- Effect of synthesis conditions on cristobalite crystallization in low-cost ceramic membranes
Nevzat Zerena and Serkan Abalıb,*
aDepartment of Bioengineering and Materials Engineering, Çanakkale Onsekiz Mart University, Çanakkale, 17100, Türkiye
bDepartment of Materials Science and Engineering, Çanakkale Onsekiz Mart University, Çanakkale, 17100, TürkiyeThis article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
The raw materials quartz, calcite, kaolin, and zeolite were used to fabricate β-cristobalite-based ceramic membranes at low temperatures. The raw materials were divided into two groups: calcite and zeolite. Calcite and zeolite raw material mixtures were prepared in different proportions by weight. Quartz-calcite-kaolin and quartz-zeolite-kaolin raw material mixtures were subjected to grinding, drying and shaping processes and then sintered separately at temperatures of 1100 °C, 1150 °C, and 1200 °C in a 7-hour furnace regime. All samples were subjected to X-ray diffraction (XRD), scanning electron microscopy (SEM), Brunauer–Emmett–Teller (BET), density, and mechanical testing. The effects of various raw materials with different compositions and sintering temperatures on the characteristics of ceramic membranes were examined. The necessary conditions for sufficient cristobalite production were created by a sintering temperature of 1200 °C with zeolite as the raw material. These criteria are also suitable for homogeneous pore size distribution and crystal structure formation. The flexural strength of the membrane containing 10 wt.% zeolite sintered at 1200 °C was 34.75 N.mm-2. The average pore diameter of the membrane sintered at 1200 °C with an initial zeolite content of 25 wt.%, was 33.95 nm.
Keywords: β-Cristobalite, Characterization, Strength, Sintering, Zeolite.
Membranes are suitable for filtration because they require significantly less energy than other separation methods such as distillation and electrodialysis [1]. Compared with commercially used polymer membranes, ceramic membranes have advantages such as a narrow pore size distribution, high porosity, and high mechanical stability [2]. Ceramic membranes with high chemical, mechanical, and thermal stabilities are prepared from pure oxides such as Al2O3, TiO2, ZrO2, and SiO2 [3]. However, the use of expensive inorganic precursors to obtain these oxides increases the cost and requires high sintering temperatures. Low-cost ceramic membrane fabrication significantly reduces fabrication costs while preserving the advantages of ceramic materials. The use of cheaper raw materials such as clay, kaolin, quartz, feldspar, and CaCO3 has been reported for the preparation of ceramic membranes suitable for microfiltration [4, 5].
Quartz is a preferred raw material in the fabrication of ceramic membranes because of its mechanical strength and environmentally friendly properties [6]. The β-cristobalite phase obtained during the processing of ceramic membranes transforms into α-cristobalite during cooling [7]. This transformation may cause microcrackings, decreased strength, and material deformation owing to the volume changes. Therefore, it is important that the quartz raw material used in the fabrication of ceramic membranes be doped with appropriate additives [8].
Kaolin contributes to ceramic membranes with small pore sizes and good mechanical stability [1]. Kaolin is utilized in investigations wherein CaCO3 is doped to produce cost-effective ceramic membranes for filtration applications [9, 10]. Kaolin minerals with Si/Al ratios of 1 can be easily used as raw materials for the synthesis of low-silica zeolites [11].
Zeolites are crystalline porous aluminosilicate minerals that are effective in pore and channel systems, catalysis, and separation [11]. Zeolites can remove many pollutants from water through their ion-exchange ability [12, 13]. Zeolite-containing membranes have two types of pore structure: nanopores and micropores [13]. Natural zeolite minerals are suitable for use as the main component in the manufacturing of mineral-based ceramic membranes, partly because they do not swell in water and easily form a suspension for coating membranes on porous supports [14]. Previous studies have shown that zeolites affect the transformation of quartz into cristobalite during sintering [15, 16]. Zeolites at the right rate are known to have a pore-forming effect and enable the transformation of quartz into β-cristobalite. The added zeolite acts as a phase-transforming agent by creating a eutectic point. Zeolite melts at a lower temperature (approximately 800 °C) than quartz and ensures that the liquid phase is well-distributed throughout the quartz particles. Thus, when the mechanism that increases the crystallization of SiO2 is activated, the cristobalite phase, which normally occurs at high temperatures (approximately 1470 °C), is formed at a higher rate at a lower temperature (1200 °C) [8].
Calcite, in the form of CaCO3, decomposes into calcium oxide (CaO) and carbon dioxide (CO2) at temperatures above 650 °C. The voids formed by the release of CO2 gas bubbles during sintering give the membrane a porous texture [17]. In addition, Kouras et al. [18] observed that the conversion of quartz to tridymite is facilitated by the addition of calcite to quartz during the fabrication of ceramic membranes. However, this study showed that calcite cannot lower the temperature at which the cristobalite phase forms. In this study, it was revealed that calcite also affects flexural strength. Although calcite increases the porosity, it may have negative effects on strength. Tong et al. reported that the fracture strength of the membrane improved when it was doped with 5 wt% CaCO3, whereas the flexural strength decreased at 15 wt% CaCO3 [19].
Apatite, one of the raw materials used in the production of low-cost ceramic membranes, requires expensive support materials such as alumina due to its low strength [1]. There are also studies using diatomite, but stabilization of diatomite is needed; otherwise, alpha formation of the cristaobalite phase occurs, making cost efficiency a disadvantage [20].
Darunee et al. [21] used calcite, kaolin and silica as raw materials to obtain the highest porosity of 43.3% in membranes sintered at 1200 °C; the average strength of these samples was between 28 and 30 MPa. Simão et al. fabricated ceramic membranes with 7-29 MPa flexural strength using low-cost calcium carbonate and different proportions of kaolin, potassium feldspar, albite, quartz and white clay [22]. In another study, Nandi et al. obtained flexural strength values of 3-8 MPa for the fabrication of membranes from low-cost raw materials containing zeolites. These studies show that, while attempting to obtain sufficient porosity in ceramic membrane fabrication, the mechanical strength cannot be improved sufficiently. The material composition and pore-forming agents significantly influence the production of cost-effective membranes. Currently, low-cost ceramic membranes are applied in limited areas such as high-temperature filtration. However, there are opportunities to expand their application [23].
In this study, the fabrication of β-cristobalite phase using different raw material additives was investigated. The mechanical and microstructural properties of all structures were examined for a membrane with homogeneous pore size distribution and sufficient mechanical properties. Increasing the sintering temperature caused the porosity to decrease and the flexural strength to increase. However, it is possible to fabricate membranes with high pore volumes and flexural strength. Therefore, the results obtained by considering the temperature and composition criteria were compared and examined.
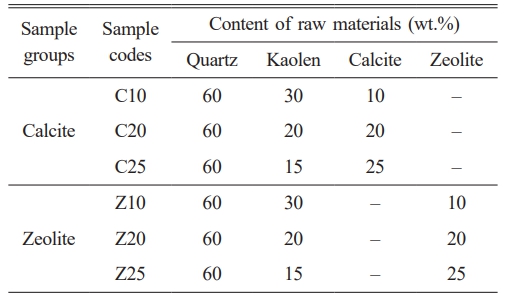
Ceramic structures were prepared from a mixture of two types of raw materials with different compositions. Table 1 lists the compositions of calcite and zeolite groups (designated with the codes C10, C20, C25, Z10, Z20, and Z25 respectively). The raw materials were wet-ball-milled for 6 h (Gabbrielli, Calenzano, Italy) and the samples were then dried in an oven (Memmert UM 600, Germany) at 105 °C for 2 h. The powders were divided into three groups at different sintering temperatures. The samples were uniaxially cold pressed under 100 MPa in a steel die (Nannetti, Faenza, Italy). After drying, the samples were sintered at 1100, 1150, and 1200 °C with a soaking time of 120 min and heated at a rate of 20 °C.min-1 (MSE Furnace, Turkey).
Mineralogical analyses of the samples were performed using X-ray diffraction (XRD, PANalytical Empyrean). The samples were scanned using Cu-Kα radiation in a 2θ range of 10-80°. Scanning electron microscopy (Zeiss GeminiSEM, Germany) was performed to evaluate the development of porosity and crystallization with variations in sintering temperature and basic composition. The samples were sputter-coated with Au-Pd (80:20 wt.%) in a coating device. To examine the effect of temperature and composition on the density, the density of each membrane was determined according to the ASTM D 792 standard test method (Metler, Switzerland). A homogeneous distribution ensured that filtration processes were performed correctly. In addition, pore size is important for minimizing the pressure drop during the filtration process. For this reason, the pore volumes, size distributions and average size of the samples were measured using a Brunauer–Emmett–Teller surface area analyzer (Micromeritics Gemini VII, USA). Nitrogen adsorption measurements were performed for all samples after a de-gassing process for 3 h at 300 °C. The porous samples also exhibited sufficient strength during filtration. The flexural strength of the samples was determined at a loading speed of 1 Mpa.s-1, according to ISO 10545-4 (Gabrielli Crometro CR5/650, Italy).
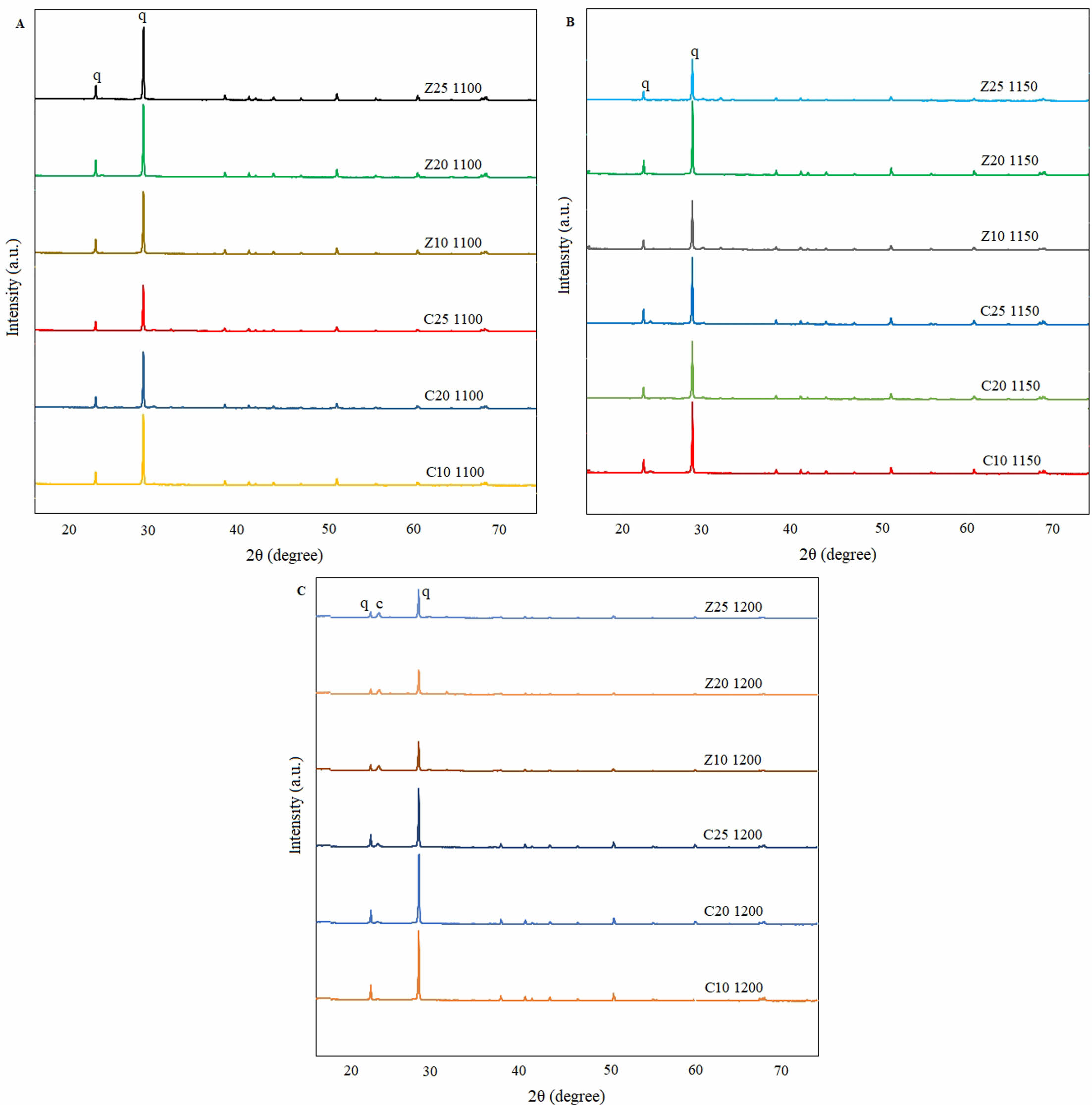
Fig. 1 shows the results of the mineralogical analysis of each sample. Fig. 1a and 1b show that the intensity of the quartz peaks (JCPDS 47-1144) is high at 1100 °C and 1150 °C, and that the cristobalite phase does not form. Fig. 1c shows that quartz began to transform into cristobalite (JCPDS 39-1425), especially in samples containing zeolite; the intensity of the quartz peak decreased and a cristobalite phase appeared [24]. Although the transformation of quartz into cristobalite occurs above 1400 °C under normal condition, the cristobalite phase is released at lower temperatures. In all samples containing zeolite sintered at 1200 °C, cristobalite appeared. Therefore, zeolites accelerate the transformation of quartz into cristobalite at low temperatures [8]. Zeolite melts at low temperatures, forms a viscous phase around the quartz grains, and begins to accelerate the dissolution of the cristobalite phase. There are two important criteria for the formation of cristobalite phases in ceramic membranes: zeolite and a sintering temperature of 1200 °C. Neither zeolite nor temperature alone were sufficient for the formation of the cristobalite phase at low temperatures. Kaolin is a raw material whose composition changes in a manner similar to that of zeolite and calcite. Hedfi et al. reported that kaolin also contributes to the formation of the cristobalite phase [25]. However, cristobalite formation was not observed in any of the K-series samples. However, cristobalite formed in the Z-series samples at 1200 °C. Thus, in the calcite group, the change in the composition of kaolin alone had no effect on the cristobalite formation at 1200 °C.
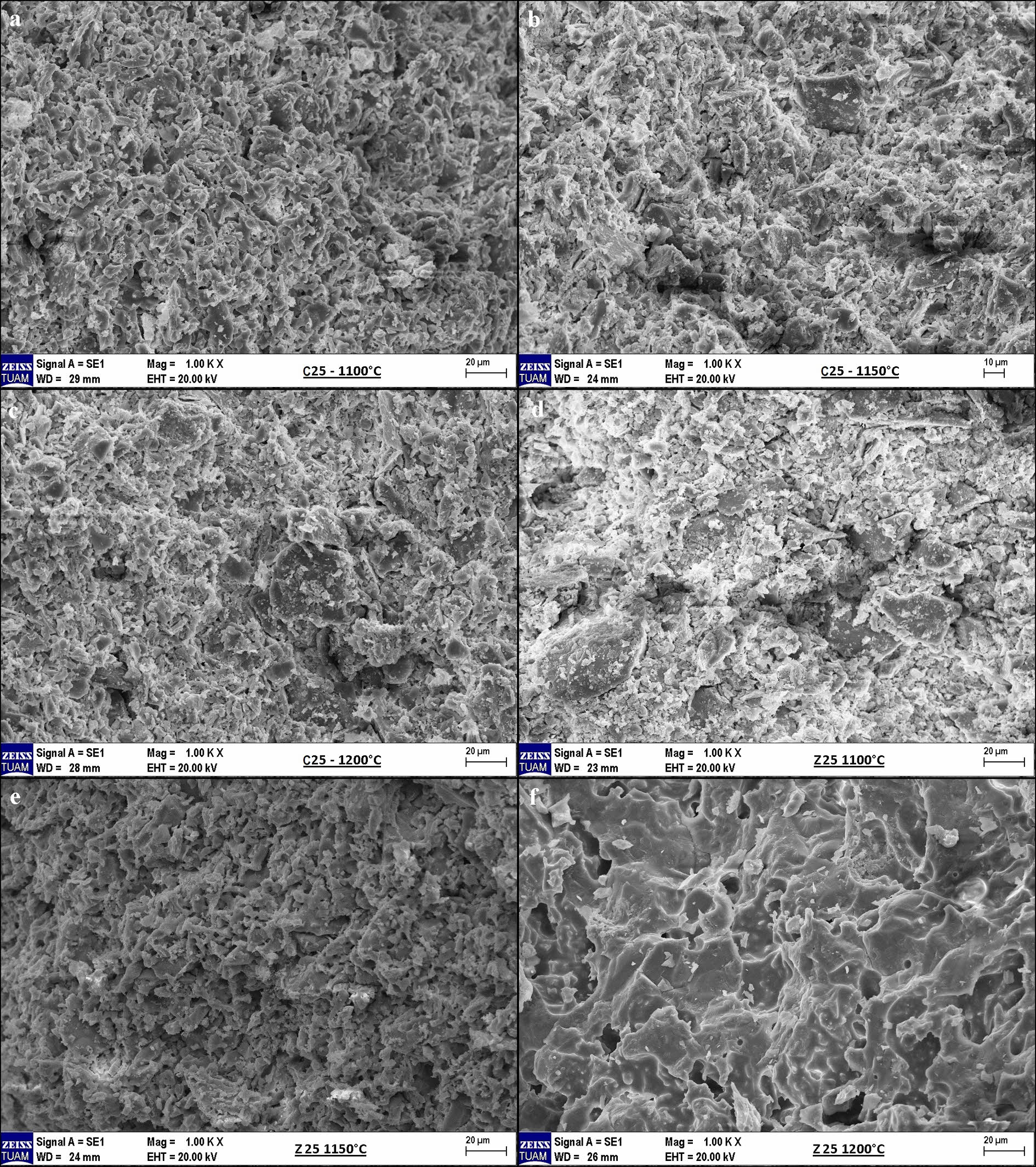
Important criteria in the surface morphology of ceramic membranes are homogeneous pore distribution and the formation of a non-localized, low amount of vitreous phase: vitrification must be completed and crystallization must occur [26]. Mineralogical analysis showed that the transformation of quartz to cristobalite occurred because of the degree of crystallization. Consequently, the type of raw material (calcite or zeolite) and temperature were the determining factors for the microstructural images. The change in the weight percentage composition of zeolite or calcite was insignificant. For this reason, Fig. 2 shows images of the membranes containing 25 wt.% calcite and zeolite raw material in the basic composition for each temperature. In particular, vitrification occurred in the zeolite-containing membrane sintered at 1200 °C. Crystallization was completed and the pores became apparent. Additionally, there were no defects such as pinholes or cracks. Zeolites have been reported to exhibit low negative thermal expansion [27]. Therefore, clinoptilolite-type zeolites may prevent deformation during the transformation from β-cristobalite to α-cristobalite during cooling. Clearly, the temperature alone is insufficient for the fabrication of ceramic membranes. This is because the same microstructure was not formed in the calcite sample sintered at 1200 °C. This indicates that the zeolite acts as a catalyst at low temperatures.
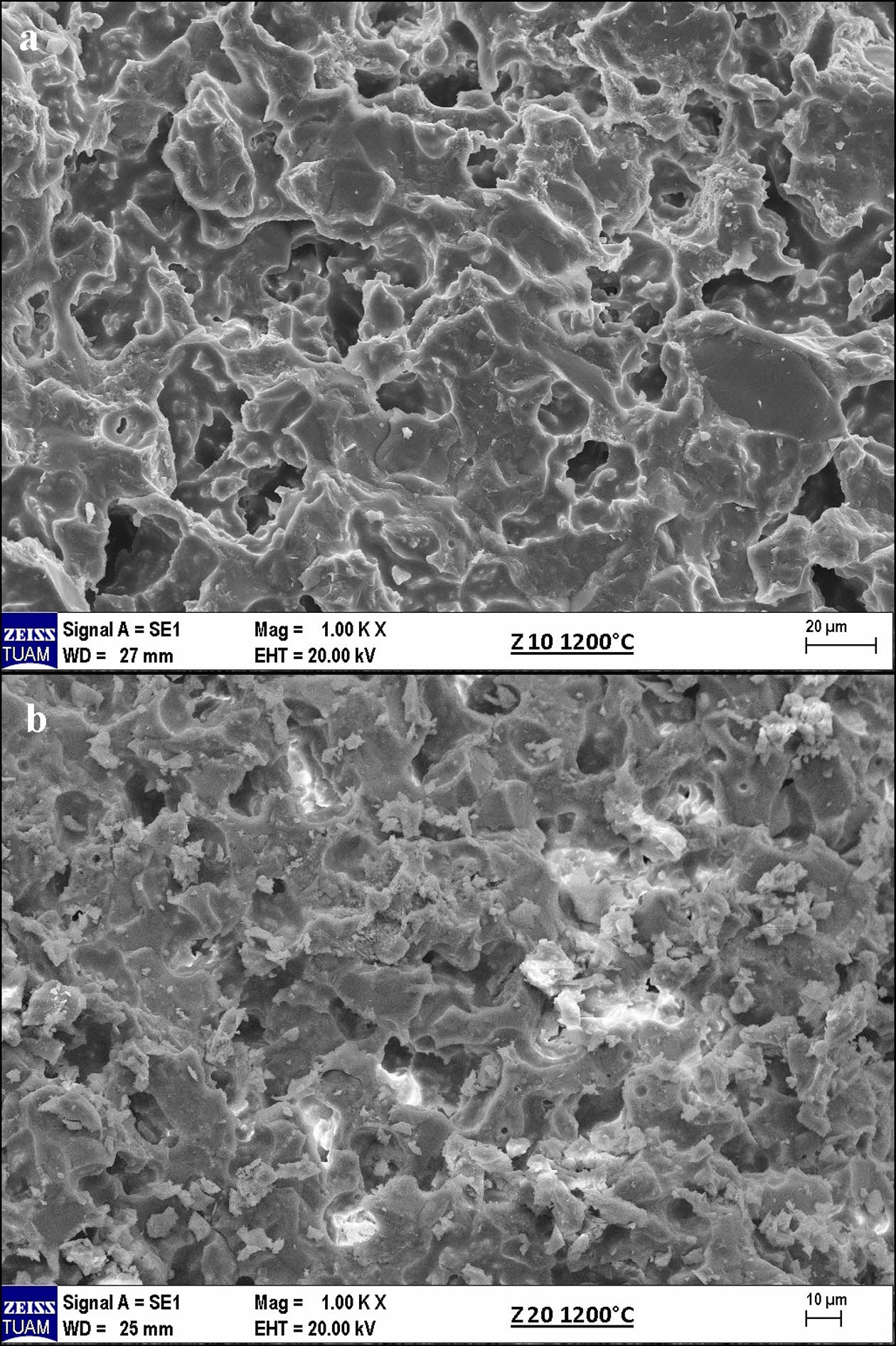
Fig. 3 shows images of another zeolite-containing membranes sintered at 1200 °C. The desired conditions were achieved, regardless of the amount of zeolite used. As shown in Figs. 3 and 4, pore formation in zeolite-containing membranes sintered at 1200 °C and, more importantly, pore formation of similar sizes occurred. This is important to ensure the homogeneity of the filtration process in ceramic membranes. The formation of porous structures as close as possible to each other is important for subsequent filtration processes.
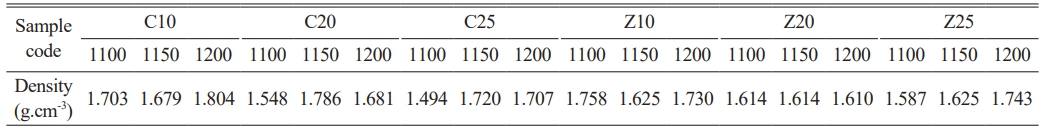
The expectation of ceramic membranes, in terms of density, is that their density is not low enough to contain high porosity. Ceramic membranes with suitable properties must be vitrified and crystallized. Therefore, the density can also be high. In particular, the porosity of high-density membranes in a class of dense membranes can be low. Importantly, the pores are homogeneous in size. Table 2 lists the density values of all samples. In the group containing calcite as a foaming agent, the density of the sample coded C10-1200 was higher than that of the sample coded Z10-1200. In this case, the foaming agent function of calcite may not be as effective as that of a zeolite catalyst at 1200 °C. In general, sufficient condensation occurred in the samples sintered at 1200 °C; whereas, in the calcite group, the sample sintered at 1200 °C and containing 10% calcite by weight had the highest density; whereas, in the zeolite group, the sample with the highest zeolite content by weight and sintered at 1200 °C had the highest density. Normally, as the temperature increases, the density is expected to increase, and the porosity decreases. However, high temperatures are important for sufficient strength and formation of the cristobalite phase. Here, it can be seen that, among the samples sintered at 1200 °C, samples containing 20% calcite and 20% zeolite by weight achieved this balance well. However, it is predicted that the density values of the samples containing 25% calcite, 10% zeolite, and 25% zeolite by weight (from the same temperature series) are not much higher than those of the sample containing 10% calcite (1.804 g.cm-3). These samples will provide a balanced density-pore ratio. When the Z20 series was examined, it was observed that, as the temperature increased, the density initially remained constant and then decreased slightly. The lowest density (1.610 g.cm-3) was observed in the series at 1200 °C, in the Z20 series. Examination of the microstructure images in Fig. 2 may also indicate more pores without compromising the strength value.
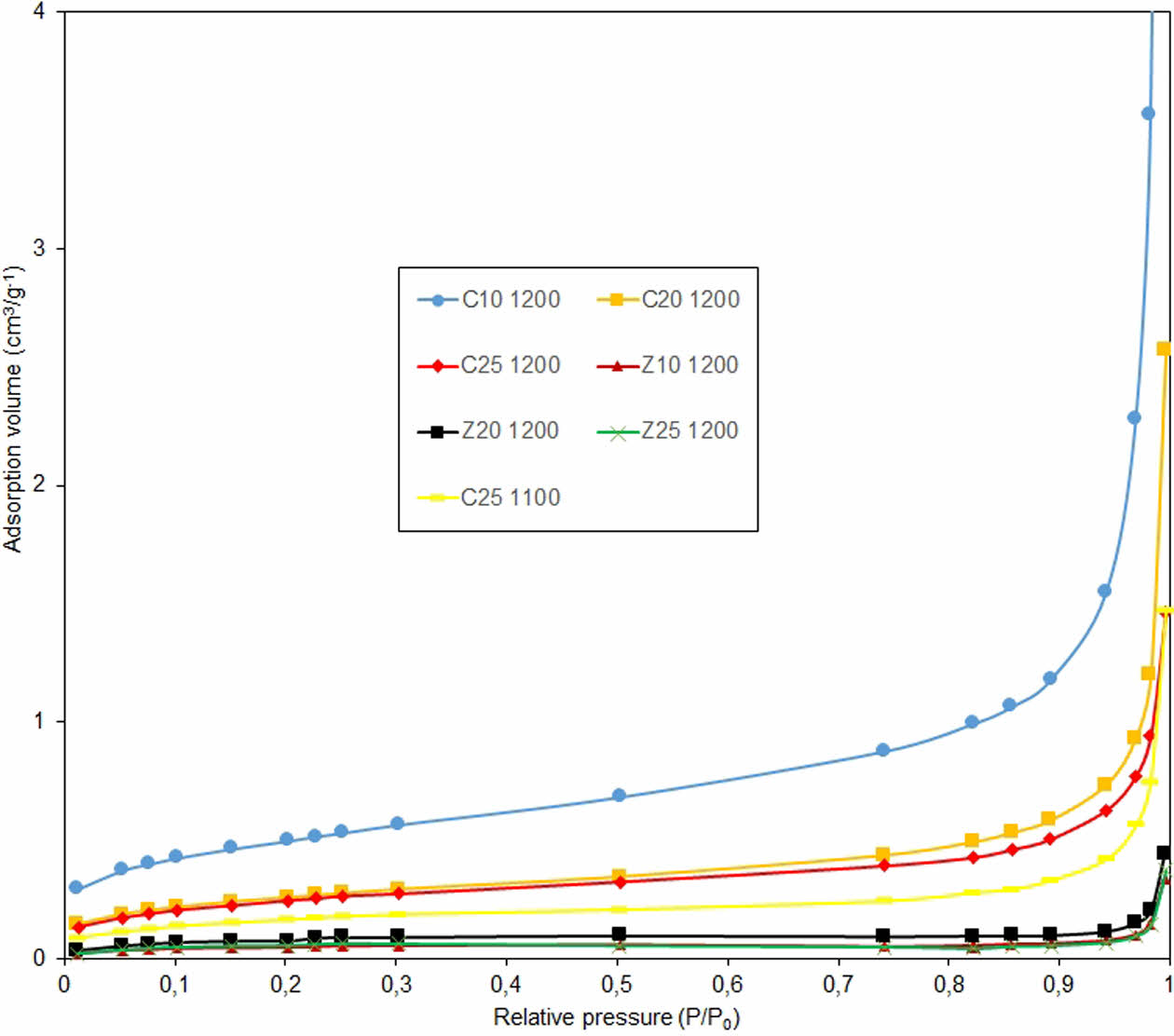
The adsorption isotherm curves of the samples sintered at 1200 °C and those of the calcite group sintered at 1100 °C are shown in Fig. 5. The adsorption isotherms were type III, according to the IUPAC classification [28] and were similar for all the samples. When sintered at 1200 °C, the zeolite-containing membranes exhibited a lower adsorption volume at the same relative pressure. This indicates a lower mesopore and higher crystallization, compared to the calcite-containing samples sintered at 1200 °C. In other words, zeolite-containing samples underwent phase transformations that could improve membrane properties. Notably, the sample coded C25-1100 had a lower volume than the calcite-containing samples sintered at 1200 °C at the same relative pressure. This shows that the temperature increase alone is not sufficient to ensure sufficient crystallization during ceramic membrane fabrication, which is where the zeolite factor comes into play. Zeolite acts as a catalyst for quartz, affecting its transformation into the cristobalite phase. This also supports the XRD and SEM results. Table 2 shows that the C25-1100 sample with lower density has an adsorption volume of approximately 0.35 cm3.gr-1, at a relative pressure value of 0.9 P/P0, meaning that the pore amount is lower than the C10-1200 sample at the same pressure value. In other words, it can be said that density measurements do not have an effect on the amount of pores. A striking feature of the C10-1200 sample was that it had sufficient porosity despite sintering at high temperatures. Adequate strength is important and, therefore, the porosity factor should not reduce the strength.
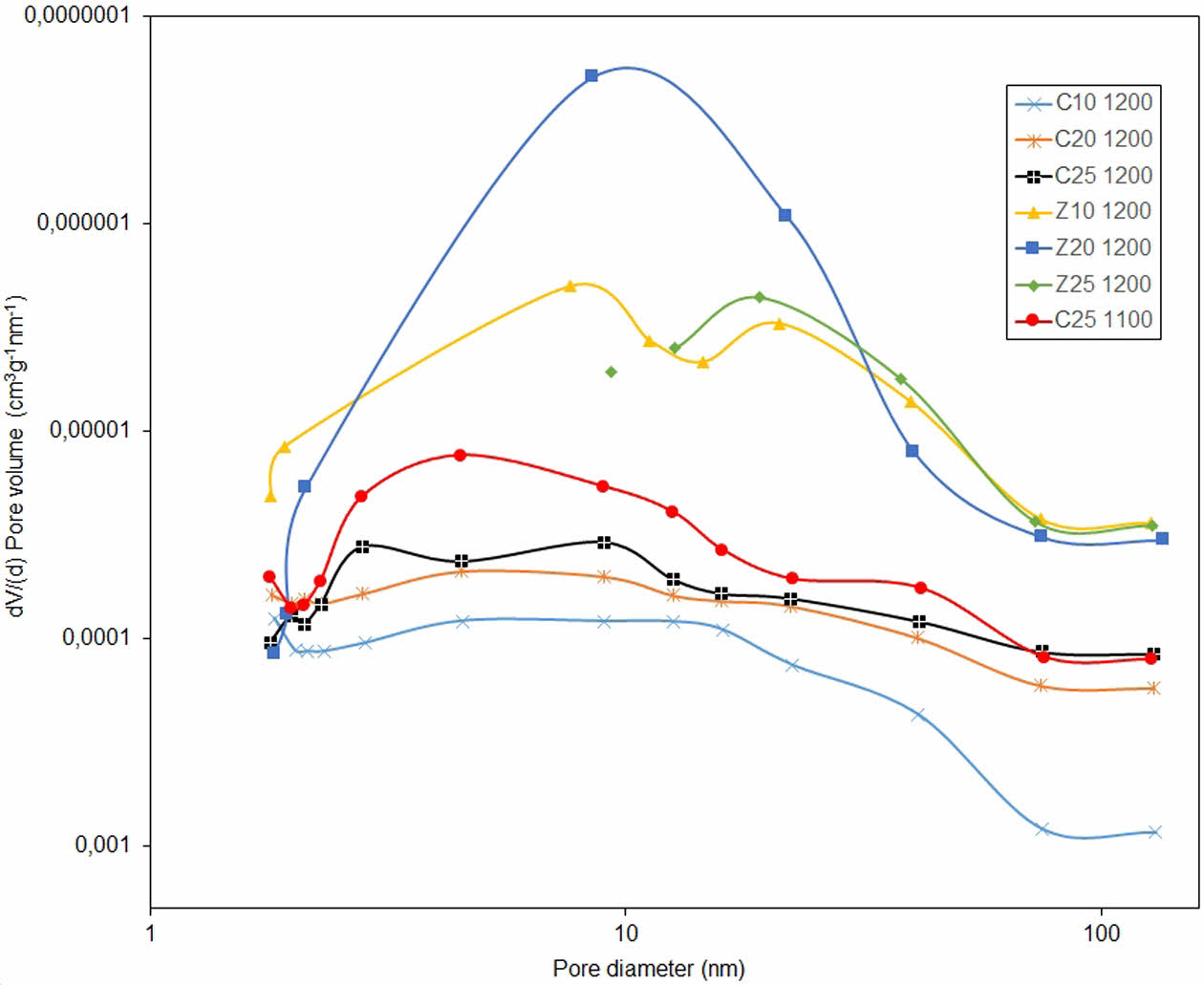
Fig. 6 shows the BJH pore size distributions of the C25-1100 coded sample, together with the samples sintered at 1200 °C. All samples containing calcite had a wide pore size distribution, regardless of the temperature at which they were sintered. This means that there are large and small pores and, in ceramic membranes, it is desirable that the pore sizes are similar, in terms of the homogeneity of the cake formed on the membrane, particularly during the filtration process. This is also important in terms of the homogeneity and strength of the ceramic membrane. In samples containing calcite, a temperature of 1200 °C was not sufficient for densification. Here, the zeolite factor ensures crystallization. Narrow-spaced high peaks were observed in the pore-size distribution graphs of all membranes containing zeolite. This indicated that there were more pores of similar sizes. For example, the Z20-1200 coded sample has a lower pore volume than the other two zeolite series, which shows that it consists of smaller pores. This situation was confirmed by the data presented in Table 3. There were many large pores in the calcite series, but there was no homogeneous pore distribution because there was no narrow range in the graph. In addition, the large pores indicate that the strength may be low and the formation of the cristobalite phase is insufficient. Z10-1200 contains two types of pores, low and slightly larger, in narrow ranges. Z25-1200, on the other hand, contains larger pores than Z10-1200 (which is not as large as calcites, as shown by the data in Table 3) and consists of single peaks. This may have provided Z25-1200 with optimum pore-size characteristics without compromising strength and homogeneity.
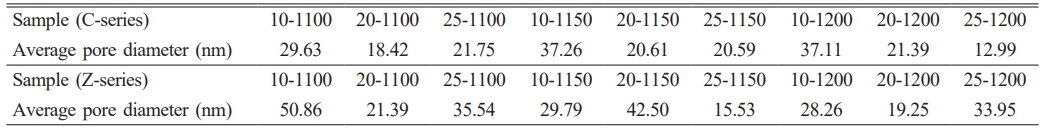
Table 3 presents the average pore sizes of the samples. In the C-series samples, the average pore diameter decreased as the amount of foaming agent calcite increased, regardless of the temperature. In this case, the presence of calcite in the kaolin and quartz recipes had no effect on pore size. The isotherm curves of the C10-1200 sample showed that it had a high pore content despite being sintered at 1200 °C. Although it is known that calcite series do not complete their crystallization, even when sintered at 1200 °C, and, therefore, have a high pore volume, the high pore volume can also be associated with an average pore diameter of 37.11 nm (Table 3). The pore diameters of the Z-series membranes were mostly larger than those of the C-series membranes. In this case, zeolite contributes to the formation of sufficiently large pores, in addition to increasing the transformation of quartz to cristobalite. Except for the Z25-1200 sample, the average pore diameter was generally larger in samples sintered at temperatures lower than 1200 °C. However, this was due to the lack of sufficient crystallization at temperatures lower than 1200 °C. The desired cristobalite phase in the membranes was formed in zeolite-containing samples sintered at 1200 °C. Although there is a decrease in the number of pores at high temperatures where crystallization is completed, the presence of a high average pore diameter in the Z25-1200 sample distinguishes it from other zeolite-containing membranes sintered at 1200 °C. Therefore, even if the number of pores is low, a high pore diameter in a phase that has completed its crystallization can create a more favorable situation in terms of suitable filtration conditions.
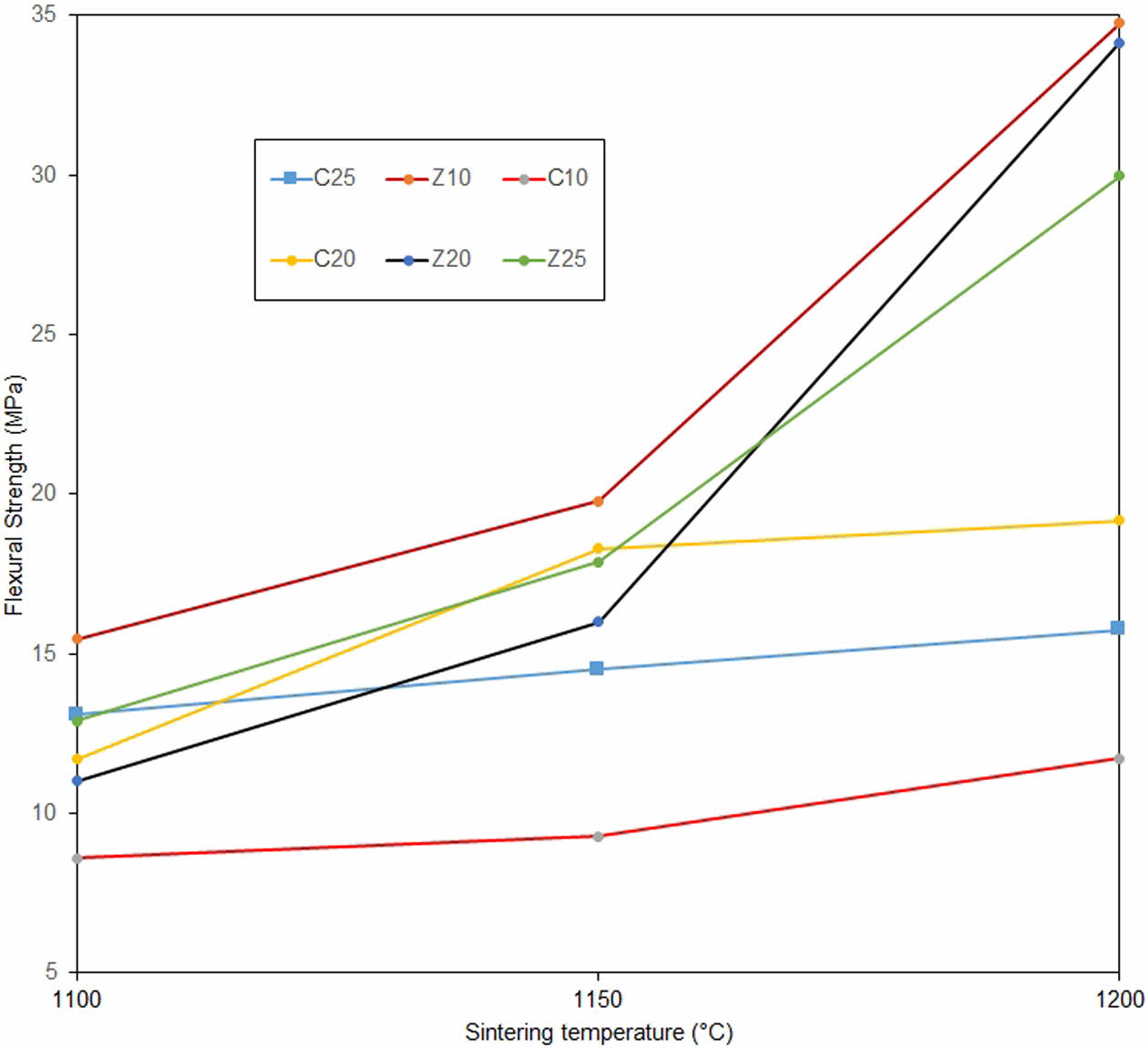
Fig. 7 shows the flexural strengths of the membranes for all compositions and sintering temperatures. For samples containing calcite, the increase in flexural strength was low above 1150 °C, i.e. at 1200 °C; whereas, for membranes containing zeolite, the increase in flexural strength was high at the same temperature values. This supports the notion that zeolite is effective in the transformation of quartz to cristobalite in the quartz-zeolite-kaolin membranes. Although the flexural strength increases with temperature in samples containing calcite, this increase remains low compared with that of zeolite membranes. This was due to the absence of the cristobalite phase and the inability to form sufficient strength. No mechanism exists by which calcite or kaolin transforms quartz into cristobalite at low temperatures. It is likely that the low silica content in the zeolite is effective in the transformation of quartz to crisrobalite [8]. Although the Z10-1200 sample has the highest strength value (34.75 MPa), the increase in strength with sintering temperature is similar in zeolite-containing membranes. Although the determining factor in flexural strength is the cristobalite phase, kaolin is known to affect mechanical stability [1]. In particular, in the Z10-1200 sample, the fact that the amount of kaolin (by weight) was initially higher than that in the other zeolite group samples sintered at 1200 °C may have caused the Z10-1200 coded sample to come to the fore in flexural strength. As shown in Fig. 7, there is no such result for the calcite group. Thus, it can be concluded that zeolite may have had the same catalytic effect on quartz as kaolin. In the calcite samples, the change in strength with increasing temperature, as a result of the compositional change, was more obvious. Although the sample with 20% calcite content by weight was insufficient, it had the highest strength value in the calcite group. The strength values of zeolite-containing membranes have yielded significantly better results than studies in the literature on membranes produced from other low-cost raw materials [8, 29]. At temperatures above 1200 °C, the possibility of increasing closed pores may slow the increase in flexural strength, which is uneconomical at higher temperatures [30].
|
Fig. 1 Mineralogical analyses of C and Z series membranes sintered at various temperatures: a) 1100 °C, b) 1150 °C, and c) 1200 °C. |
|
Fig. 2 Surface morphologies of samples containing 25 wt.% calcite and zeolite in the basic composition and sintered at various temperatures: a,d) 1100 °C, b,e) 1150 °C, and c,f) 1200 °C. |
|
Fig. 3 Surface morphologies of samples containing 10 and 20 wt.% zeolite in the basic composition and sintered at various temperatures: a) 1100 °C, b) 1150 °C. |

|
Fig. 4 Pore structure, distribution, and size in SEM micrographs of sample coded Z10-1200. |
|
Fig. 5 Nitrogen adsorption isotherms of samples sintered at 1200 °C and sample coded C25-1100. |
|
Fig. 6 BJH pore size distributions of samples sintered at 1200 °C and sample coded C25-1100. |
|
Fig. 7 Flexural strengths of samples as functions of temperature and composition. |
|
Table 3 Average pore sizes of samples at various temperatures and compositions. |
Because the clinoptilolite-type zeolite was doped to quartz and kaolin, the zeolite contributed to the formation of the cristobalite phase at a low temperature (1200 °C) and enabled the production of a membrane that completed its crystallization. The foaming agent calcite did not exhibit the same function at the same temperature. Membranes with high strength and homogeneous pore distribution at low temperatures can be developed using low-cost zeolite, quartz, and kaolin formulations. The membrane prepared from the sample containing 25 wt.% zeolite and sintered at 1200 °C attracted attention because of both a unimodal pore distribution and a larger pore volume and size, without compromising the flexural strength. This study revealed promising outcomes regarding the flexural strengths exhibited by samples containing zeolite. Furthermore, it was observed that an increase in kaolin content resulted in a positive effect on the strength of zeolite-containing samples. This observation is correlated with the distinct peak formation of cristobalite in the mineralogical analysis of the Z-series sample, which was synthesized at 1200 °C and exhibited a high kaolin content. This investigation demonstrated that kaolin, analogous to zeolite, functions as a catalyst in the formation of cristobalite. Although the strength remained low in the calcite-containing samples, an increase in the amount of calcite also had a positive effect on strength.
The authors are grateful for financial support by Çanakkale Onsekiz Mart University, The Scientific Research Coordination Unit, Project number: FYL-2022-3889.
- 1. A. Abdullayev, M. Bekheet, D. Hanaor, and A. Gurlo, Membranes 9[9] (2019) 105.
-

- 2. B. Hofs, J. Ogier, D. Vries, E.F. Beerendonk, and E.R. Cornelissen, Sep. Purif. Technol. 79[3] (2011) 365-374.
-

- 3. R. Das, K. Sondhi, S. Majumdar, and S. Sarkar, J. Asian Ceram. Soc. 4[3] (2016) 243-251.
-

- 4. A. Potdar, A. Shukla, and A. Kumar, J. Membr. Sci. 210[2] (2002) 209-225.
-

- 5. A. Belouatek, N. Benderdouche, A. Addou, A. Ouagued, and N. Bettahar, Microporous Mesoporous Mater. 85[1-2] (2005) 163-168.
-

- 6. А.I. Ivanets, А.I. Rat׳ko, Т.А. Azarova, S.М. Azarov, S.H. Al-Khowaiter, О. Al-Harbi, S.V. Shemchonok, V.А. Dobysh, V.А. Tarasevich, V.Е. Agabekov, and А.А. Rat׳ko, Ceram. Int. 40[8] (2014) 12343-12351.
-

- 7. E. Ringdalen, JOM 67[2] (2014) 484-492.
-

- 8. O. Şan, S. Abalı, and Hoşten, Ceram. Int. 29[8] (2003) 927-931.
-

- 9. N. Ediz, I. Tatar, and A. Aydın, J. Ceram. Process. Res. 16[1] (2015) 129-136.
-

- 10. B.K. Nandi, R. Uppaluri, and M.K. Purkait, Appl. Clay Sci. 42[1-2] (2008) 102-110.
-

- 11. M. Kazemimoghadam, J. Ceram. Process. Res. 17[9] (2016) 978-984.
-

- 12. M.R. Adam, M.H. Othman, S.K. Hubadillah, M.H. Abd Aziz, and M.R. Jamalludin, Mater. Today Proc. (2023).
-

- 13. Z. He, Z. Lyu, Q. Gu, L. Zhang, and J. Wang, Colloids Surf. A 578 (2019) 123513.
-

- 14. Y. Dong, S. Chen, X. Zhang, J. Yang, X. Liu, and G. Meng, J. Membr. Sci. 281[1-2] (2006) 592-599.
-

- 15. M. Hernandez-Velez, O. Raymond-Herrera, A. Alvarado-Martin, A. Jacas-Rodriguez, and R. Roque-Malherbe, J. Mater. Sci. Lett. 14[23] (1995) 1653-1656.
-

- 16. S.-H. Xue, H. Xie, H. Ping, Q.-C. Li, B.-L. Su, and Z.-Y. Fu, RSC Adv. 5[88] (2015) 71844-71848.
-

- 17. H. Aripin, E. Priatna, I.N. Sudiana, and S. Sabchevski, Int. J. Eng. 35[2] (2022) 300-306.
-

- 18. N. Kouras, A. Harabi, F. Bouzerara, L. Foughali, A. Policicchio, S. Stelitano, F. Galiano, and A. Figoli, J. Eur. Ceram. Soc. 37[9] (2017) 3159-3165.
-

- 19. Z. Tong, M. Li, D. Li, K. Wu, and X. Yang, J. Ceram. Process. Res. 24[2] (2023) 308-320.
-

- 20. O. Şan and C. Özgür, J. Alloy. Compd. 484[1-2] (2009) 920-923.
-

- 21. B. Darunee, C. Nucharee, and B. Tripob, J. Appl. Membr. Sci. Technol. 9[1] (2017) 27-35.
-

- 22. L. Simão, R.F. Caldato, M.D.M. Innocentini, and O.R. K. Montedo, Ceram. Int. 41[3] (2015) 4782-4788.
-

- 23. Z. Li, Y. Ren, J. Hao, J. Xu, P. Yan, X. Zhang, and L. Li, J. Ceram. Process. Res. 25[3] (2024) 389-403.
-

- 24. H. Mao, H. Cao, W. Fan, W. Li, Y. Wu, X. Chen, M. Qiu, H. Verweij, and Y. Fan, Ceram. Int. 48[12] (2022) 16599-16610.
-

- 25. I. Hedfi, N. Hamdi, E. Srasra, and M.A. Rodríguez, Appl. Clay Sci. 101 (2014), 574-578.
-

- 26. K. Zhang, M. Liu, Z. Han, H. Sun, X. Li, J. Bai, Q. Du, and C. Li, Int. J. Appl. Ceram. Technol. 20[3] (2022) 1701-1714.
-

- 27. W. Miller, C.W. Smith, D.S. Mackenzie, and K.E. Evans, J. Mater. Sci. 44[20] (2009) 5441-5451.
-

- 28. K.S. Sing, D.H. Everett, R.A.W. Haul, L. Moscou, R. A. Pierotti, and J. Rouquerol, Pure Appl. Chem. 57[4] (1985) 603-619.
-

- 29. B.K. Nandi, R. Uppaluri, and M.K. Purkait, Appl. Clay Sci. 42[1-2] (2008) 102-110.
-

- 30. R. Durgun and S. Abalı, Mater. Test. 64[3] (2022) 391-400.
-

 This Article
This Article
-
2025; 26(2): 231-238
Published on Apr 30, 2025
- 10.36410/jcpr.2025.26.2.231
- Received on Nov 20, 2024
- Revised on Feb 19, 2025
- Accepted on Mar 20, 2025
 Services
Services
- Abstract
introduction
experimental procedure
results and discussion
conclusions
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Serkan Abalı
-
Department of Materials Science and Engineering, Çanakkale Onsekiz Mart University, Çanakkale, 17100, Türkiye
Tel : 902862180018 Fax: 902862180541 - E-mail: sabali@comu.edu.tr






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.