- Effects of sintering temperature and compressive stress on the ionic conductivity of β''-alumina pellets
Su-Bin Shina, Chea-Yun Kanga, Ji-Won Park, Kyeoung-min Park, Jeong-haeng Heo, In-Ho Imb,* and Seung-Hwan Leea,*
aDepartment of Battery Convergence Engineering, Kangwon National University, Chuncheon 24341, Republic of Korea
bDepartment of Electrical Engineering, Shinansan University, Ansan 15435, Republic of KoreaThis article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
This study systematically analyzed the effects of sintering temperature and compressive stress on the ionic conductivity of β''-alumina pellets. The pellets were prepared at three sintering temperatures: 1500 °C, 1550 °C, and 1600 °C, with some undergoing a separate pre-sintering compression process to induce initial densification. The pellets subjected to the pre-sintering compression process were sintered under 4 MPa pressure, and their ionic conductivity was measured under compressive stresses of 6 N, 8 N, and 10 N after sintering. In contrast, the pellets without the pre-sintering compression process were analyzed under a 10 MPa sintering condition and compressive stresses of 0 N, 1 N, 2 N, and 4 N. At 1550 °C, the pellets exhibited optimal ionic conductivity, reaching a maximum of 3.99766×10−6 S/cm without the pre-sintering compression process and 2.50621×10−6 S/cm with it. At 1500 °C, the conductivity was low due to the small grain size, while at 1600 °C, it decreased due to insufficient densification. Compressive stress further enhanced conductivity, with the highest performance observed at stress levels of 4 N and 10 N. These results highlight the importance of optimizing the manufacturing process for β''-alumina and suggest its potential for application in all-solid-state batteries.
Keywords: β''-alumina, Ionic conductivity, Sintering temperature, Compressive stress.
Secondary batteries have become a core technology for energy storage and utilization in modern society due to their high energy efficiency and minimal design constraints. They are essential in various fields, including IT devices, energy storage systems (ESS), and electric vehicles (EVs), serving as a foundational technology that supports the shift to a low-carbon society and sustainable energy transition. In particular, the role of ESS in complementing the intermittency of renewable energy and ensuring stable power supply is becoming increasingly significant, driving the demand for high-performance secondary battery technologies.
Currently, the secondary battery market is dominated by lithium-ion batteries; however, issues such as resource depletion and high costs associated with their high integration are emerging. To address these challenges, extensive research is being conducted into new secondary battery technologies that can serve as alternatives to lithium [1, 2]. Among these, sodium-based secondary batteries have gained attention as an economical and stable alternative, owing to the cost-effectiveness and extensive reserves of sodium relative to lithium. Particularly in large-scale energy storage systems like ESS, where stable and cost-effective storage technologies are more critical than high energy density, sodium-based batteries can provide sufficient performance.
However, when using organic liquid electrolytes, safety issues arise due to the continuous degradation of the solid electrolyte interface (SEI) layer [3]. Similar to lithium-ion batteries, sodium-ion batteries are not entirely free from the risks of fire and explosion. To overcome these challenges, solid-state batteries are gaining traction as a viable solution, with the development of solid electrolytes being a key technology for their advancement [4].
Solid electrolytes used in solid-state batteries are primarily classified into oxide-based, polymer-based, and sulfide-based categories, each with its own strengths and weaknesses. Polymer-based electrolytes suffer from low thermal stability due to severe flammability, limiting their application. Sulfide-based electrolytes readily interact with moisture in the air, producing H and emitting H₂S gas, a highly toxic substance that raises major safety issues [5, 6]. In contrast, oxide-based electrolytes offer high ionic conductivity and superior stability, making them a focal point as next-generation solid electrolyte materials for solid-state batteries [7, 8, 9].
Among oxide-based electrolytes, beta-alumina has garnered attention as a representative sodium-ion conductive oxide material. Beta-alumina exhibits superior ionic conductivity, high chemical and thermal stability, and excellent electrochemical stability compared to other materials. Its structure comprises conduction planes and spinel blocks, which support its high performance. The conduction planes, composed of loose-packed layers, allow sodium ions to move freely, providing excellent ionic conductivity. The spinel blocks consist of densely packed slabs, where aluminum ions occupy octahedral and tetrahedral interstices between four oxygen ion layers [10]. Based on its stable structure and exceptional durability, beta-alumina is recognized as a key material suitable for solid-state batteries.
This study aims to optimize the manufacturing process of beta-alumina pellets and analyze their ionic conductivity characteristics under varying sintering temperatures and compressive stress conditions. These findings are expected to explore the practical feasibility of beta-alumina-based solid-state batteries and contribute to accelerating the commercialization of sodium-based solid-state batteries in the future.
In this study, 0.5 g of β''-alumina powder was used to form samples by applying uniaxial pressure in a 14Φ-shaped mold, followed by sintering. The sintering process was conducted at temperatures ranging from 1500 °C to 1600°C in 50 °C intervals, with a heating rate of 5 °C/min, and held for 10 h at each temperature. The experimental conditions were divided into two categories based on the pre-sintering compression process.
In Condition 1, the samples were subjected to uniaxial pressure of 10 MPa before sintering, followed by polishing to a thickness of 1 mm and Au sputtering after sintering. The ionic conductivity was then measured under external compressive stresses of 0 N, 1 N, 2 N, and 4 N. In Condition 2, a separate pre-sintering compression process was applied, where the samples were subjected to uniaxial pressure of 4 MPa, followed by 60 MPa of isostatic pressure for 1 minute. After sintering, the samples were polished to a thickness of 1 mm, sputtered with Au, and the ionic conductivity was measured under external compressive stresses of 6 N, 8 N, and 10 N. An impedance analyzer was utilized to measure the ionic conductivity of the prepared samples, and the correlation between applied pressure and ionic conductivity was evaluated.
Impedance measurements were performed in the frequency range of 7 MHz to 100 mHz. The measurements were conducted at room temperature. After the impedance measurements, the ionic conductivity values were calculated by observing the Nyquist plot (Fig. 1).
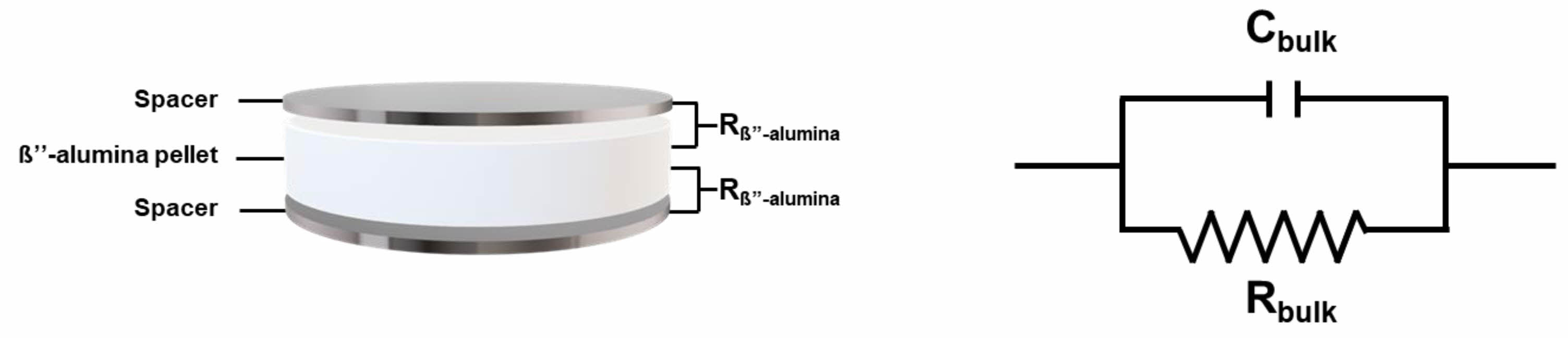
|
Fig. 1 Equivalent circuit for the EIS of a cell and Schematic of the symmetric cell. |
To determine the optimal conditions, thermal analysis (DSC/TGA) was conducted. As shown in Fig. 2, no significant weight loss was observed above 500 °C, indicating thermal stability of the material within this range. Although the analysis was limited to 800 °C, it provides useful insight into the absence of low-temperature phase transitions prior to sintering. This supports the selection of 1500-1600 °C as the sintering range, as no precursor decomposition or intermediate phase changes were detected below 800 °C. For high-temperature phase evolution, structural analysis such as XRD was employed to complement the thermal analysis.
The sintering temperature was identified as a key factor significantly influencing the bulk resistance and ionic conductivity of β''-alumina pellets. For the pellet sintered at 1500 °C, the ionic conductivity was measured to be 2.24687×10⁻⁶ S/cm. This is attributed to the grain boundaries acting as physical barriers in the ion transport process, thereby limiting ionic conductivity. In particular, grain boundaries, which are structurally defective and inherently imperfect, exhibit significantly higher resistance at lower temperatures due to the pronounced effect of these defects. This phenomenon suggests that insufficient sintering reactions occur at low sintering temperatures, leading to a substantial increase in contact resistance between particles. Such an analysis aligns with previous research findings and underscores the critical importance of sintering temperature in determining the microstructure and electrochemical properties of materials [11]. At low sintering temperatures, inadequate bonding between particles hinders the proper formation of the microstructure, thereby obstructing ion transport pathways and significantly reducing conduction performance (Fig. 3).
In contrast, the pellet sintered at 1550 °C exhibited a substantial reduction in bulk resistance, and its ionic conductivity improved markedly to 3.99766×10⁻⁶ S/cm. This result indicates that an appropriate sintering temperature significantly improves the pellet's microstructure and facilitates densification, enabling the formation of efficient ion transport pathways. Under optimal sintering conditions, the bonding strength between particles increases, contact resistance decreases significantly, and obstacles that hinder ionic conductivity are removed. At this temperature, the pellet demonstrates a uniform structural characteristic, which effectively ensures smoother ion transport pathways [11] (Fig. 3).
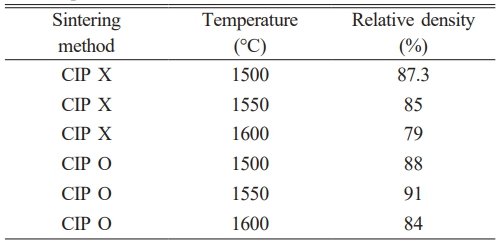
On the other hand, the pellet sintered at 1600 °C showed an ionic conductivity of 2.09595×10⁻⁶ S/cm, which is the lowest among all samples. This trend is interpreted as a result of insufficient densification at high sintering temperatures. At 1600 °C, the pores were not completely eliminated, leading to a lower relative density, which in turn disrupted the ionic conduction pathways and reduced conductivity. These results indicate that excessive sintering temperatures can negatively affect the densification of the electrolyte [12] (Fig. 3, 4, Table 1).
When compressive stresses of 0 N, 1 N, 2 N, and 4 N were applied, the ionic conductivity of β''-alumina pellets showed a distinct improvement with increasing stress. Notably, the ionic conductivity measured at 0 N was the lowest under all sintering conditions, indicating that the high interparticle contact resistance and pores within the pellet limited the ion migration pathways. In the absence of compressive stress, the weak bonding between particles indicates that ion transport does not occur efficiently. For compressive stresses above 1 N, the ionic conductivity gradually increased. This phenomenon is likely due to the improved interparticle contact as compressive stress was applied. Particularly, at 4 N of compressive stress, the bulk resistance was minimized, and the ionic conductivity reached its highest value (Fig. 3).
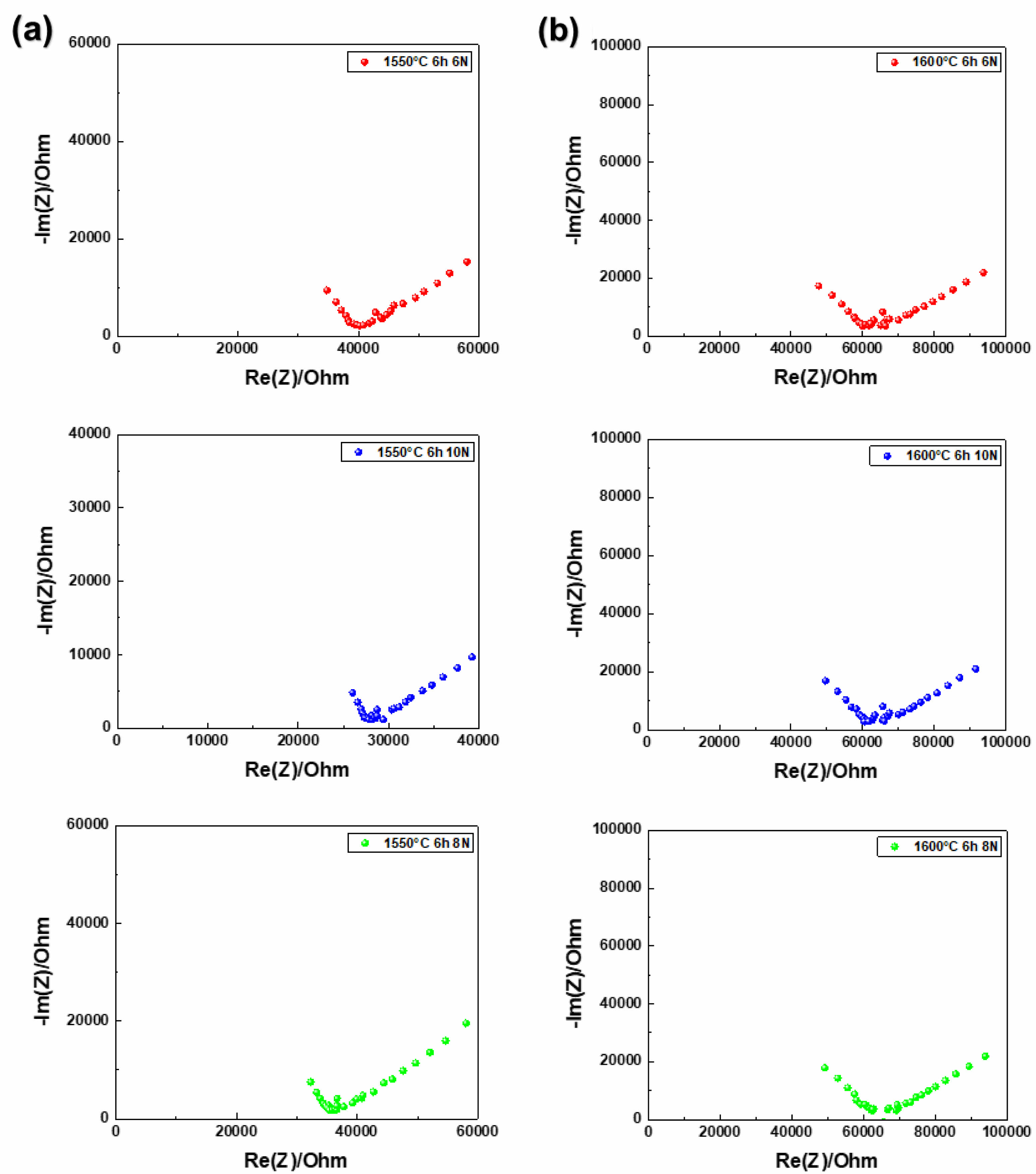
In the case of pellets sintered at 1550 °C, those subjected to a separate pre-sintering compression process demonstrated a gradual increase in ionic conductivity with increasing external compressive stresses (6 N, 8 N, 10 N). The ionic conductivity was measured at 1.75435×10⁻⁶ S/cm under 6 N and improved to 2.50621×10⁻⁶ S/cm under 10 N [13] (Fig. 5).
These results suggest that compressive stress plays a positive role in stabilizing the internal microstructure and enhancing ion migration pathways. They align with earlier research demonstrating the positive impact of compressive stress on enhancing ionic conductivity in solid electrolytes. Fig. 6
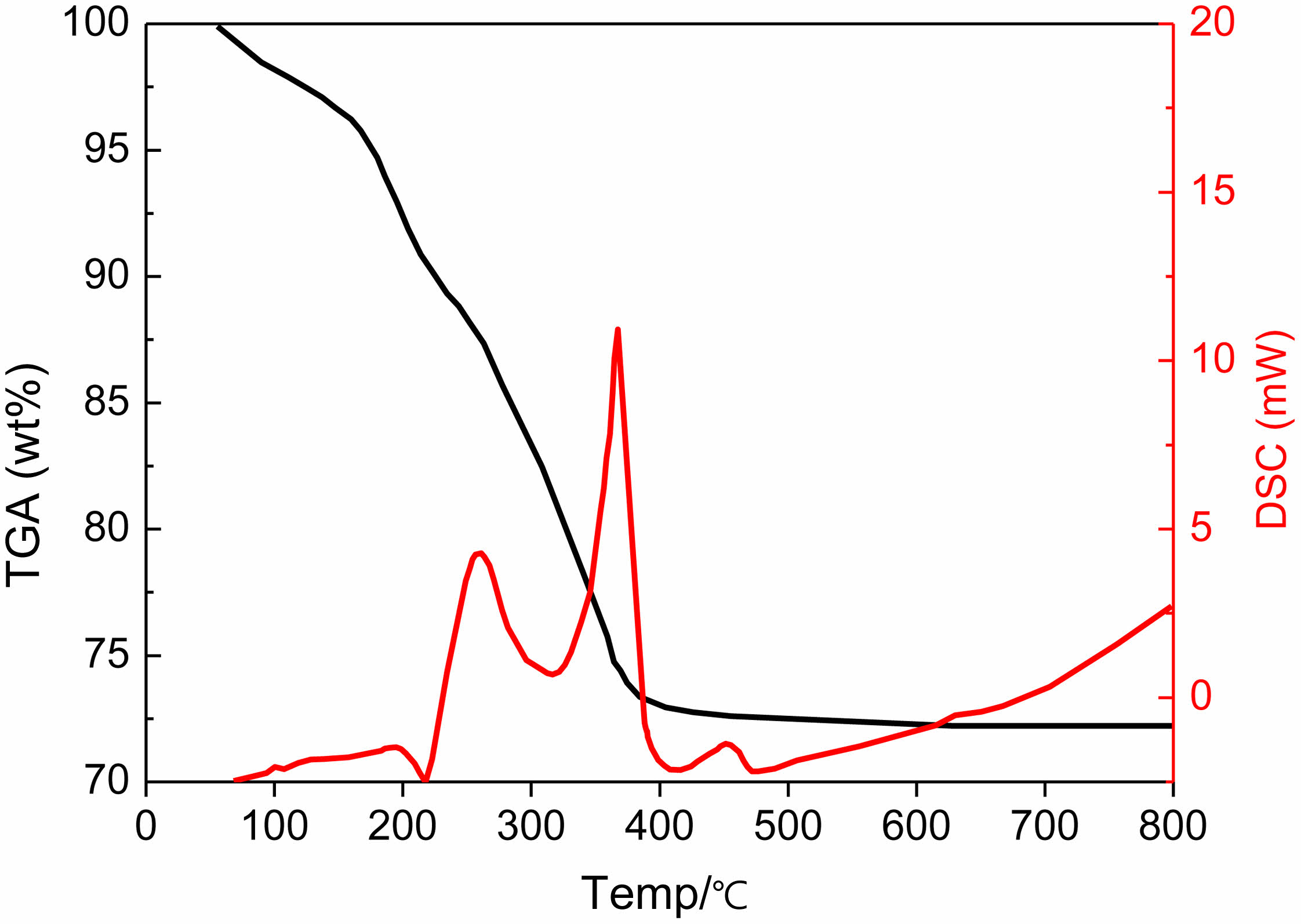
|
Fig. 2 TGA/DSC curves of β''-alumina. |
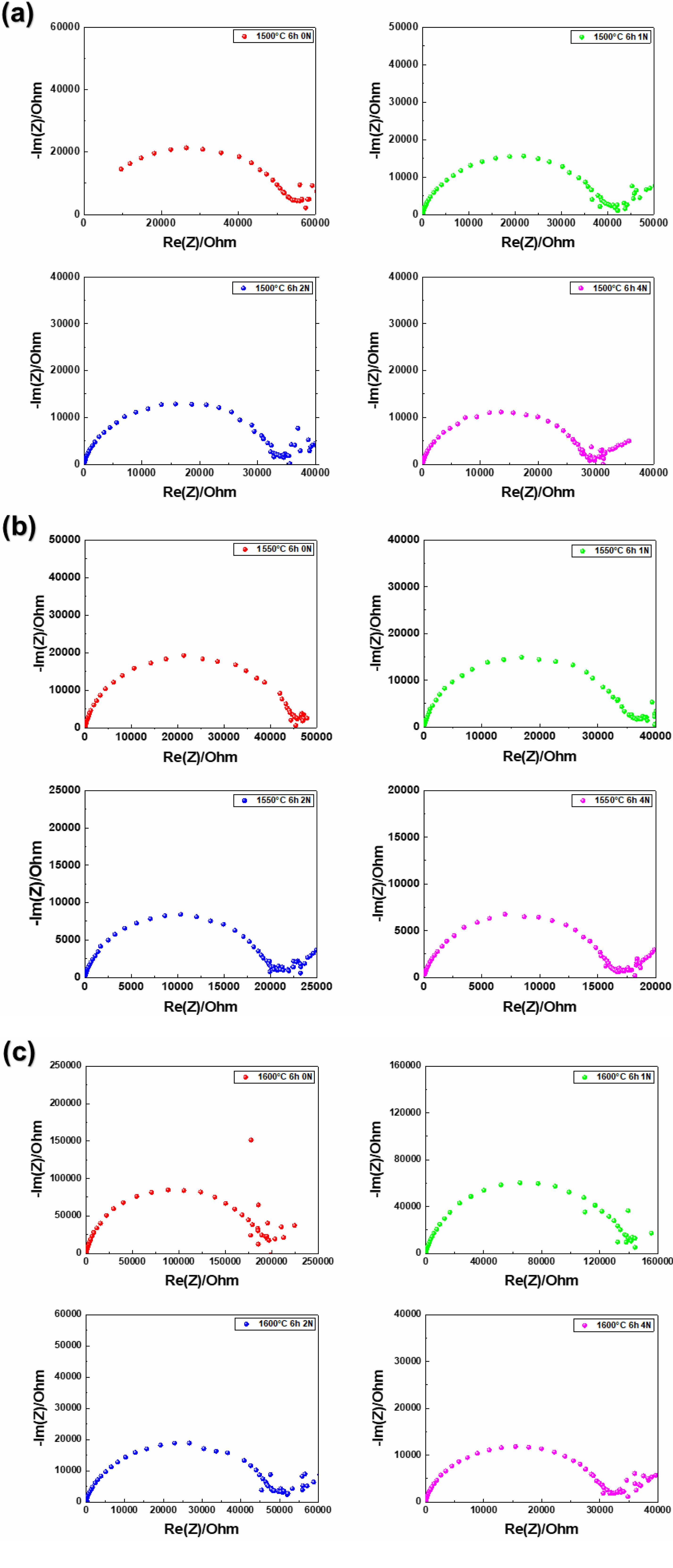
|
Fig. 3 Nyquist plots of β''-alumina pellets sintered at (a) 1500 °C, (b) 1550 °C, (c) 1600 °C, measured under compressive forces of 0 N, 1 N, 2 N, 4 N. |
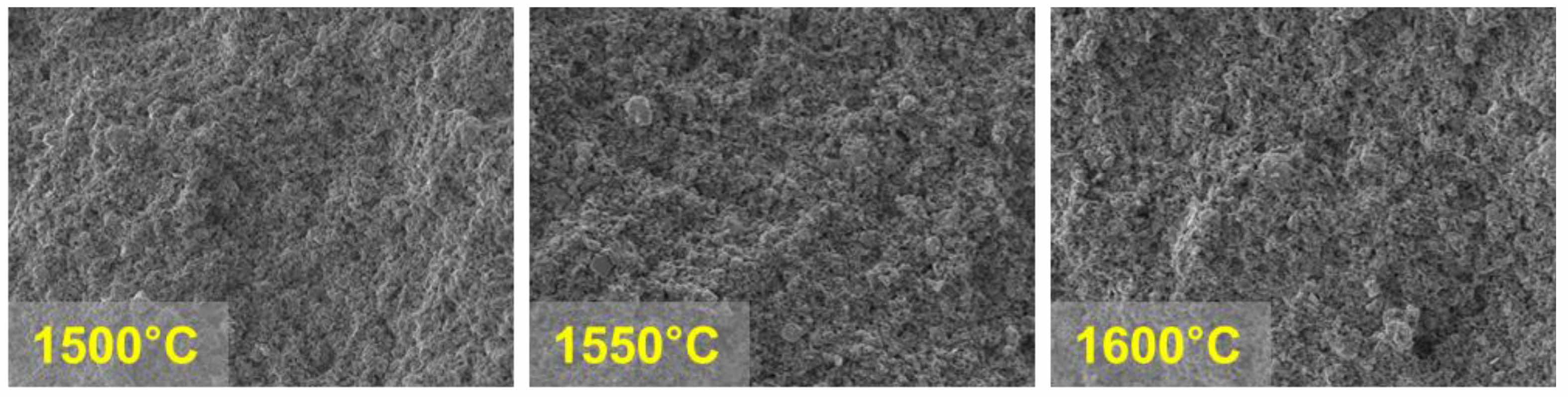
|
Fig. 4 SEM Images of β-Alumina Sintered at Different Temperatures: (a) 1500 °C, (b) 1550 °C, (c) 1600 °C. |
|
Fig. 5 Nyquist plots of β''-alumina pellets sintered at (a) 1550 °C, (b) 1600 °C, measured under compressive forces of 6 N, 8 N, 10 N. |
|
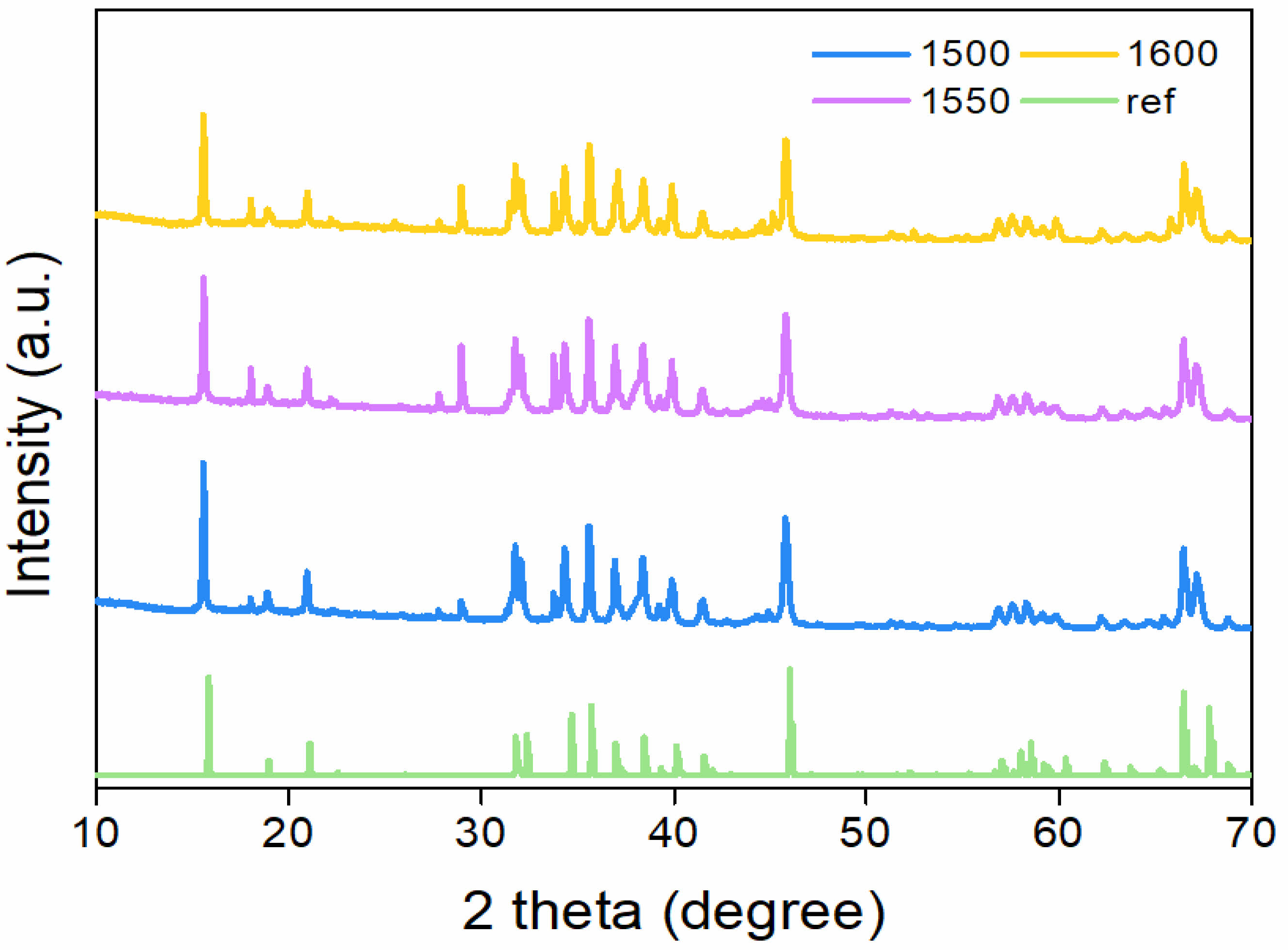
Fig. 6 XRD patterns of β-Alumina pellets sintered at 1500 °C, 1550 °C, 1600 °C. |
In this paper, the effects of sintering temperature and compressive stress on the ionic conductivity of β''-alumina pellets were systematically analyzed. The results showed that sintering temperature significantly impacts the microstructure and ionic conductivity, with 1550 °C identified as the optimal condition for achieving high conductivity due to improved densification and reduced porosity. Compressive stress played a crucial role in reducing interparticle resistance and stabilizing the microstructure, thereby enhancing ionic conductivity. Higher compressive stress resulted in superior performance. These findings underscore the importance of optimizing sintering and compressive stress conditions to maximize the ionic conductivity of β''-alumina pellets.
The authors declare that they have no competing interests.
- 1. S.W. Seo, M. Yoon, J.H. Lee, J.H. Seo, and J. Lee, Trans. Electr. Electron. Mater. 26[1] (2025) 29-36.
-

- 2. Y.W. Yoo, H.S. Oh, J.K. Lee, J.R. Yoon, and S.H. Lee, Energy Materials Advances 5 (2024) 0147.
-

- 3. E.J. Kim, Master’s Thesis, Kyungpook National University, 2023.
- 4. J.Y. Hwang, S.T. Myung, and Y.K. Sun, Chem. Soc. Rev. 46 (2017) 3529-3614.
-

- 5. M. Shoji, E.J. Cheng, T. Kimura, and K. Kanamura, J. Phys. D: Appl. Phys. 52 (2019) 103001.
-

- 6. Y.W. Yoo, C.Y. Kang, H.K. Kim, J.K. Lee, R.V. Kumar, K.N. Kim, J.R. Yoon, and S.H. Lee, Energy & Environmental Materials 8 (2025) e12778.
-

- 7. C. Zhao, L. Liu, G. Xing, X. Lu, F. Wu, J. Zhao, Y. An, S. Hu, and Q. Chen, Adv. Energy Mater. 8 (2018) 1703012.
-

- 8. S. Polat, M. Mashrah, and A. Maksur, Trans. Electr. Electron. Mater. 25 (2024) 801-810.
-

- 9. R.H. Lee, H.N. Jo, C.Y. Kang, J.K. Lee, H.S. Kim, B.S. Jin, K.N. Kim, J.R. Yoon, and S.H. Lee, Chem. Eng. J. 502 (2024) 158175.
-

- 10. X. Lu, G. Xia, J.P. Lemmon, and Z. Yang, J. Power Sources 195 (2010) 2431.
-

- 11. G.M. Lee, Master’s Thesis, Konkuk University, 2013.
- 12. Y.W. Rhee, S.W. Jung, and S.J.L. Kang, J. Korean Powder Metall. Inst. 5[1] (1998) 2-14.
- 13. U.M. Attia, Crit. Rev. Solid State Mater. Sci. 46[6] (2021) 587-610.
-

 This Article
This Article
-
2025; 26(2): 261-266
Published on Apr 30, 2025
- 10.36410/jcpr.2025.26.2.261
- Received on Jan 15, 2025
- Revised on Mar 27, 2025
- Accepted on Apr 1, 2025
 Services
Services
- Abstract
introduction
experimental
result and discussion
conclusion
- Conflict of Interest
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- In-Ho Im b and Seung-Hwan Lee a
-
aDepartment of Battery Convergence Engineering, Kangwon National University, Chuncheon 24341, Republic of Korea
bDepartment of Electrical Engineering, Shinansan University, Ansan 15435, Republic of Korea
Tel : +82-33-250-6265 - E-mail: iminho@sau.ac.kr, shlee@kangwon.ac.kr






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.