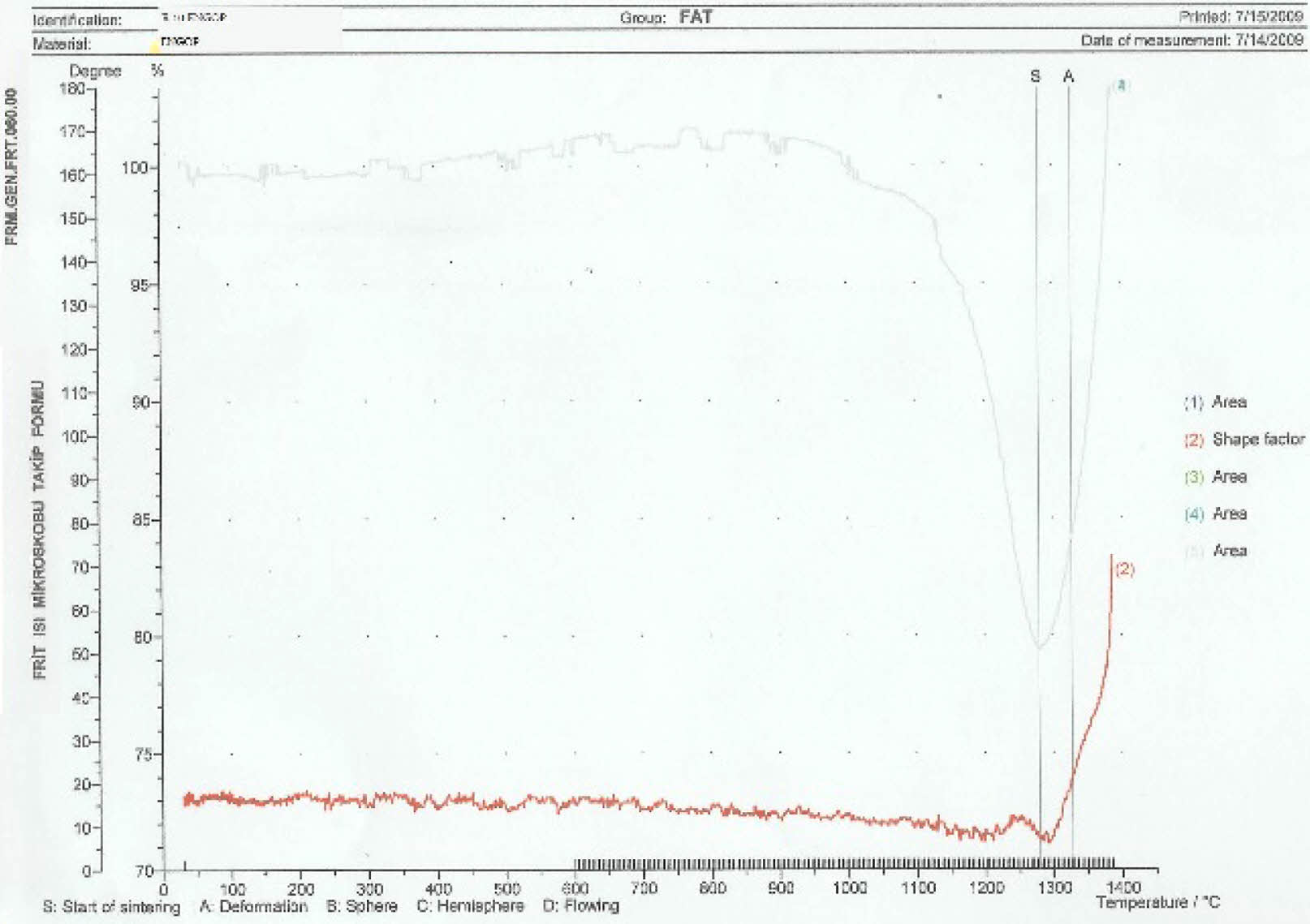
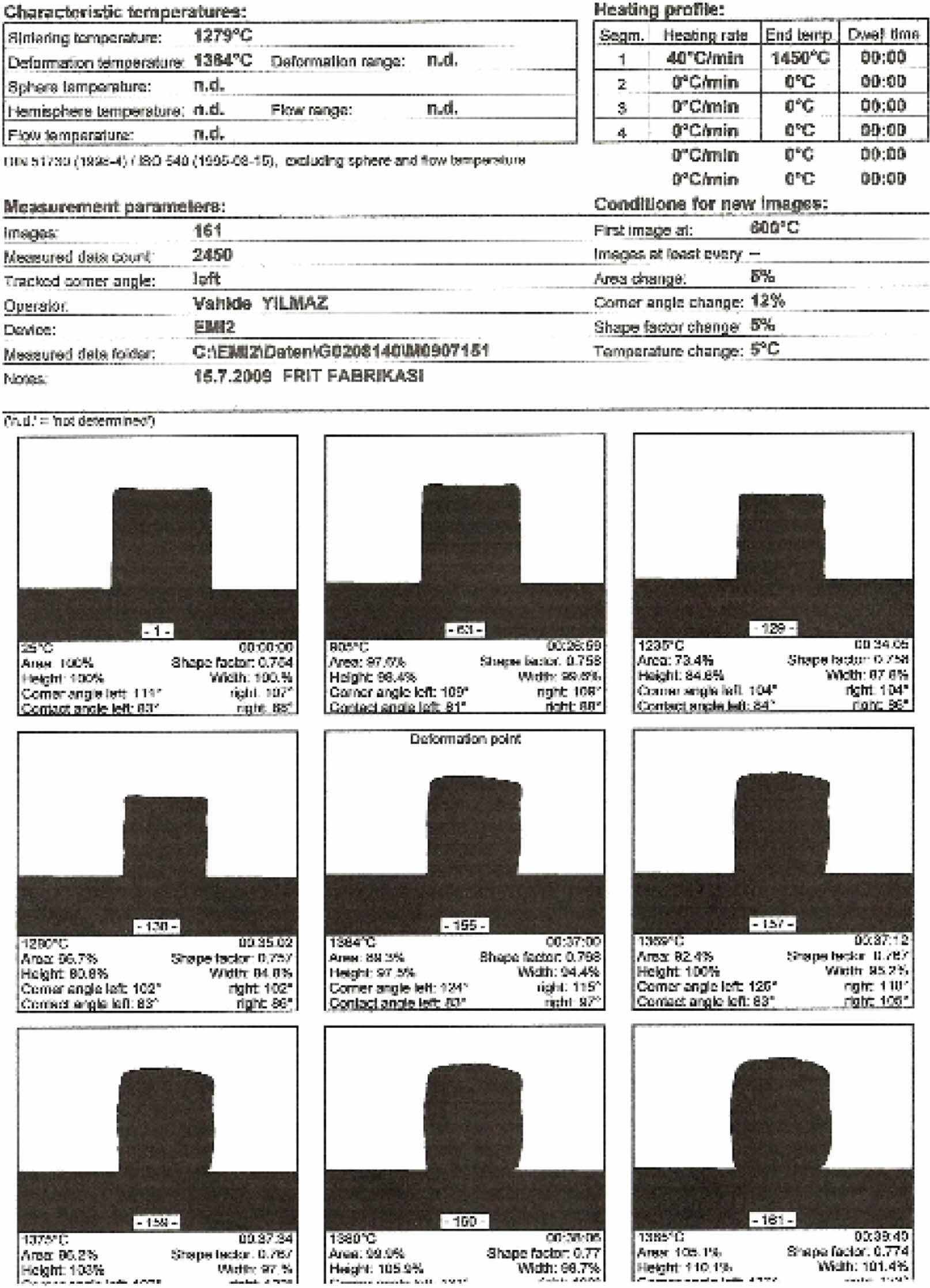
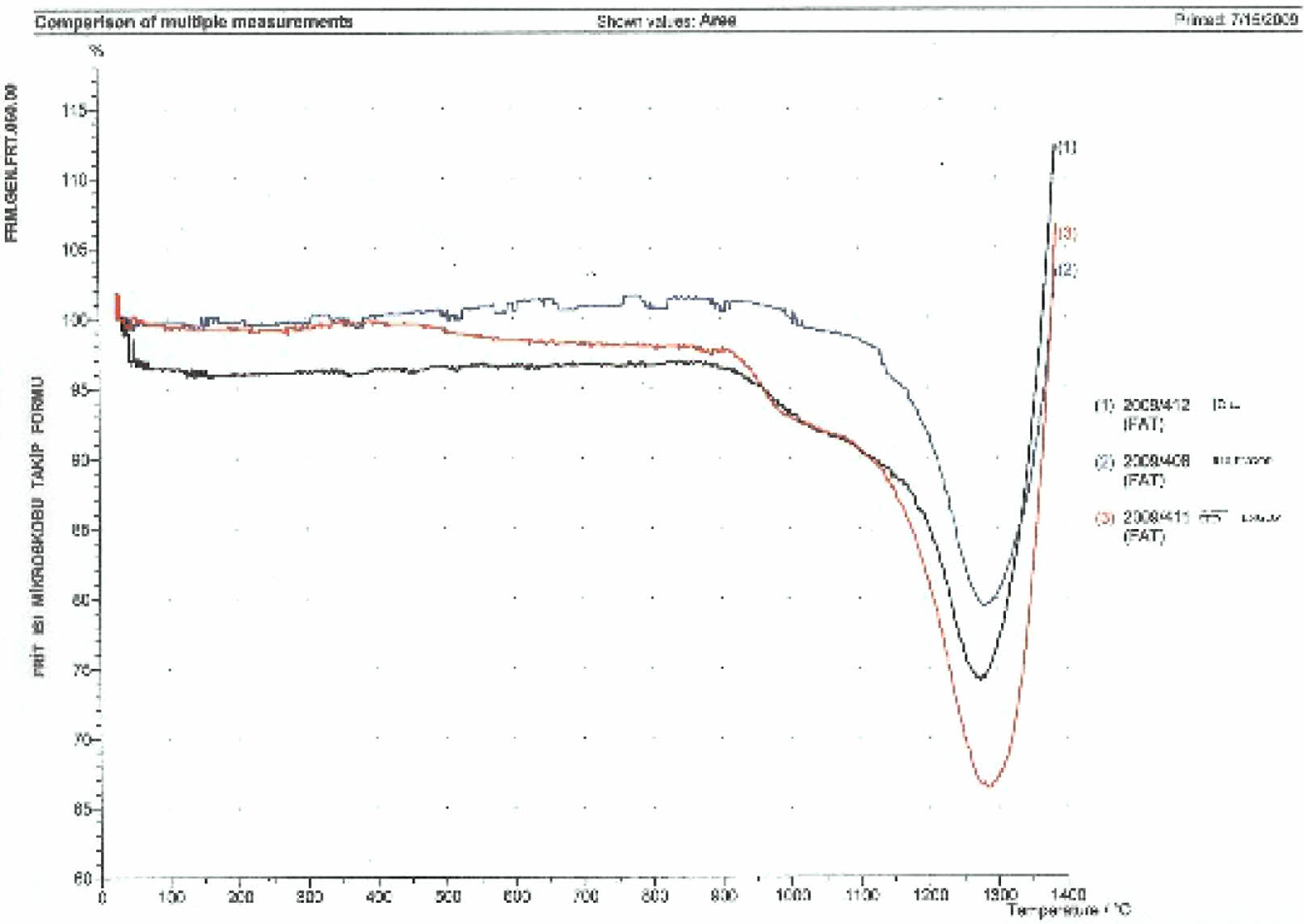
According to the heat microscope analysis getting sphere heat for standard engobe could not be observed. Figure 18-20. Standart engobe melting rate for the sphere heat is higher. This shows that sintering temperature of the E11 engobe is lower than standart engobe’s. So E11 engobe contains low porosity (Fig. 16).
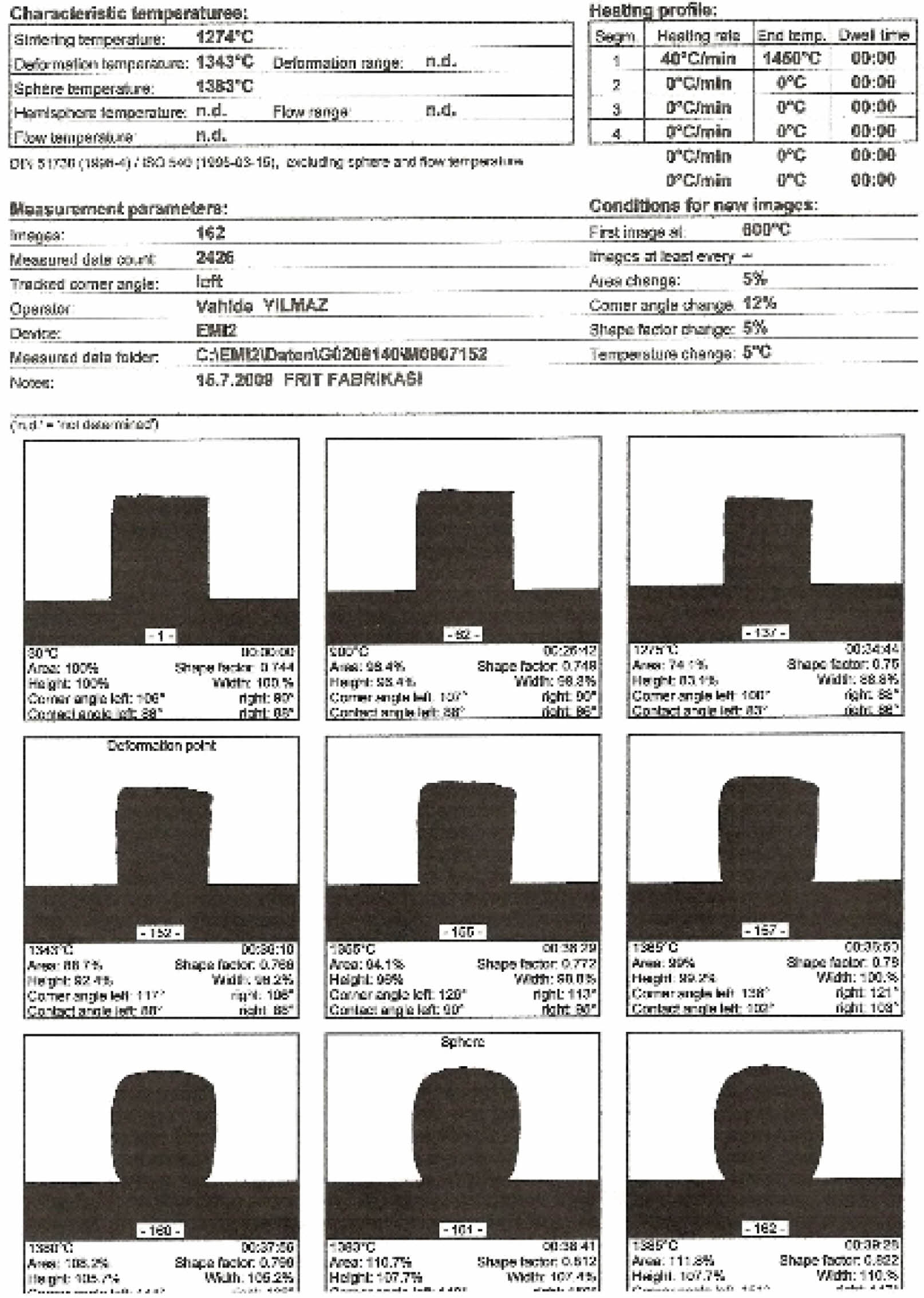
E11 engobe melting rate for the sphere heat is lower. So the sphere can be observed as shown in Fig. 19.
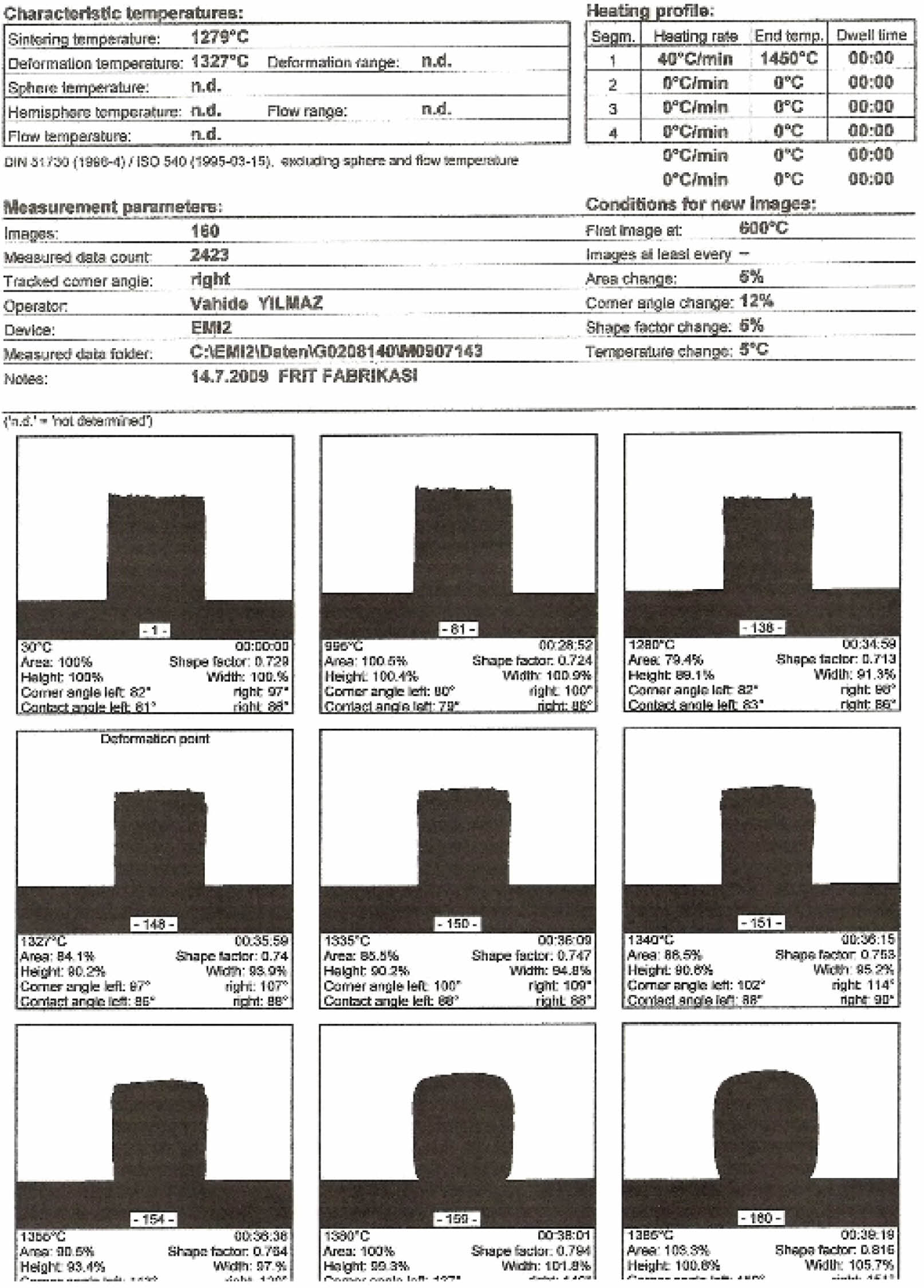
Sphere heat for E10 engobe could not be observed by heat microscope analysis E10 engobe melting rate for the sphere heat is higher has been shown in Fig. 20.
The sintering and deformation temperatures for the engobes has been shown in Fig. 18, Fig. 19, and Fig. 20. Also, the comparative thermal microscopy graphics of the E10, E11 and the standard engobe have been shown in Fig. 21 [15].
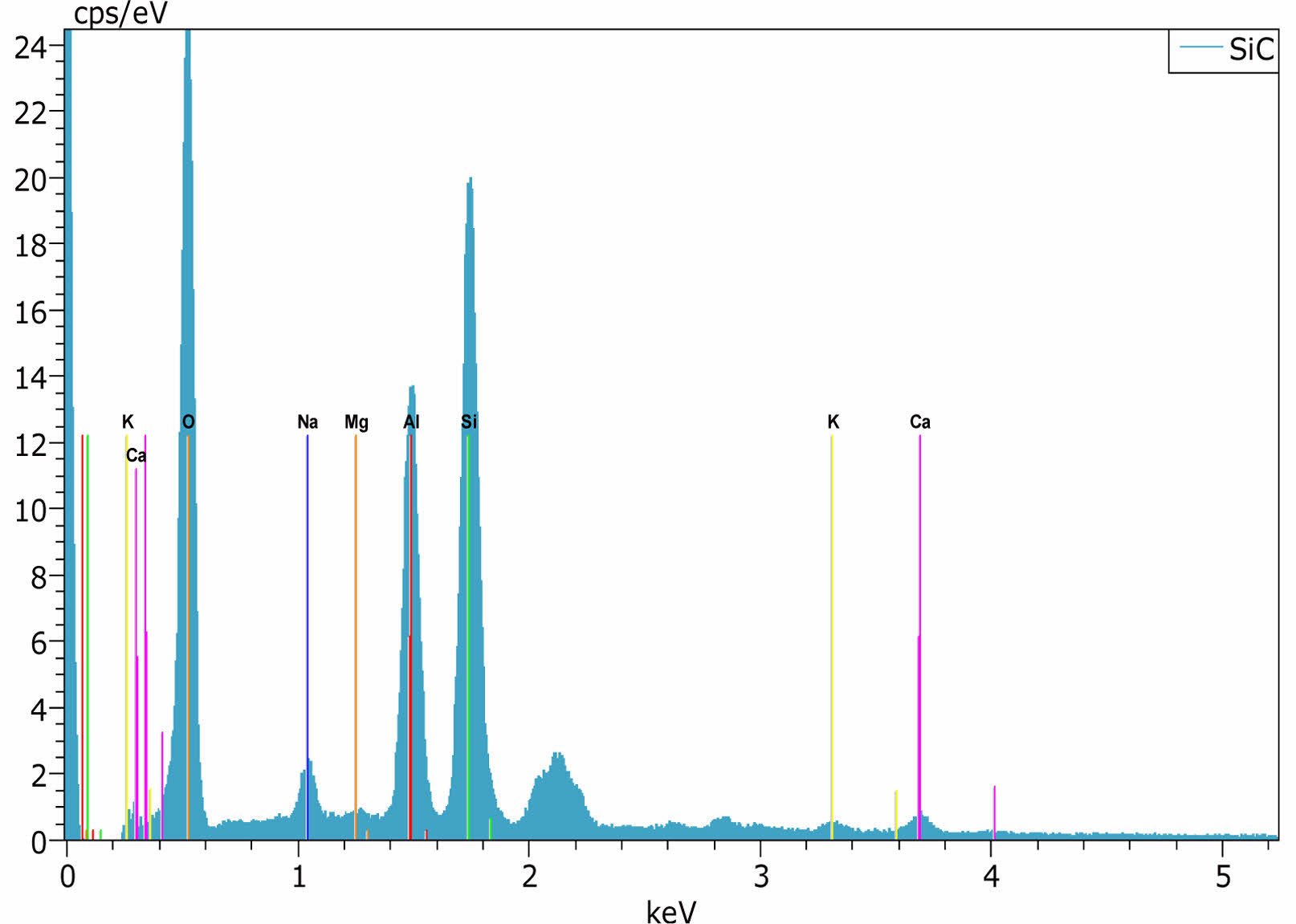
|
Fig. 11 Edx Analysis Image of E11 Engobe. |
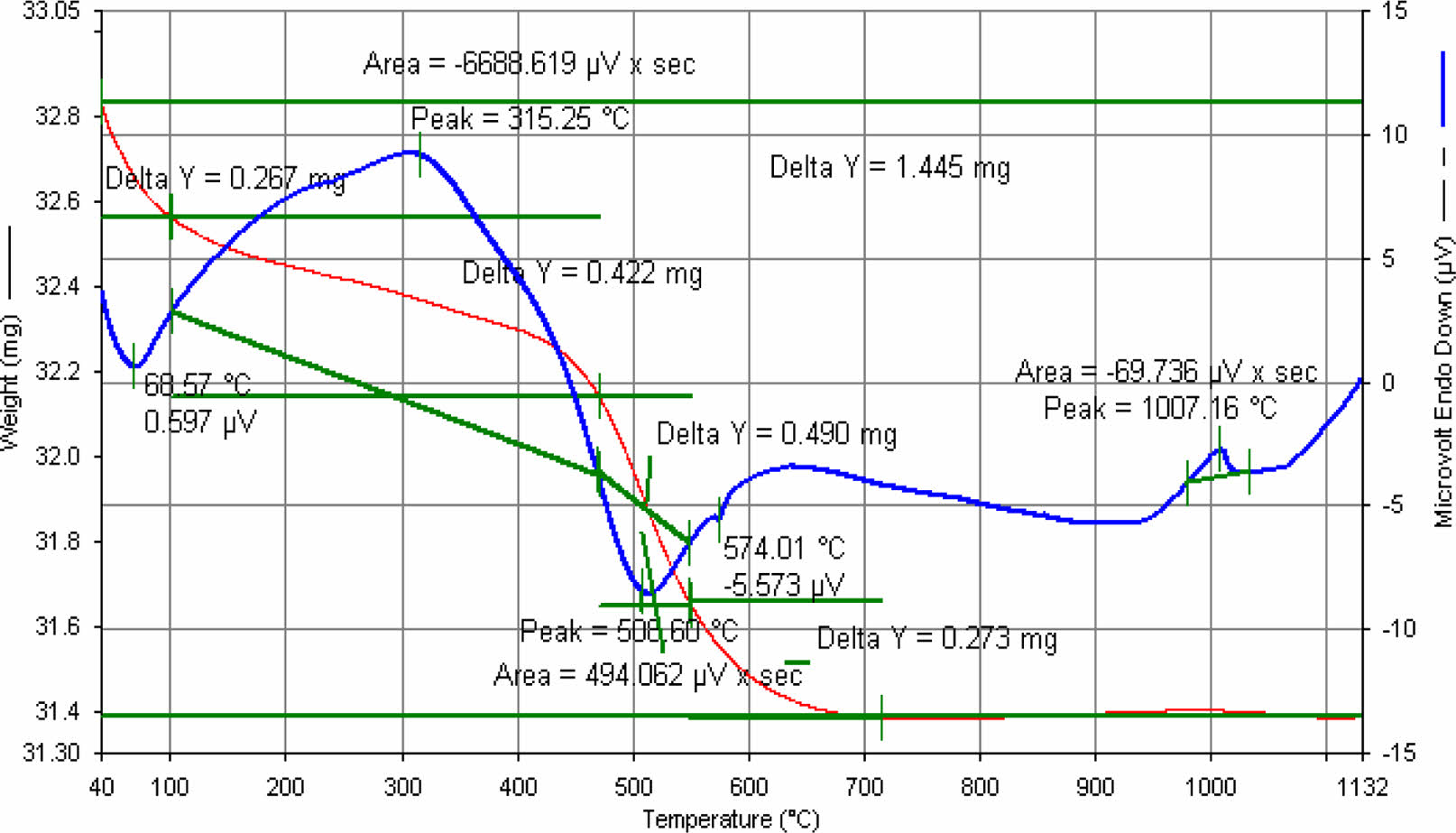
|
Fig. 12 E11 engobe DTA. |
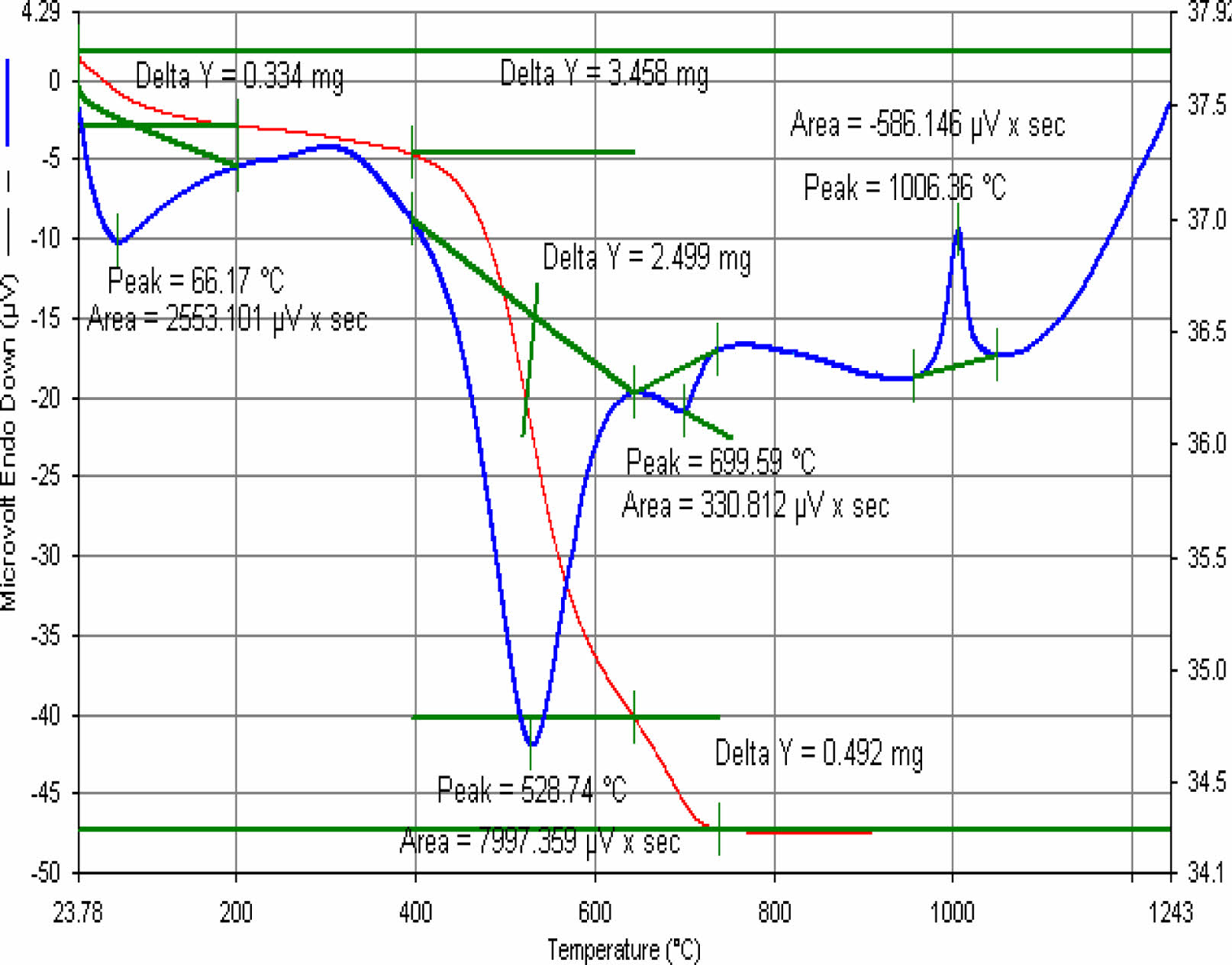
|
Fig. 13 E5 engobe DTA. |
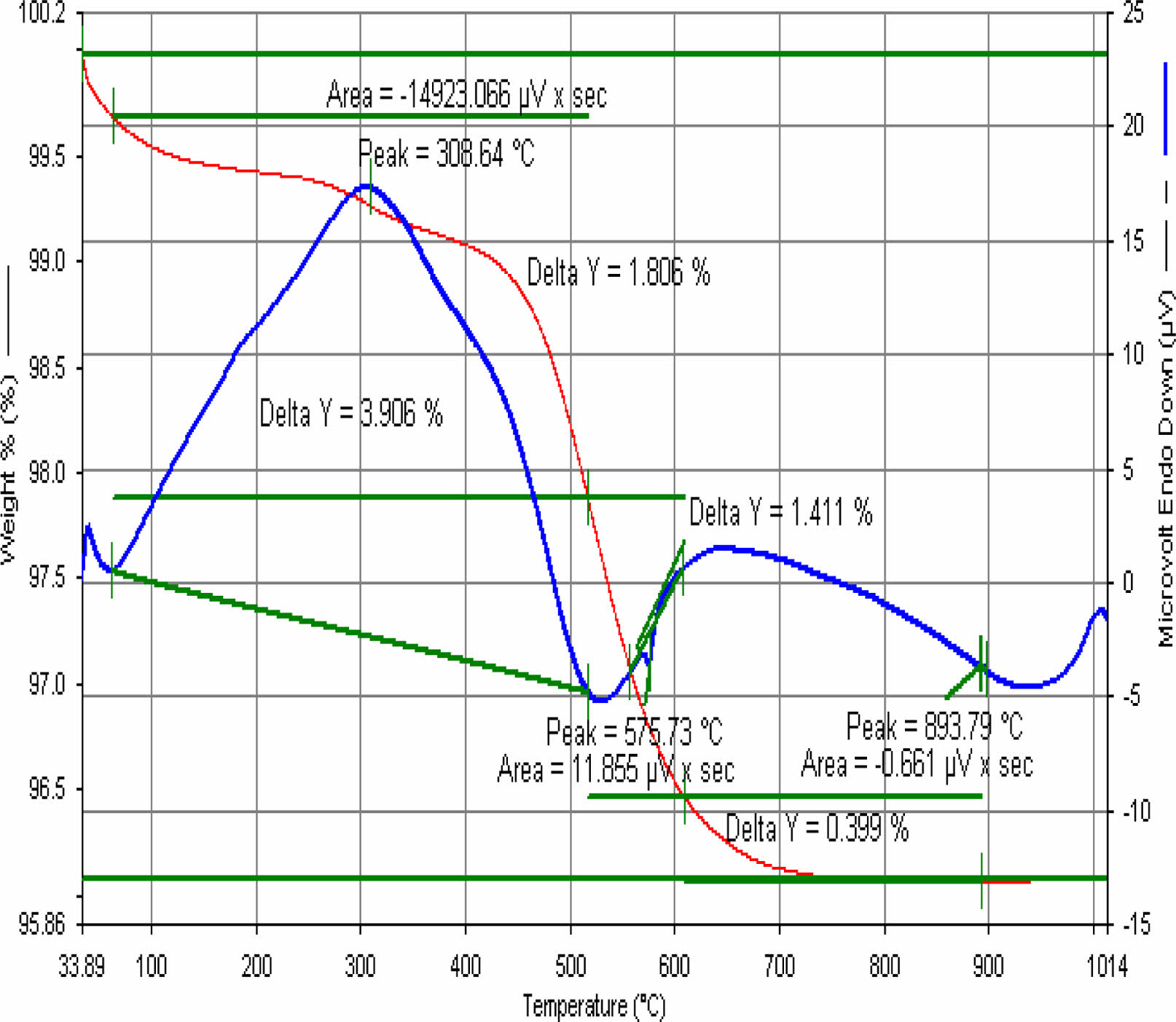
|
Fig. 14 E10 engobe DTA. |
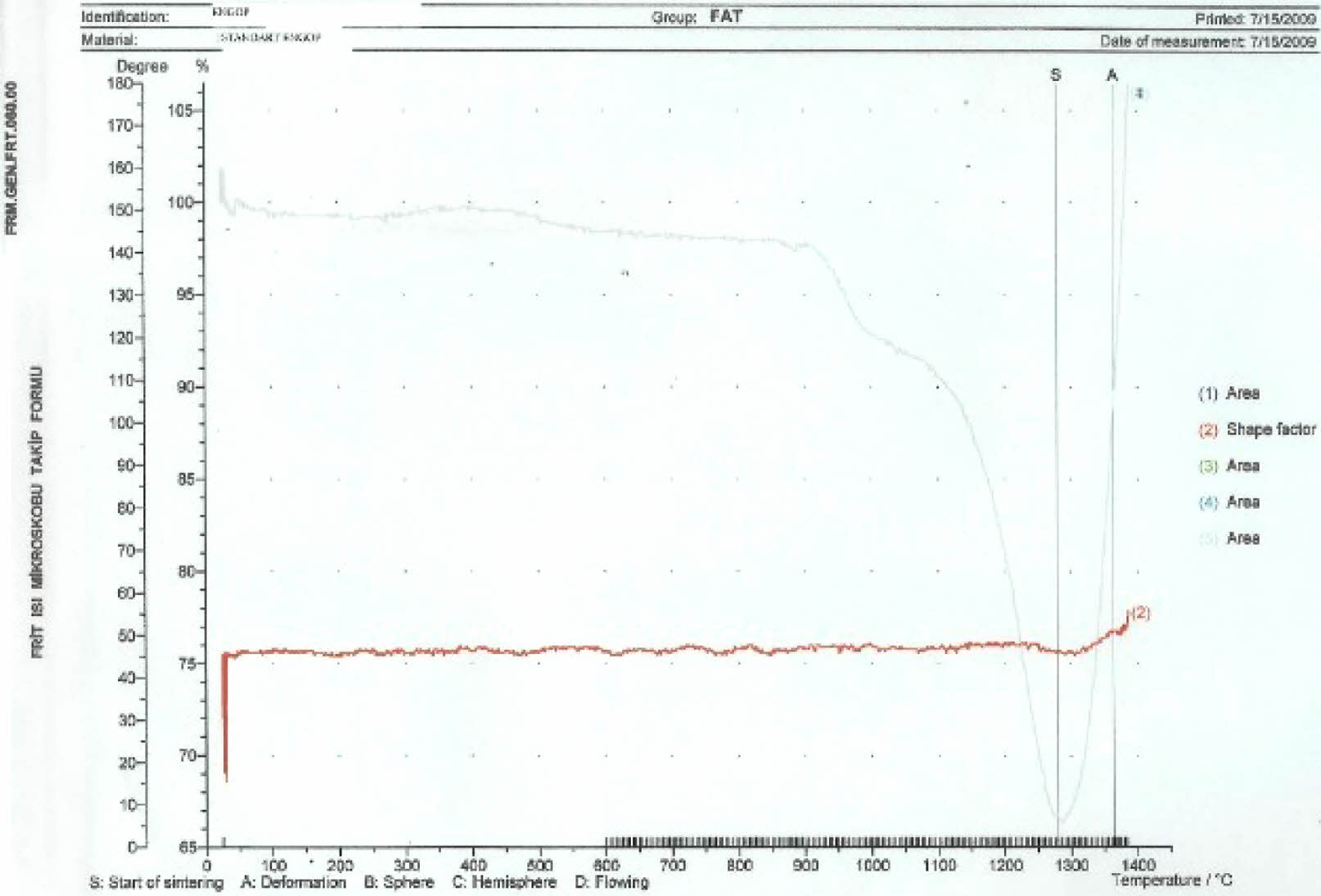
|
Fig. 15 Thermal microscopy graphic of the standart engobe |
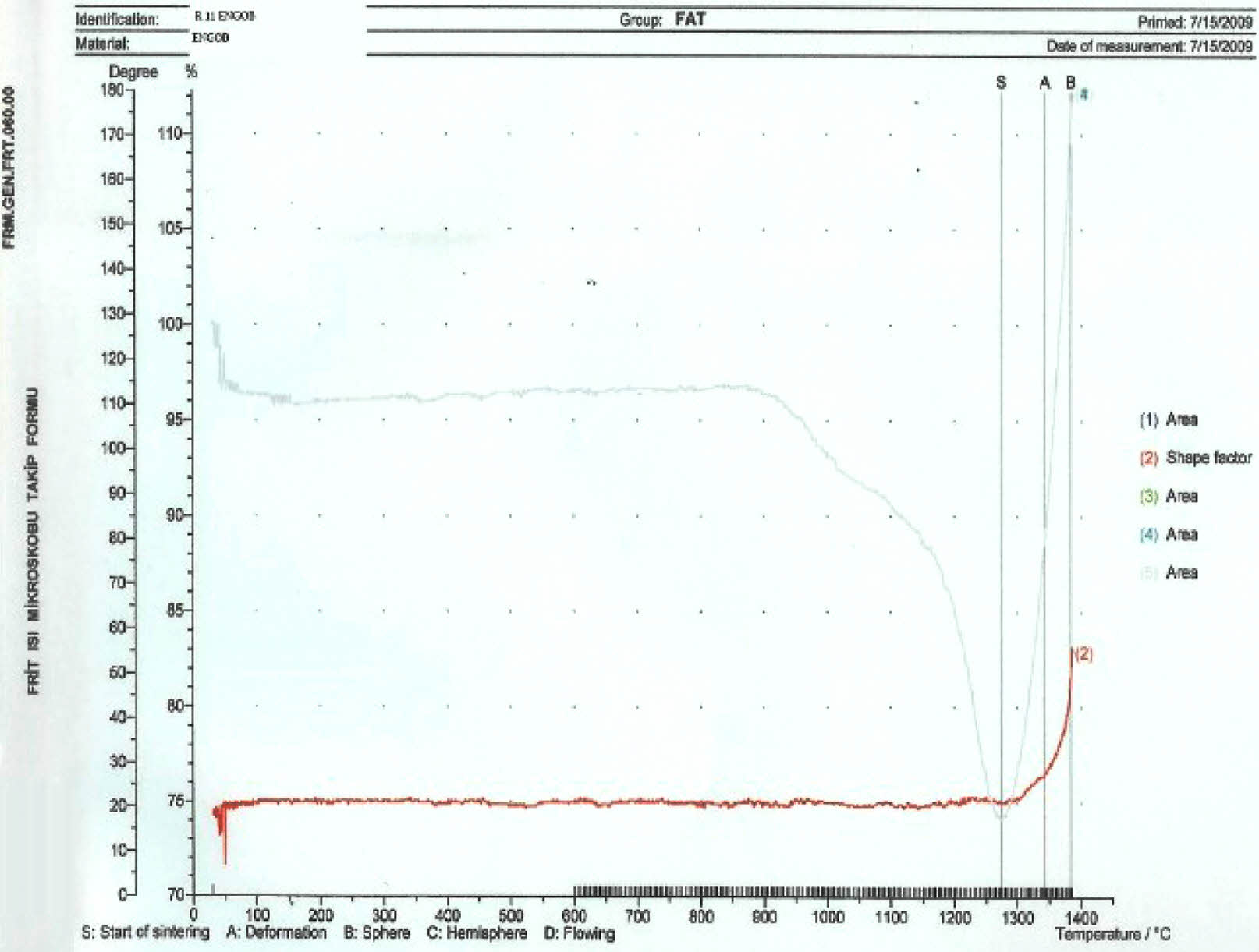
|
Fig. 16 Thermal microscopy graphic of the E11 engobe |
|
Fig. 17 Thermal microscopy graphic of the E10 engobe. |
|
Fig. 18 Thermal microscopy image of the standart engobe. |
|
Fig. 19 Thermal microscopy image of the E11 engobe. |
|
Fig. 20 Thermal microscopy image of the E10 engobe. |
|
Fig. 21 The comparative Thermal microscopy graphics of the E10, E11 and the standart engobe. |
Impervious engobe prescriptions have been researched in this study. It is necessary to obtain the vitreous stage in order to get an impervious engobe. By this means better sintering will be provided. Therefore, the boron frit rate has been increased. Than the permeability problem can be solved. According to the test results, E7, E8 and E9 engobs have been determined to be impermeable. However, it has been determined that the whiteness of E9 engob developed without zircon is far from the standard engob. E7 and E8 engobs are grayer than standard engobs. Studies have continued because engobs need to be brought closer to standard engobs in terms of whiteness. The whiteness value of E10 engobe is very close to standard engobe, but a small amount of permeability is observed. E11 engob has no permeability at all. However, there is greyness. Although E12 engob does not have a permeability problem, it has a grayness problem. Although the whiteness value of E5 engob is quite good, it has a permeability problem. As a result, the perviousness problem has been solved with addition min. %20 frit according to the prescription rates. In the following studies, whiteness of the engobe can be increased and cost of the engobe can be reduced.
- 1. A. Arcasoy, “Ceramic Chemistry” (Marmara University Press, 1983) p. 20.
- 2. F. Más, J.M. Estela, V. Cerdà, and E. Ochandio, J. Automat. Chem. 13[3] (1991) 107-110.
-

- 3. H. Yildizay, J. Ceram. Process. Res. 24[2] (2023) 237-241.
-

- 4. H. Norsker and J. Danisch, “Glazes” (Vieweg Teubner Verlag, Wiesbaden,1993) p. 44.
-

- 5. S. Aksan, “Master Thesis” (DPÜ, 2009) p. 50.
- 6. E. Gunay, J. Ceram. Process. Res. 11[5] (2010) 591-597.
-

- 7. R. Blonski, Ceram. Eng. Sci. Proc. 15[1] (2008) 249-265.
-

- 8. C.W.F Jacobs, J. Am Ceram. Soc. 37[5] (1954) 216-220.
-

- 9. I.A. Levitskii, Glass Ceram 60[3] (2003) 83-86.
-

- 10. R.J. Castilone, D. Sriram, W.M. Carty, and R.L. Snyder, J. Am Ceram. Soc. 82[10] (1999) 2819-2824.
-

- 11. J.L. Amoros, A. Escardino, M.J. Orts, A Moreno. Br Ceram. Trans. 93[6] (1994) 224-228.
- 12. K. Pekkan and B. Karasu, J. Eur. Ceram. Soc. 29[9] (2009) 1571-1578.
-

- 13. F. Karimi, S. Baghshahi, M.N. Khezrabad, and N.R. Noori, J. Ceram. Process. Res. 20[4] (2019) 357-362.
-

- 14. M. Romero, J. Ma. Rincon, and A. Acosta, J. Eur. Ceram. Soc. 22[6] (2002) 883-890.
-

- 15. S. Aksan. 5th Int. Tc. Symp. Proc. (2011) 9-19.
 This Article
This Article
-
2024; 25(3): 332-344
Published on Jun 30, 2024
- 10.36410/jcpr.2024.25.3.332
- Received on Dec 6, 2023
- Revised on Apr 18, 2024
- Accepted on Apr 22, 2024
 Services
Services
- Abstract
introduction
experimental methods
results and discussions
differential thermal analysis
conclusion
appendix
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Sinem Aksan
-
Kütahya Dumlupınar University, Metallurgical and Materials Engineering Department, Kutahya Türkiye
Tel : +90-274-443-4289 Fax: +90-274-265-2066 E-mail: sinem.aksan@dpu.edu.tr - E-mail: sinem.aksan@dpu.edu.tr







 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.