- Nanoscale SiC powder synthesized by NaCl-assisted combustion and its formation mechanism
Ru Bai, Faqi Zhan*, Hua Zhang, Min Zhu, Yuehong Zheng and Peiqing La*
State Key Laboratory of Advanced Processing and Recycling of Nonferrous Metals, School of Materials Science & Engineering, Lanzhou University of Technology, Lanzhou, 730050, China
This article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Silicon carbide (SiC) is a high-melting-point carbide that exhibits properties such as high-temperature ablation resistance, high thermal conductivity, and excellent thermochemical stability. As a high-temperature structural material, the performance of SiC is significantly influenced by its particle size and purity. This paper employs the combustion synthesis method to produce SiC using a magnesium thermal reduction-based technique, which is characterized by simple equipment, low energy consumption, and suitability for large-scale production. In the SiO2-C-Mg system, utilizing combustion synthesis with NaCl as a diluent effectively prevented particle growth caused by excessive combustion temperatures, thereby enabling the scalable production of high-purity nano SiC. Experimental results demonstrated that the addition of NaCl significantly reduced the product particle size, achieving a minimum of 26 nm. As a diluent, NaCl influenced the nucleation and growth processes of SiC, further regulating the particle sizes. During nucleation, NaCl absorbed heat through phase change, effectively lowering the adiabatic temperature of the system, increasing undercooling, and enhancing the nucleation rate. During growth, NaCl acted as a dispersant to inhibit the growth of particles. In addition, NaCl provided a liquid phase environment that accelerated mass transfer and heat transfer processes, and exhibited a high solubility for active species, the formation of SiC followed the dissolution-precipitation mechanism. Large-scale production of high-purity nano SiC can lay the foundation for the high-end applications of SiC-based materials.
Keywords: Nano SiC, Combustion synthesis, Diluent, Formation mechanism.
Carbides are a class of materials with high melting points [1, 2], high hardness [3, 4], excellent thermal conductivity [5], mechanical strength [6-8], exceptional thermal stability, and outstanding wear and corrosion resistance [9]. They are also ultra-high temperature structural materials widely used in the aerospace field [10, 11]. Among them, SiC represents carbides with significant commercial value [12]. Due to its excellent physical and chemical properties, SiC is widely used not only in the aerospace field but also in ceramics [13, 14], abrasives, chemicals, and semiconductors [15, 16]. The quality of the raw powder significantly impacts the sintering densification of ceramic materials and the performance of the devices. Research by Bjørk et al. [17] found that during the sintering process of carbide powders, the smaller the particle size of the raw powder, the higher density of the sintered body and the more uniform the microstructure. Shinde's [18] research indicates that the smaller the particle size of the carbides, the higher the hardness and better wear resistance of the bulk materials produced. These studies suggest that smaller particle sizes enhance the physical and chemical properties as well as the sintering performance of the materials, making the preparation of nanoscale carbide powders crucial.
Common methods for preparing SiC currently include direct synthesis, carbothermal reduction, calciothermic reduction, spark plasma sintering, the sol-gel method, and combustion synthesis [19-22]. Hidehiko [23] utilized silicon and carbon powders as raw materials to prepare SiC powder with a particle size of less than 0.5 μm through direct synthesis. However, this method requires a temperature of 3000 ℃ and consumes over 7000 kW of electricity per ton of SiC powder, which presents drawbacks such as excessive temperature and high energy consumption. Jung et al. [24] utilized tetraethyl orthosilicate and phenyltrimethoxysilane as raw materials to produce β-SiC powder with a purity of 99.7% and a particle size of approximately 10 μm through carbothermal reduction at 1800 ℃. Nonetheless, this method suffers from the disadvantages of high temperature and relatively low purity. Chen et al. [25] employed ethyl orthosilicate and phenolic resin as raw materials to prepare highly dispersed SiC powder through a solvothermal-assisted sol-gel method. Micromorphology analysis and particle size detection indicate that the average particle size of the SiC powder is 200 nm. However, this method necessitates a high temperature of 1400 ℃ to 1600 ℃ and requires 66 h, presenting drawbacks such as high temperature and long duration. Yun et al. [26] utilized rice husks and molten silicon powder as raw materials to synthesize β-SiC fine powder via carbothermal reduction, achieving a SiC particle size of less than 500 nm. Nonetheless, this method requires a temperature of 1850 ℃ and generates CO gas during the process, presenting specific drawbacks. Gyu et al. [27] employed tetraethyl orthosilicate and phenolic resin as raw materials to prepare SiC powder with particle sizes all below 1 µm through the sol-gel method. However, this method is characterized by excessive temperature, larger particle sizes, and unsuitability for large-scale preparation. In summary, these methods face issues such as complex processes, slow reaction rates, high energy consumption, unsuitability for large-scale production, and poor economic viability, which further limit the application of ultra-high temperature SiC materials [28, 29]. Therefore, there is an urgent need to develop low-cost, simple methods for large-scale preparation. Our research group previously proposed a salt-assisted combustion synthesis method, which has successfully prepared nanoscale ZrC, ZrB2, and other materials [30, 31], demonstrating promise for the large-scale synthesis of nano-SiC.
This paper utilizes a salt-assisted combustion synthesis method within the SiO2-C-Mg system, incorporating NaCl as a diluent, to thoroughly investigate the mass production of SiC with finer particle sizes and higher purity by adjusting thermodynamic and kinetic conditions. The study elucidates the role of NaCl as a diluent in controlling SiC particle size and analyzes the reaction process and specific mechanisms, ultimately producing high-purity nanoscale SiC powder with an average particle size ranging from 155 to 28 nm.
Experimental Materials
The combustion synthesis method was employed to scale up the production of nano-sized SiC, utilizing both silicon dioxide (SiO2) and carbon black in nano-scale to minimize the impact of raw materials on the synthesized powder. Detailed specifications of the raw materials are provided in Table 1.
Sample Preparation
The experimental setup comprises a planetary ball mill, a hydraulic press, a crusher, an ultrasonic cleaner, a heating magnetic stirrer, a vacuum drying oven, and a centrifuge. The preparation of SiC samples primarily involves two processes: combustion synthesis of the powder and washing purification. Carbon black serves as the carbon source, metallic magnesium as the reducing agent, and molten salt NaCl as the diluent, measured according to the stoichiometric ratio of reaction eq. (1), where x represents the mass percentage of NaCl in the total reactants (SiO2, C, Mg). During the preparation of SiC powder, varying amounts of NaCl are added, resulting in synthesized products labeled SiC0, SiC30, SiC60, SiC80, and SiC90. Given the low melting and boiling points of magnesium powder, an excess amount is added to ensure complete reaction.
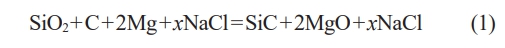
The prepared mixed powder is placed into a ball mill jar with a ball-to-powder ratio of 2:1 and milled continuously at 200 rpm for 8 h. Following ball milling, the mixture is pressed into a cylindrical billet measuring Φ80×40 mm under specific pressure. The igniter is formed into a thin sheet, placed atop the cylindrical billet, and covered with a small amount of mixed powder. This assembly is then placed into a sealed copper crucible within the reaction container. Argon is introduced into the reaction vessel at room temperature to displace air. Upon reaching 200 °C, the gas is vented and argon is reintroduced. The temperature is subsequently increased to 280-300 °C in an Ar atmosphere, initiating the reaction upon igniter ignition. After the reaction, the combustion-synthesized sample cools naturally, is collected, and then crushed.
The sample is washed and purified according to reaction eq. (2) to remove MgO by-products and repeatedly washed to eliminate residual molten NaCl. After diluting standard hydrochloric acid to 3 mol/L, the powder is gradually added and stirred using a magnetic stirrer to maintain solution acidity. The sample is then repeatedly washed with distilled water to remove NaCl and other impurities. Finally, the precipitated powder is vacuum-dried at 70 °C for 12 h, resulting in a SiC powder sample.
Characterization
The phase composition of the samples, both before and after washing, was identified using X-ray diffraction (XRD). By analyzing the phase composition and transformation characteristics of the products, microcrystal size and lattice strain can be further analyzed. The grain size and lattice strain of the powder can be estimated using the full width at half maximum (FWHM) of the diffraction peaks in the XRD pattern. The particle size of the combustion-synthesized powder can be estimated using the Debye-Scherrer formula (3) [32].

In the formula, D represents the grain size, λ is the wavelength of the X-ray source, θ is the diffraction angle, k is 0.89, and β is the full width at half maximum (FWHM) of the diffraction peak, measured in radians.
The microstructural morphology of the prepared powders was analyzed using an electron microscope and evaluated according to eq. (4).

In the formula, DBET represents the equivalent average diameter (µm), ρ is the theoretical density of the carbide phase, and SBET is the surface area determined by BET (m2/g).
The phase and composition of nanoscale SiC
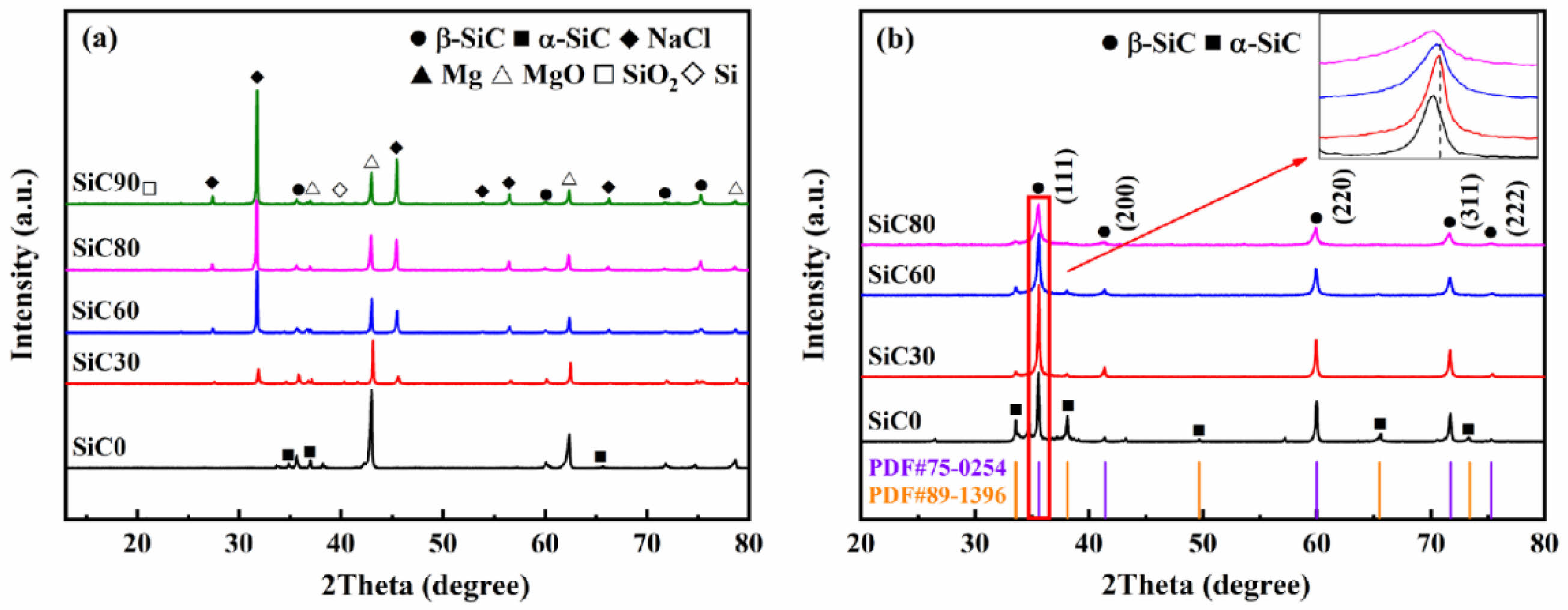
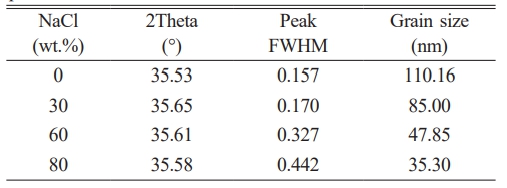
Fig. 1a shows that as the NaCl content increases to 90 wt.%, crystalline phases like SiO2 and Si are detected, suggesting that the reduction reaction between carbon and silica is incomplete due to the system's low reaction temperature. Furthermore, the Si ions reduced from SiO2 depict high activity, but the solubility of graphite carbon particles in the molten salt decreases at lower temperatures. This limits the contact between carbon atoms and highly active Si ions, leading to reduced SiC synthesis and increased elemental Si. The results suggest that the maximum NaCl content in this reaction system is around 80% of the total reactant mass. The XRD pattern of the washed SiC in Fig. 1 depicts that MgO, NaCl, and by-products have been removed, resulting in a high-purity SiC phase. Five main peaks appear at approximately 2θ angles of 35.7 °, 41.4 °, 60.0 °, 71.8 °, and 75.5 °, corresponding to the β-SiC (3C-SiC) (PDF#75-0254) phase. Peaks at 33.6 °, 35.5 °, 38.1 °, 49.7 °, and 60.0 ° correspond to the α-SiC phase (PDF#89-1396). As the NaCl content increases, the intensity of the α-SiC diffraction peaks gradually decreases. When the NaCl content reaches 80 wt.%, the peak intensity of α-SiC at high angles in the synthesized SiC80 sharply diminishes. The increase in diluent content lowers the system's adiabatic temperature, leading to an increase in the low-temperature stable β-SiC and a decrease in the high-temperature stable α-SiC. The upper right of Fig. 1b shows an enlarged view of the SiC (111) crystal plane diffraction peak, indicating a systematic shift to lower angles as the NaCl content increases. The microcrystalline size of the combustion-synthesized SiC powder is calculated using Eq. (3), with the results presented in Table 2. Within a certain range, increasing the amount of NaCl leads to smaller particle sizes of the SiC powder [33].
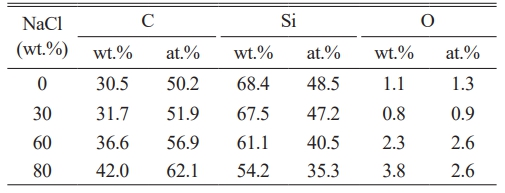
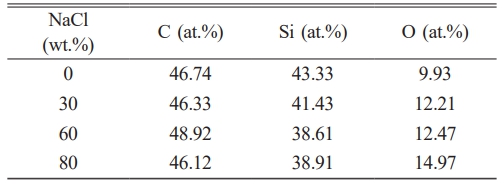
Table 3 displays the EDS results of SiC synthesized with varying NaCl contents. The table shows that the synthesized SiC contains only C, Si, and O elements, detailing the content of each. The table reveals that the oxygen content increases as the NaCl content in the system rises. Energy dispersive spectroscopy (EDS) analysis of the stoichiometric composition of the synthesized samples revealed that when 0 to 30 wt.% NaCl was added, the resulting silicon oxide approached the standard stoichiometric ratio. Further addition of NaCl increases the proportion of carbon, and when the total mass ratio of silicon and carbon exceeds 96%, high-purity nano SiC powder can be obtained.
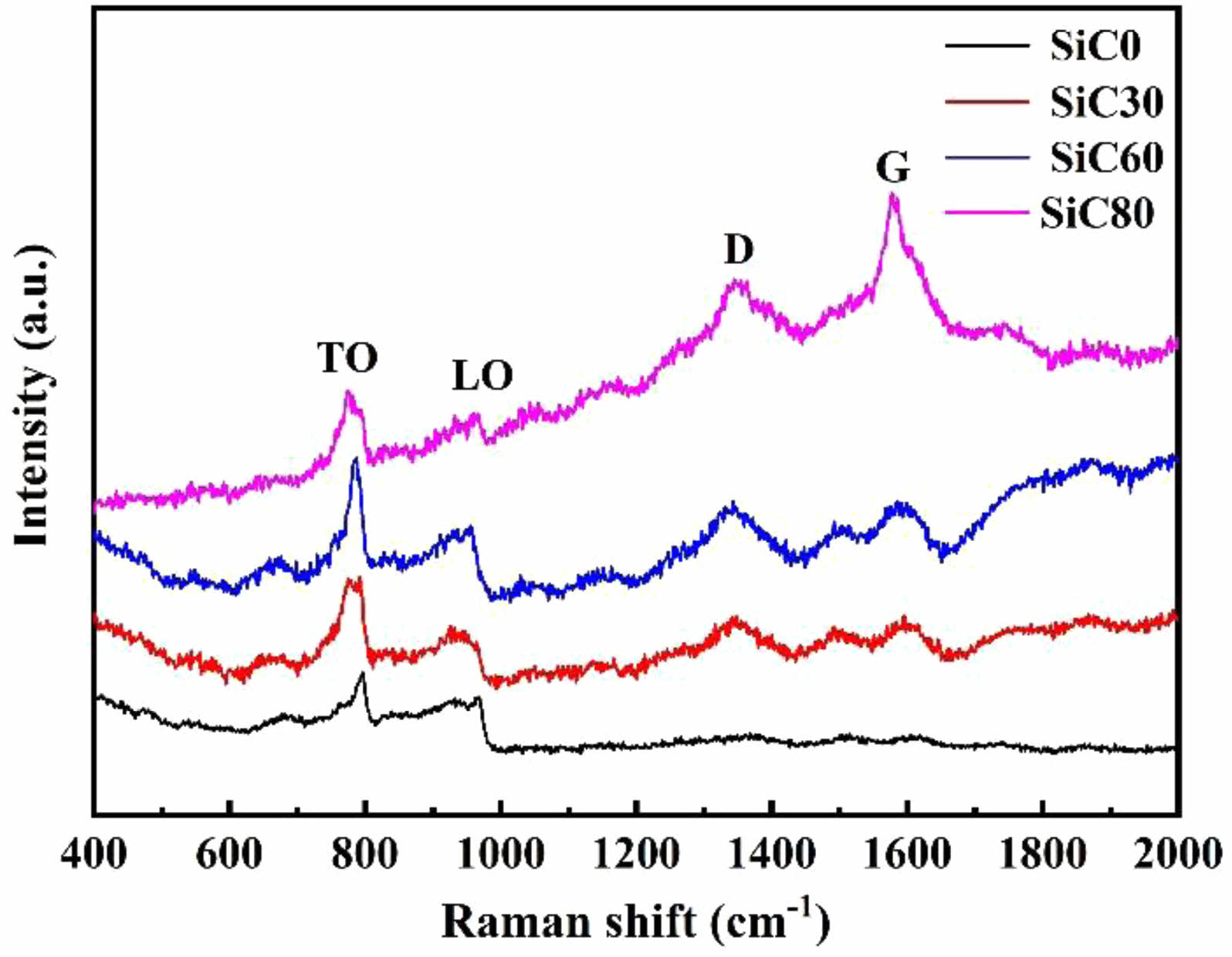
Fig. 2 presents the Raman spectrum of synthesized nano SiC. The Raman spectrum of disordered carbon exhibits the graphite peak (G peak) at 1600 cm-1 and the disorder-induced peak (D peak) at 1350 cm-1, indicating free carbon in the SiC powder. Additionally, the characteristic Raman modes of cubic SiC (β-SiC) are observed, with the TO (transverse optical) phonon peak at 796 cm-1 and the LO (longitudinal optical) phonon peak at 972 cm-1. The ultrafine nano size of the synthesized SiC powder leads to further broadening of the main spectral bands [34, 35].
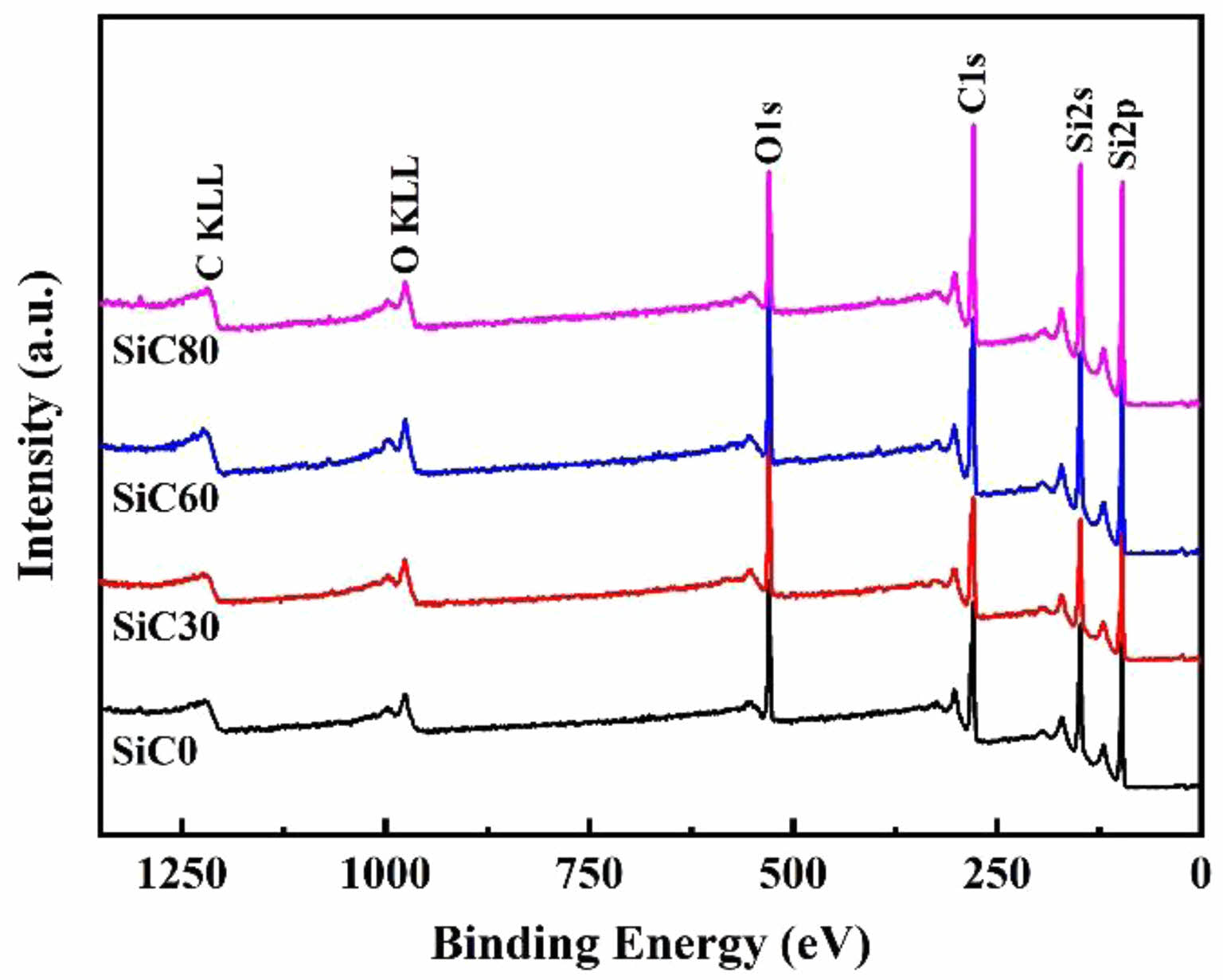
Nano SiC powders were synthesized using a combustion method in NaCl media of varying concentrations, and their surface chemical states were qualitatively and quantitatively analyzed using XPS, as shown in Fig. 3 and Table 4. A full spectrum scan was conducted on each group of samples, revealing four strong characteristic peaks of Si2p, Si2s, C1s, and O1s near 101 eV, 284 eV, and 533 eV, respectively, indicating the presence of Si, C, and O elements on the surface of the synthesized SiC powders. The atomic ratios of elements in Table 4. indicate that the surface of SiC particles is rich in oxygen. Additionally, as the salt content in the system increases, the carbon-to-silicon atomic ratio also increases, which is consistent with previous phase analysis results.
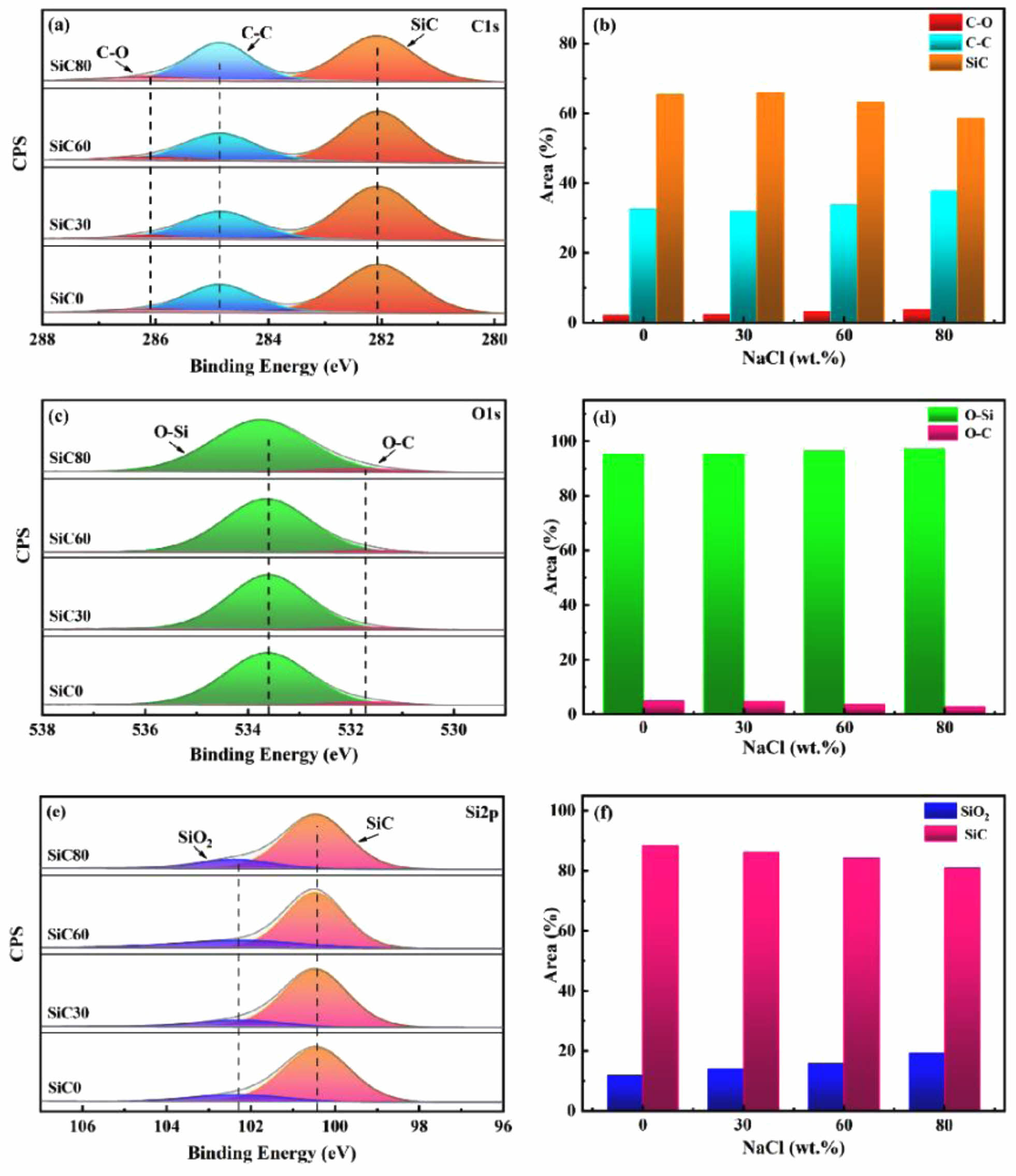
To investigate the influence of varying NaCl concentrations on the atomic bonding states of nano-SiC, the C1s, O1s, and Ti2p peaks in the XPS spectra, as illustrated in Fig. 4, were analyzed in detail. In Fig. 4a, peak fitting of the C1s spectrum identified three peaks located at 282.0 eV, 284.8 eV, and 286.1 eV, corresponding to the SiC, C-C, and C-O bonding states, respectively. As the molten salt content increased, slight variations were observed in the integrated intensities of the C-O and C-C sub-peaks. This phenomenon is attributed to a reduction in the system's adiabatic temperature, which impeded grain growth and slowed the chemical reaction rate, resulting in increased surface-adsorbed oxygen and free carbon. The O1s spectrum was peak-fitted to reveal two peaks, O-C and O-Si, located at 531.7 eV and 533.6 eV, respectively. The quantitative analysis in Fig. 4d indicates that the chemical states of the synthesized SiC samples undergo minimal changes. As illustrated in Fig. 4e, fitting the Si2p spectrum revealed binding energies at 100.4 eV and 102.2 eV, corresponding to the SiC and SiO2 states, respectively [36]. Due to the reduction in particle size, increase in specific surface area, and the presence of surface oxidation, the intensity of the oxide peak gradually increases.
The micromorphology and particle size of SiC
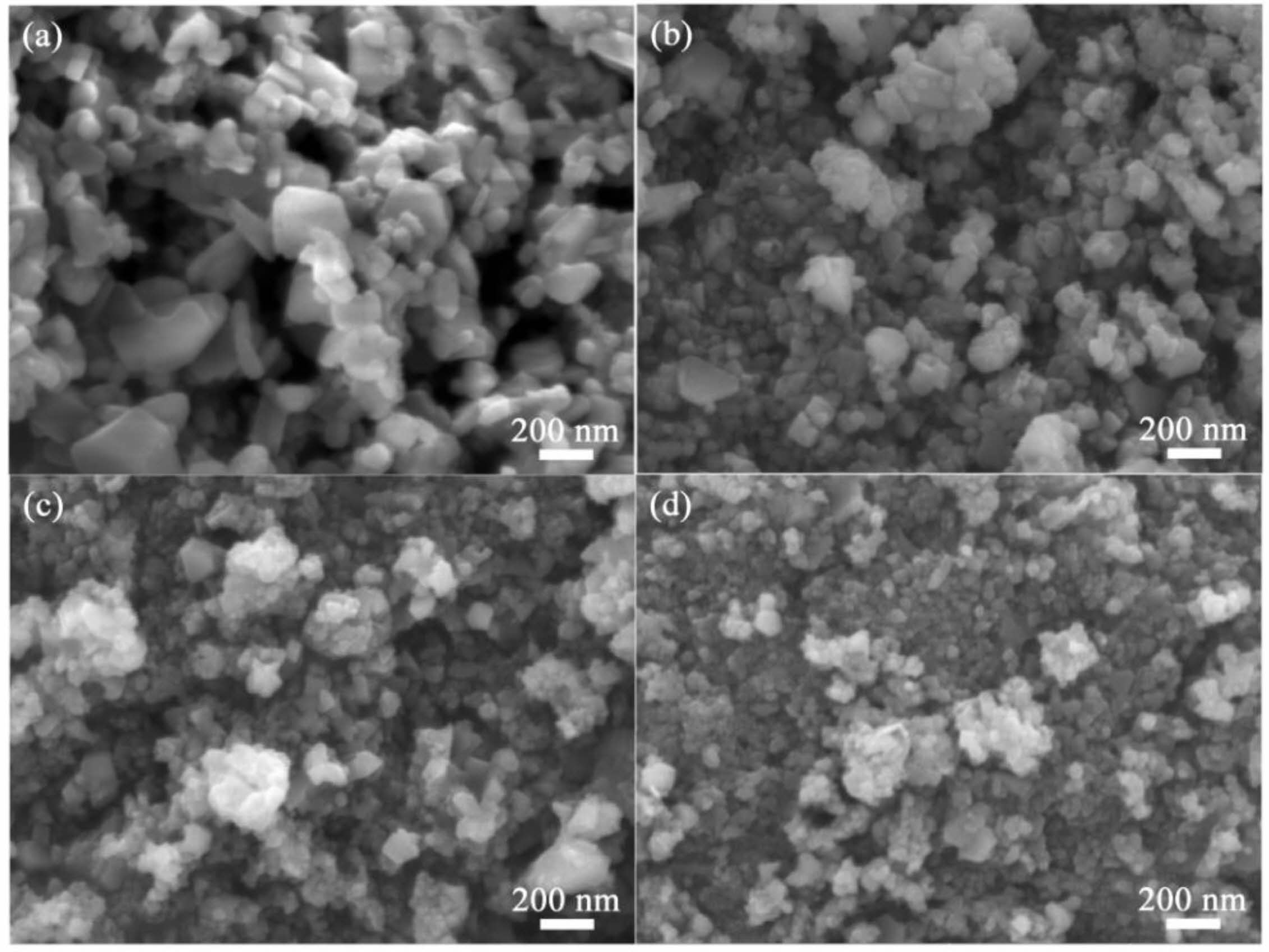
Fig. 5 displays scanning electron microscope (SEM) images of SiC powders synthesized using varying amounts of NaCl. The SiC powders are primarily composed of nanoscale square particles, with some displaying agglomeration and stacking. Fig. 5a illustrates SiC synthesized without molten salt, showing uneven particle sizes with smaller particles adhering to larger ones. When NaCl is added as a diluent, the system absorbs the released heat, inhibiting particle growth and significantly reducing particle size. With 80 wt.% NaCl added, the SiC particles become more distinct, averaging around 30 nm in size, and the particle shape transitions from cubic to nearly spherical. Compared to SiC0 and SiC30, the SiC80 particles exhibit greater size uniformity [37, 38].
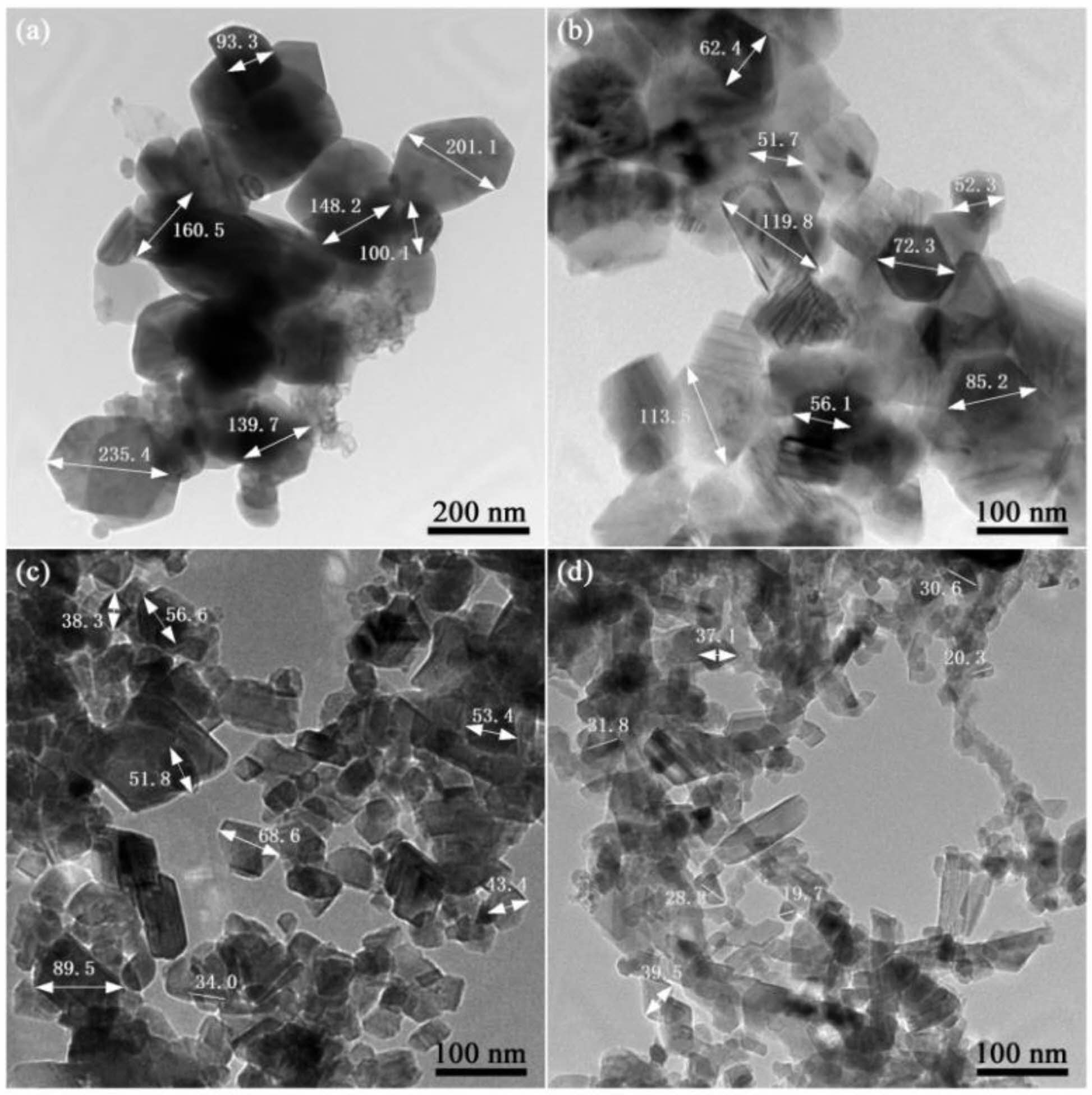
The size and morphology of SiC powder were examined using transmission electron microscopy (TEM), as shown in Fig. 6. Phase analysis indicates that the synthesized SiC primarily consists of α-SiC and β-SiC. Cubic β-SiC is predominant, with a minor amount of hexagonal α-SiC. As indicated in Fig. 6, when 0~30 wt.% NaCl is added to the system, some SiC particles exhibit a hexagonal shape; as the NaCl content continues to increase, the structure of SiC changes, with most particles adopting a cubic structure and a few depicting a near-spherical morphology. This further confirms the characterization analyses such as XRD. Additionally, the size of the SiC particles significantly decreases with increasing molten salt content, and the particle size distribution becomes more uniform, with a controllable size range of approximately 20 to 350 nm.
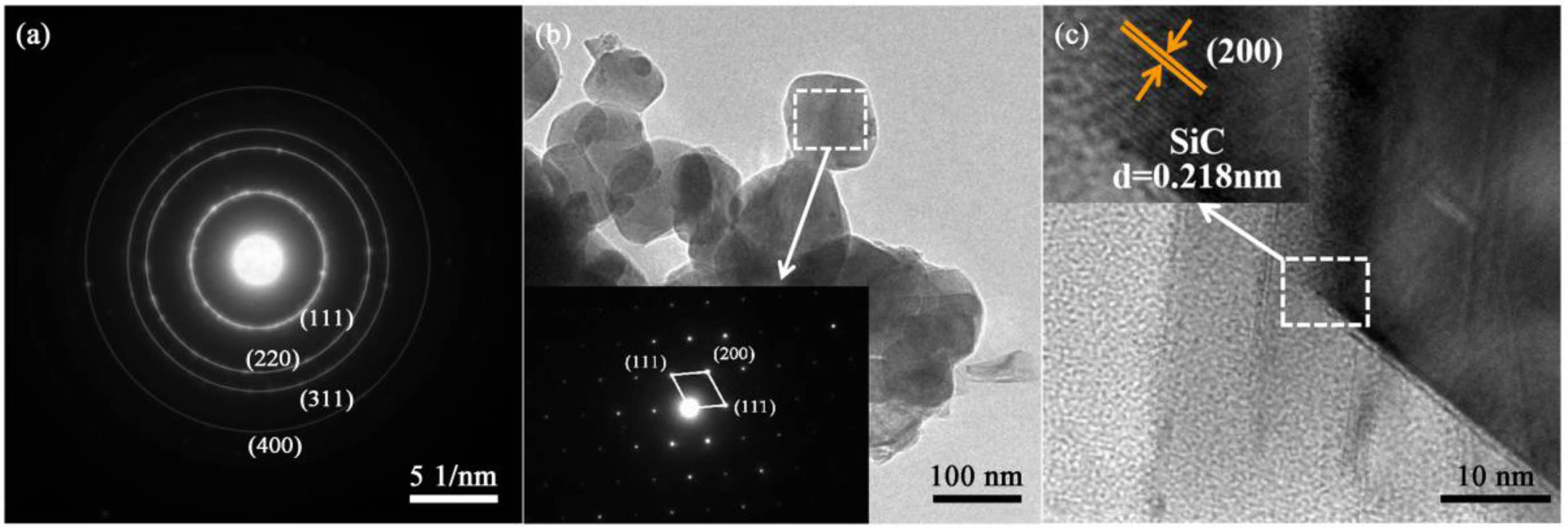
Fig. 7 presents the characteristic High-Resolution Transmission Electron Microscopy (HRTEM) and Selected Area Electron Diffraction (SAED) images of SiC. The SAED image in Fig. 7a displays polycrystalline diffraction rings corresponding to the (111), (220), (311), and (400) planes of cubic β-SiC, indicating the high crystallinity of the SiC particles. The SAED image in Fig. 7b reveals that the particle is single crystalline, with its crystal structure indexed to cubic β-SiC. The crystal structure of SiC80 was characterized by HRTEM, and the lattice fringe spacing measured in Fig. 7c is 0.218 nm, consistent with the d-spacing of 0.218 nm in the (200) direction of standard β-SiC [39].
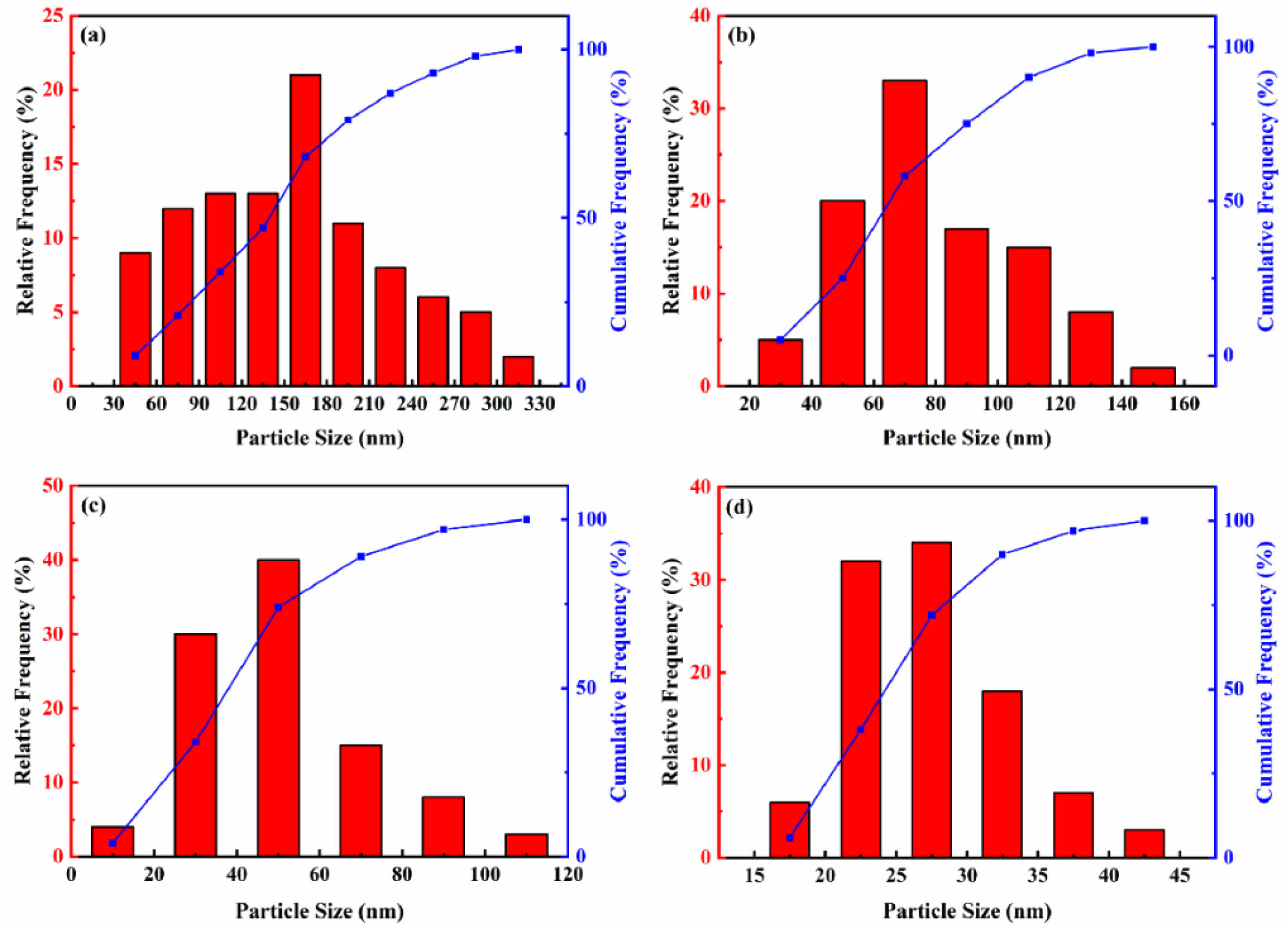
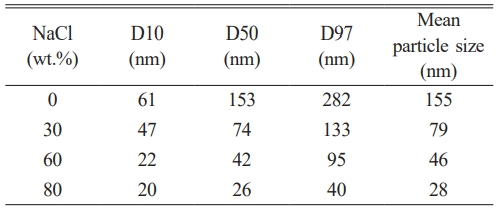
Fig. 8 illustrates the particle size distribution of nano-SiC powders synthesized with varying NaCl contents. The figure reveals that the particle sizes of the four synthesized SiC samples exhibit a normal distribution. Table 5 presents the particle size parameters of the synthesized SiC. The median diameters of combustion-synthesized SiC0, SiC30, SiC60, and SiC80 are 153 nm, 74 nm, 42 nm, and 26 nm, respectively. Based on the data from the figure and table, the overall trend indicates that the particle size of SiC powder decreases as the NaCl content increases, with the average size reducing from 155 nm to 28 nm. When no molten salt diluent is added, the particle size distribution is uneven, with the largest particles exceeding 300 nm, while the smallest are around 35 nm. With the addition of NaCl to the system, the particle size distribution becomes narrower and more concentrated, with approximately 75% of the SiC80 powder particles ranging between 20 and 30 nm, exhibiting uniform particle sizes. The results indicate that adding molten salt NaCl to the system can regulate particle size. As the NaCl content increases, the adiabatic temperature of the system decreases, thereby inhibiting further particle growth [30].
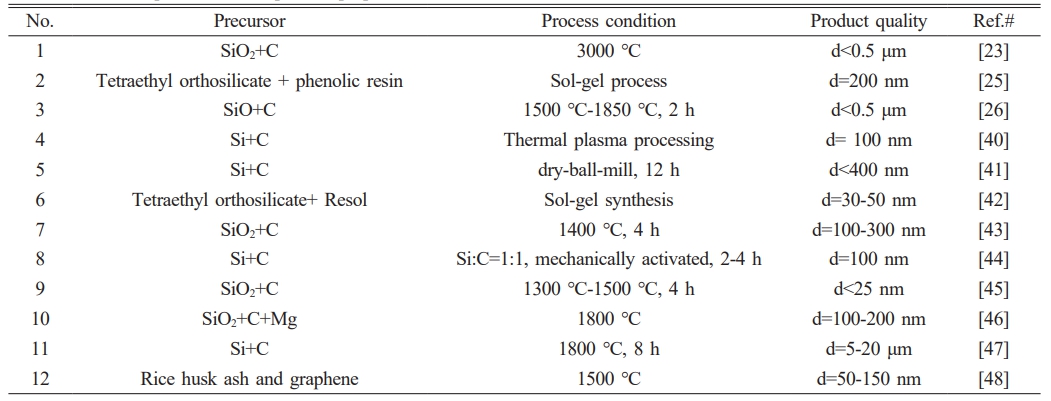
Table 6. provides a summary of the raw materials, preparation methods, and particle size distribution of SiC powder products prepared by other researchers. A comparison of the raw materials, methods, and particle size of the SiC powder prepared in this study with the data in Table 6. indicates that the materials used in this study are more readily available, the preparation method is simpler, and the resulting product has a smaller particle size.
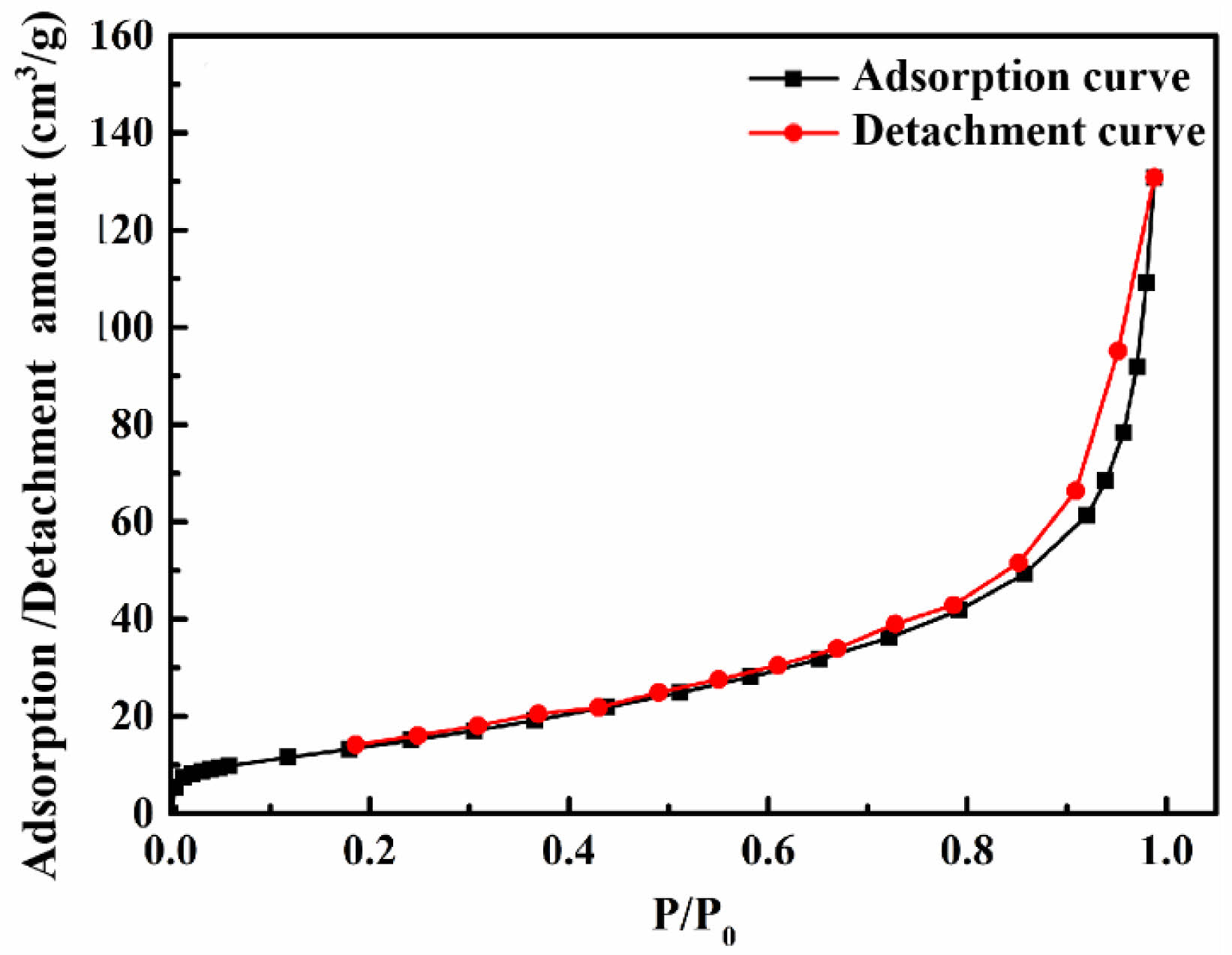
Fig. 9 illustrates the adsorption-desorption isotherms of SiC powder synthesized with 80 wt.% NaCl. The specific surface area of SiC80, an important indicator of nanopowders, was measured to be 53.452 m2/g using the static capacity method, and its average particle size was calculated to be 35 nm using formula (4). The actual particle size is slightly larger than the theoretical calculation. Further refinement of the SiC nanopowder particle size caused some small-sized particles to agglomerate, leading to changes in particle size.
|
Fig. 1 XRD patterns of SiC synthesized with different amounts of NaCl: (a) before washing, (b) after washing. |
|
Fig. 2 Raman spectra of SiC synthesized by different amounts of NaCl. |
|
Fig. 3 XPS maps of SiC synthesized by different amounts of NaCl. |
|
Fig. 4 The XPS spectra and chemical state proportions of SiC: (a-b) C1s, (c-d) O1s, and (e-f) Si2p. |
|
Fig. 5 SEM images of SiC synthesized by different amounts of NaCl: (a) SiC0, (b) SiC30, (c) SiC60, and (d) SiC80. |
|
Fig. 6 TEM images of SiC synthesized by different amounts of NaCl: (a) SiC0, (b) SiC30, (c) SiC60, (d) SiC80. |
|
Fig. 7 (a) SAED and (b) HRTEM image of SiC30, (c) HRTEM image of SiC80. |
|
Fig. 8 Particle size distribution of SiC synthesized by different amounts of NaCl: (a) SiC0, (b) SiC30, (c) SiC60, and (d) SiC80. |
|
Fig. 9 The adsorption and desorption isotherm curves of SiC were synthesized by adding 80 wt.% NaCl. |
|
Table 3 The EDS elements content of SiC synthesized by different amounts of NaCl. |
The effect of NaCl content on the adiabatic temperature of the synthesis system
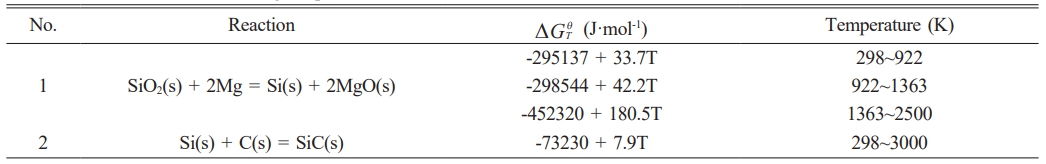
The thermodynamic analysis of the reaction system forms the foundation for thermodynamic parameters and product composition of the reaction system can be examined, providing data support for understanding product formation mechanisms in various reaction systems. Gibbs free energy(∆GθT)is the key parameter for determining whether a reaction occurs spontaneously, and the reaction can proceed spontaneously only when ∆GθT<0, according to the chemical equation [37]. The ∆GθTis calculated using eqs. (5) and (6), ∆Hθ298 represents the standard molar enthalpy of formation, ∆S denotes the entropy change, and T is the reaction temperature.
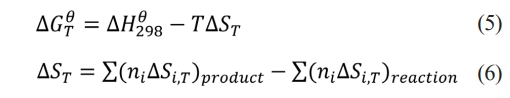
The combustion synthesis reaction utilizing the SiO2-C-Mg system to produce SiC adheres to the overall reaction eq. (1). The reaction progresses in two stages: the initial stage involves the reduction of SiO2 by Mg, followed by the synthesis of the final product, SiC, from the reduced Si and C. The reaction equations for synthesizing SiC are represented by eqs. (7) and (8).
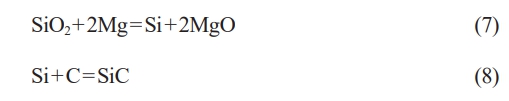
The thermodynamic data of the SiO2-C-Mg system can be obtained from the Inorganic Thermodynamics Handbook. The relationship between ∆GθT and temperature for the two system reactions was calculated using eqs. (5) and (6), as shown in Table 7.
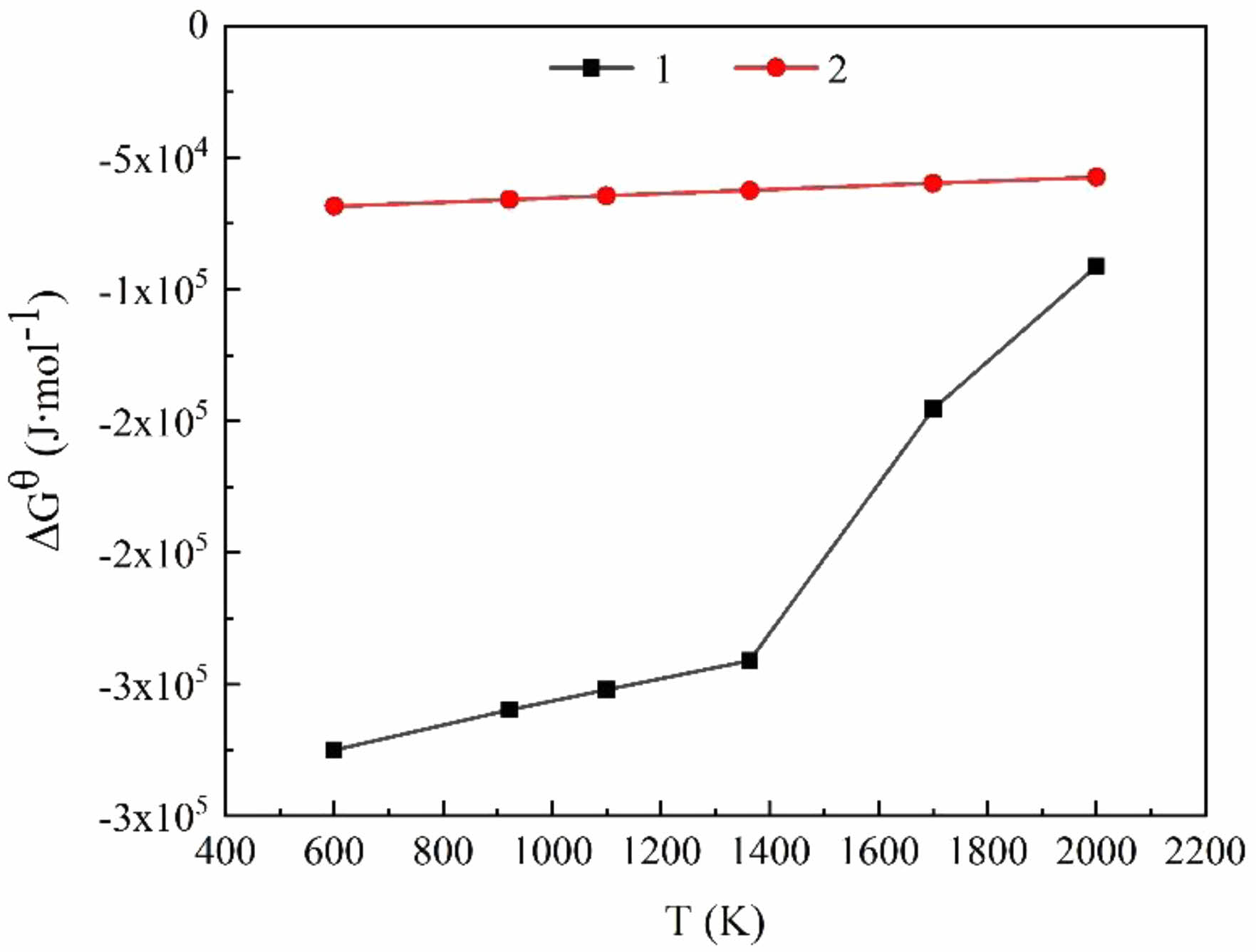
Fig. 10 depicts the relationship between the ∆GθT change of the specified reaction and temperature. In the SiO2-C-Mg system, at temperatures below 1400 K, reaction 1 shows the highest tendency for formation, and the products remain stable. The trend of the ∆GθT change, ∆GθT in the second stage of the reaction ensures it remains negative within the experimental temperature range, indicating the feasibility of the reaction in the SiO2-C-Mg system.
The combustion synthesis process releases significant thermal energy, and the entire reaction can be completed in just a few seconds. Once initiated, a key issue in the study of self-propagating reactions is whether the reaction can sustain itself. It is established that the combustion synthesis reaction occurs spontaneously only when the adiabatic temperature (Tad) is ≥ 1800 K. When Tad is < 1800 K, additional heat may be supplied via preheating or a chemical furnace to facilitate the reaction [31]. By consulting the inorganic thermodynamics manual [40] and calculating the system's adiabatic temperature using Formula (9):
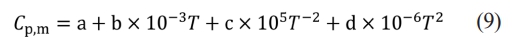
In the formula, ∆Hθ298 (kJ·mol-1) represents the standard enthalpy of formation of the product at 298 K, ∆Cp,m (J·K-1) represents the heat capacity of the product, Tm(K) represents the melting point of the product, and ∆Hm (J·kg-1) represents the heat of fusion of the product. Cp,m can be approximately calculated using the following formula:
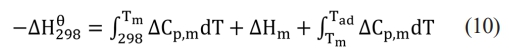
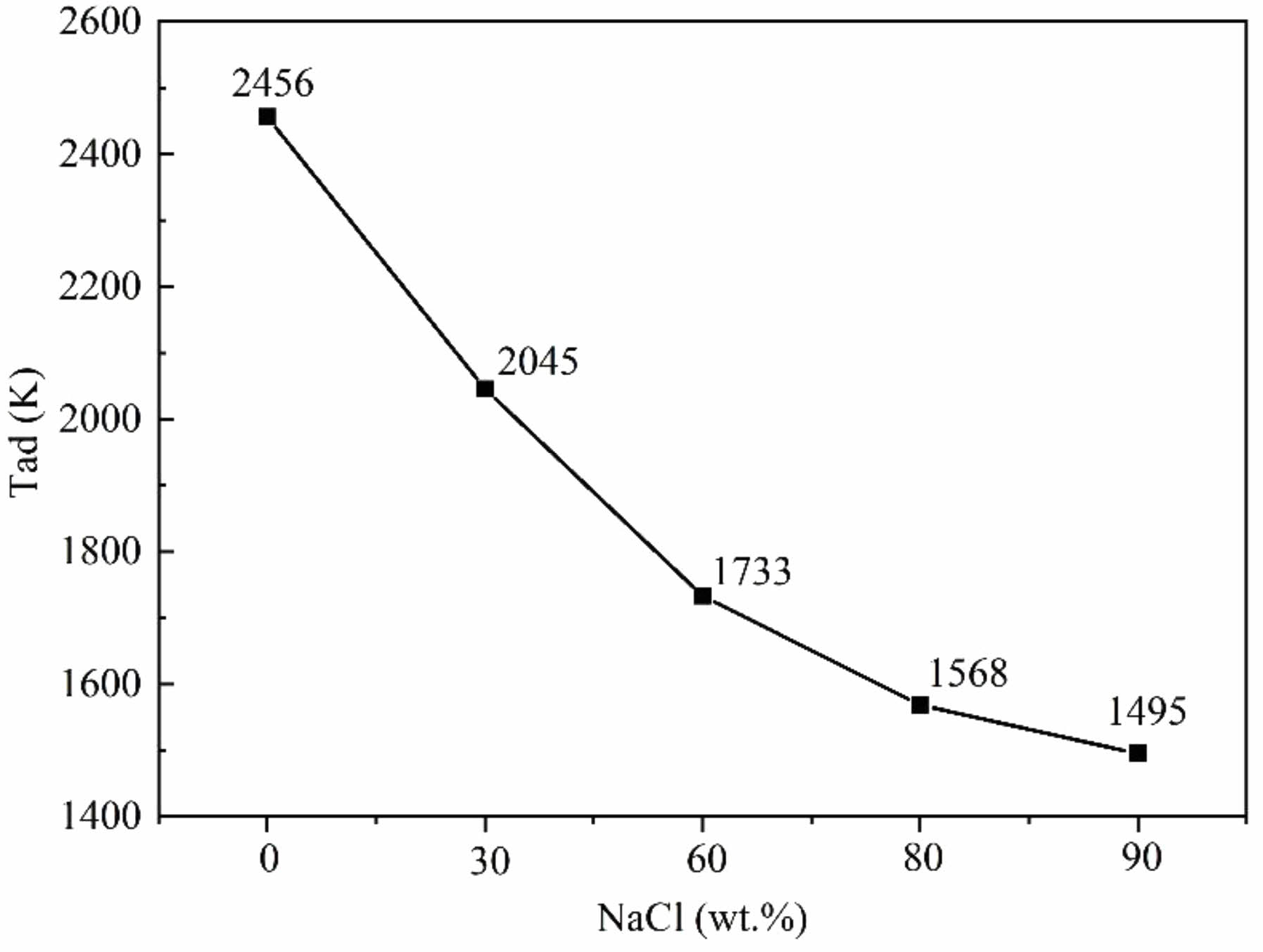
Fig. 11 illustrates the adiabatic temperatures calculated for the SiO2-C-Mg system with varying amounts of NaCl. In the combustion synthesis experiments of SiC, the system's adiabatic temperature decreases as the NaCl content increases. During the reduction reaction, the system releases a significant amount of heat. Because of NaCl's low melting point (1074 K) and boiling point (1735 K), some heat is absorbed by NaCl to undergo phase changes, resulting in a decrease in the system's adiabatic temperature. Experimental analysis indicates that, under the same conditions, SiC powder exhibits smaller particle sizes and a broader range of particle size control. In the SiO2-C-Mg system, the heat released by the magnesiothermic reduction reaction is minimal. Thermodynamic analysis shows that without a diluent, the adiabatic temperature of the SiC synthesis system is 2456 K. Therefore, in a synthesis system with lower heat, the product particle size decreases, highlighting the importance of controlling the system's adiabatic temperature with a diluent for nanopowder synthesis. Additionally, it was found that the synthesized SiC exists in two crystalline forms: α-SiC and β-SiC. At a synthesis temperature of approximately 2100 °C, the high-temperature stable α-SiC begins to transform into the low-temperature stable β-SiC at a very slow rate [49]. As the system's adiabatic temperature decreases, the synthesized product predominantly consists of β-SiC. However, due to local temperature inhomogeneity, a small amount of α-SiC phase is still formed even if the system's theoretical calculated temperature is below the SiC transformation temperature, further corroborating the phase analysis results.
By calculating the adiabatic temperature of the SiO2-C-Mg system, it was determined that the system can sustain a spontaneous reaction even when the theoretical adiabatic temperature is as low as 1500 K. The reasons for this phenomenon can be analyzed as follows: (1) Prior to igniting the combustion synthesis reaction, the external equipment must be preheated to approximately 250 ℃ in a 2 MPa argon environment to ignite the igniter, thereby initiating the self-propagating reaction. Consequently, when the actual system temperature is Tad < 1800 K, it is sufficient to sustain the self-propagating reaction; (2) Achieving the ideal adiabatic state in the actual reaction system remains challenging, as factors such as preheating temperature, pressure, and phase changes substances also affect the temperature; (3) The ultrafine particle size of the reactants increases their contact area, allowing the reaction to proceed at lower temperatures. The results indicate that the diluent NaCl absorbs the heat released during combustion, thereby reducing the temperature of the reaction zone and subsequently lowering the adiabatic temperature of the system.
The effect of NaCl content on the purity and particle size of nano SiC
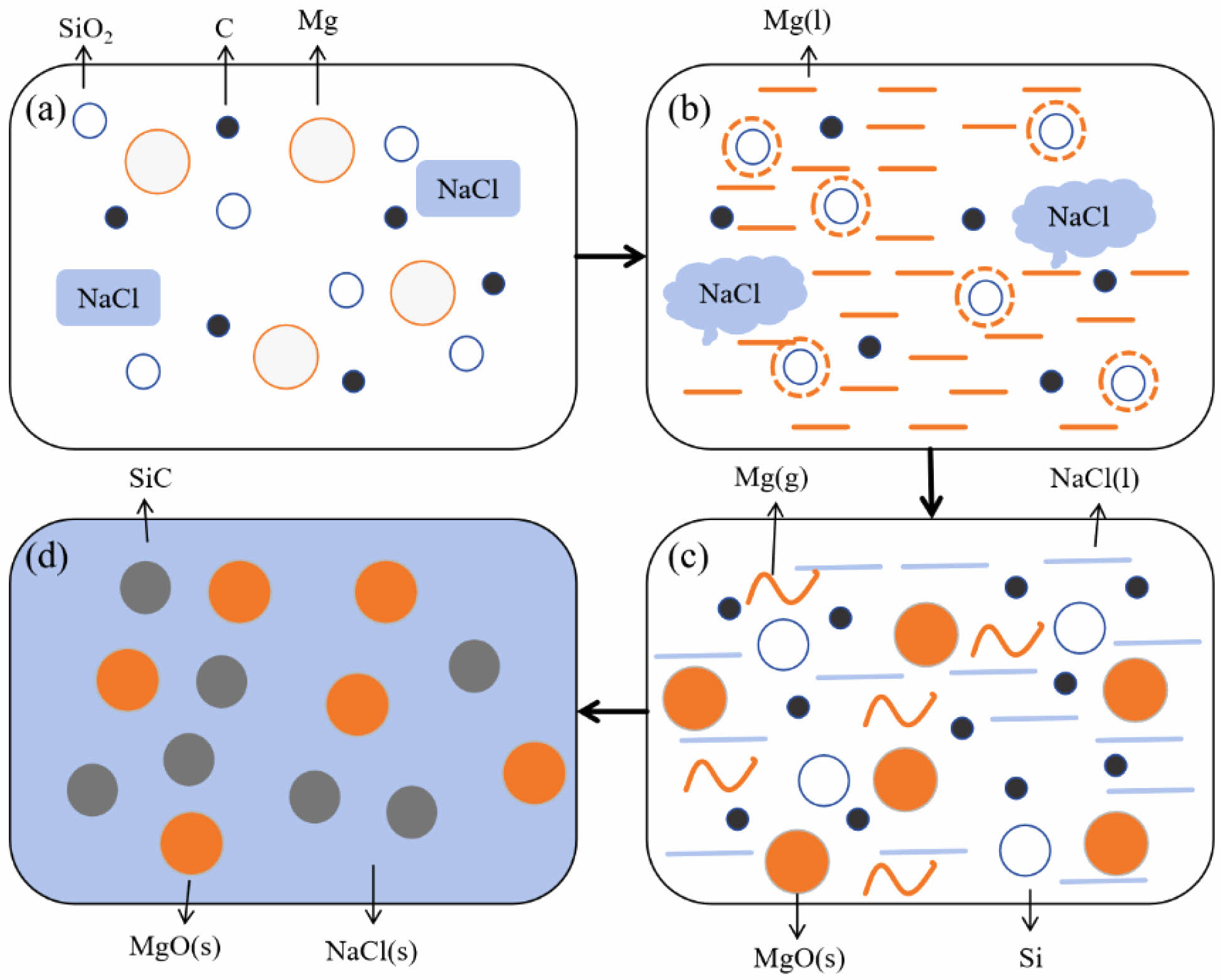
In a reaction system primarily consisting of SiO2, C, and Mg, all reactions occur within a closed environment. Thermodynamic analysis indicates that during the combustion synthesis of SiC, the adiabatic temperature decreases from 2456 K to 1495 K. NaCl has a higher melting point than the reducing agent Mg powder, which is advantageous for the reduction reaction. During the preheating stage, as illustrated in Fig. 12a, nanoscale reactants are dispersed within the auxiliary materials. When the preheating system temperature exceeds 500 K, the igniter activates, releasing a substantial amount of heat. When the material temperature in the closed system reaches approximately 1800 K, a self-propagating reaction ensues. Mg has the lowest melting point among the reactants and begins to melt when the system temperature reaches 923 K. Liquid Mg infiltrates the solid particles, forming a coating layer, as depicted in Fig. 12b. The reduction of oxides by Mg to form MgO is an exothermic reaction, releasing a substantial amount of heat in a short period. When the temperature reaches the melting point (1076 K) of the diluent NaCl, the molten salt absorbs heat, slowing the system's temperature increase and consequently the grain growth rate. Simultaneously, local temperatures in the system may reach Mg's boiling point of 1378 K, causing Mg to vaporize and the reduction reaction to proceed fully, as depicted in Fig. 12c. When the system temperature approaches NaCl's boiling point of 1700 K, the heating rate sharply decreases as a significant amount of NaCl vaporizes, absorbing most of the heat in the system. When the exothermic rate of the magnesiothermic reaction equals the endothermic rate of NaCl's phase change, the system temperature stabilizes, reaching the adiabatic temperature. As the system begins to cool and drops to NaCl's melting point, the molten salt crystallizes, covering the formed carbide and preventing further growth of carbide powder particles, as illustrated in Fig. 12d.
During the combustion synthesis of nano-SiC powders, the molten salt diluent NaCl plays a crucial role in the formation and growth of the powders. As a molten salt diluent, NaCl is introduced into the reaction system. Although it does not directly participate in the reaction, it melts and absorbs heat at high temperatures, thereby reducing the temperature at the combustion wavefront and suppressing the reaction's intensity. This effectively addresses issues such as excessive heat release, excessive heat accumulation, and overly rapid reaction rates in the combustion synthesis process. Meanwhile, by regulating the temperature, NaCl also helps inhibit the crystal growth rate of SiC, thereby controlling the particle size of the resulting SiC [50, 51]. The molten NaCl forms a homogeneous precursor liquid phase at high temperatures, enhancing the contact efficiency between reactants, thereby promoting a thorough reaction and improving reaction efficiency and product purity [52]. Additionally, NaCl molten salt has a high specific heat capacity, allowing it to absorb a large amount of reaction heat, thereby extending the combustion duration and promoting reaction completeness [53]. The addition of low melting-point NaCl during carbide formation melts at lower temperatures, providing a liquid medium that enhances reactant flow and significantly increases the diffusion rate. Due to the low exothermicity of the SiO2-C-Mg, SiC achieves early nucleation and grain growth at lower temperatures, resulting in smaller particle sizes. The diluent not only regulates the particle size of the powder but also significantly influences its purity. As NaCl content in the system increases, thermal conductivity decreases, leading to an increase in free carbon content on the surface of the powder particles. Additionally, the reduction in particle size and the increase in specific surface area promote oxidation on the particle surfaces. Changes in NaCl content also cause variations in NaCl volatilization during the reaction. When NaCl content in the SiC synthesis system increases to 110 wt.% and 90 wt.%, NaCl volatilization peaks, leading to insufficient reaction heat to sustain complete reduction reactions, which results in residual reactants and reducing agents that lower the final product purity.
|
Fig. 10 Diagram of ∆GθT - T of the reaction. |
|
Fig. 11 The relationship between NaCl addition and Tad of system: SiO2-C-Mg system. |
|
Fig. 12 Action mechanism diagram of molten salt diluent in the system of SiO2-C-Mg: (a) initial state, (b) temperature-rise period, (c) constant temperature stage and (d) cooling stage. |
The large-scale (≥ 1 kg) production of nano SiC powder was successfully facilitated in the SiO2-C-Mg reaction system via the combustion synthesis method with the addition of NaCl as a diluent. This study investigated the effect of NaCl content on the particle size and purity of the product, well as its mechanism of action in the system. With an increase in NaCl content, the particle size of the synthesized SiC powder decreased, with the average size reducing from 155 nm to 28 nm. When 80 wt.% NaCl was added, most SiC powder particles were concentrated in the 20-30 nm range, with a specific surface area of 53.452 m2/g. The diluent NaCl controlled the particle size of the SiC powder by influencing its nucleation and growth. The addition of NaCl created a liquid-phase environment that enhanced the diffusion rate, and the subsequent molten salt crystallization process prevented nanoparticle agglomeration. As the NaCl content increased, the molten salt absorbed heat and undergone a phase change, resulting in decreased adiabatic temperature and increased supercooling, thus enhancing nucleation rate and slowing the growth rate.
This work was supported by the Gansu Province Science and Technology Major Project (24ZD13GA018), the College Industry Support Plan of Gansu Province (2023CYZC-29), the Gansu Provincial Department of Education young doctor support project (2023QB-036), the China Postdoctoral Science Foundation (2022MD723787), and the Tamarisk Outstanding Young Talents Program of Lanzhou University of Technology (062202).
- 1. H.K. Wei, H.J. Qiu, H.Z. Li, Y.K. Wang, C.A. Wang, and Z.P. Xie, J. Ceram. Process. Res. 24[1] (2023) 1-7.
-

- 2. Y. Li, S.X. Li, S. Wang, C.C. Tian, and O.T. Ayode, J. Ceram. Process. Res. 24[2] (2023) 367-373.
-

- 3. L. Gao, Y. Zhang, X. Yang, Y.B. Heb, and L.H. Song, J. Ceram. Process. Res. 21[6] (2020) 615-621.
-

- 4. H.J. Qiu, H.K. Wei, S.F. Ren, L.J. Sun, J. Li, Z.H. Wang, L. Zhao, C.A. Wang, and Z.P. Xie, J. Ceram. Process. Res. 25[2] (2024) 212-219.
-

- 5. Z.Y. Fan, Y.T. Zhang, S.Y. Xie, and Y.F. Pan, J. Ceram. Process. Res. 25[6] (2024) 964-974.
-

- 6. J.C. Kwon and S.J. Lee, J. Ceram. Process. Res. 25[6] (2024) 1115-1121.
-

- 7. X.L. Zhang, Y.H. Chen, L.M. Liu, W.B. Qi, W.X. Hai, M. Xue, and T.X. Hong, J. Ceram. Process. Res. 25[4] (2024) 483-489.
-

- 8. S. Ahn, S. Jeong, and K. Nam, J. Ceram. Process. Res. 17[9] (2016) 994-998.
-

- 9. J. Yang, C.F. Wang, G.L. Liu, H. Gao, and M.L. Chen, J. Ceram. Process. Res. 23[4] (2022) 466-475.
-

- 10. H.X. Liu, W.H. Song, Q. Xu, W. Ma, and Y. Bai, Int. J. Electrochem. Sci. 15[7] (2020) 6238-6248.
-

- 11. L.X. Yang, Y. Wang, R.J. Liu, H.J. Liu, X. Zhang, C.L. Zeng, and C. Fu, J. Mater. Sci. Technol. 37 (2020) 173-180.
-

- 12. H.K.M. Al-Jothery, T.M.B. Albarody, P.S.M. Yusoff, M.A. Abdullah, and A.R. Hussein, MSE. 863[1] (2020) 1-8.
-

- 13. X.W. Pan, S.H. Yan, and M.T. Yang, J. Ceram. Process. Res. 2025, 26[1] (2025) 172-176.
-

- 14. A. Noviyanto, A. Wibowo, G. Sukmarani, R. Kusumaningrum, F. Fauzi, A.M. Habieb, M.I. Amal, and N.T. Rochman, J. Ceram. Process. Res. 21[3] (2020) 326-330.
-

- 15. C.Z. Cao, W.Q. Liu, A. Javadi, H.N. Ling, and X.C. Li, Procedia. Manuf. 10 (2017) 634-640.
-

- 16. A.H. Oh, H.S. Lee, B.G. Kim, S.C. Choi, Y.G. Jung, and G.S. An, J. Ceram. Process. Res. 21[4] (2020) 400-406.
-

- 17. R. Bjørk, V. Tikare, H.L. Frandsen, N. Pryds, and J. Blendell, J. Am. Ceram. Soc. 96[1] (2013) 103-110.
-

- 18. T. Shinde, Int. J. Comput. Mater. Sci. Surf. Eng. 37[9] (2020) 1206-1214.
-

- 19. M. Jiang, J.W. Zheng, H.Y. Xiao, Z.J. Liu, and X.T. Zu, Sci. Rep. 7[1] (2017) 9344-9357.
-

- 20. D.O. Moskovskikh, Y. Song, S. Rouvimov, A.S. Rogachev, and A.S. Mukasyan, Ceram. Int. 42[11] (2016) 12686-12693.
-

- 21. Z.M. Xie, T. Zhang, R. Liu, Q.F. Fang, S. Miao, X.P. Wang, and C.S. Liu, Int. J. Refract. Met. H. 51 (2015) 180-187.
-

- 22. T. Chanadee, J. Ceram. Process. Res. 18[5] (2017) 389-393.
-

- 23. H. Tanaka, J. Ceram. Soc. Jpn. 119[3] (2011) 218-233.
-

- 24. E.J. Jung, Y.J. Lee, S.R. Kim, W.T. Kwon, J.K. Kim, D.J. Choi, and Y. Kim, J. Ceram. Process. Res. 15[6] (2014) 447-450.
-

- 25. H.M. Chen, J.M. Jiang, and H.Y. Zhao, Appl. Phys. A. 124[7] (2018).
-

- 26. Y.H. Yun, J. Ceram. Process. Res. 23[3] (2022) 247-251.
-

- 27. G.S. Cho, G.M. Kim, D.S. Lim, and S.W. Park, J. Ceram. Process. Res. 11[3] (2010) 377-383.
-

- 28. G.A. Karim, A. Ghosh, R. Rao, and M. Krishnan, Adv. Appl. Ceram. 120[1] (2020) 24-31.
-

- 29. M. Song, Y.F. Yang, M.Q. Xiang, Q.S. Zhu, and H.D. Zhao, Adv. Powder. Technol. 380 (2021) 256-264.
-

- 30. F.Q. Zhan, H. Zhang, K. Xv, M. Zhu, Y.H. Zheng, and P.Q. La, J. Ceram. Process. Res. 23[5] (2022) 694-708.
-

- 31. F.Q. Zhan, R. Bai, X. Liu, K. Xu, H. Zhang, M. Zhu, Y.H. Zheng, S.P. Xu, J. Sheng, and P.Q. La, J. Ceram. Process. Res. 25[4] (2024) 490-498.
-

- 32. J.H.J. Kim, J. Song, C.J.V. Tyne, H. Cho, and H. Lee, J. Ceram. Process. Res. 16[5] (2015) 619-623.
-

- 33. A. Da, F. Long, J.L. Wang, W.H. Xing, Y. Wang, F. Zhang, W.M. Wang, and Z.Y. Fu, J. Wuhan Univ. Technol. 30[4] (2015) 729-734.
-

- 34. S. Nakashima and H. Harima, Phys. Status. Solidi. (a). 162[1] (1997) 39-64.
-

- 35. Y.Y. Wang, S. Dong, X.T. Li, C.Q. Hong, and X.H. Zhang, Ceram. Int. 48[7] (2022) 8882-8913.
-

- 36. G.D. Sorarù, A. Glisenti, G. Granozzi, F. Babonneau, and J.D. Mackenzie, Int. J. Mater. Res. 5[9] (2011) 1958-1962.
-

- 37. W.J. Choyke and G. Pensl, MRS. Bulletin. 22[3] (2013) 25-29.
-

- 38. D. Davoodi, S. A. Hassanzadeh-Tabrizi, A. Hossein Emami, & S. Salahshour, Ceram. Int. 41[7] (2015) 8397-8401.
-

- 39. X. Zhang, J. He, J.B. Lian, X. Zhang, and M.X. Lei, J. Ceram. Process. Res. 25[3] (2024) 404-412.
-

- 40. Z. Károly, I. Mohai, Sz Klébert, A. Keszler, I.E. Sajó, and J. Szépvölgyi, Adv. Powder. Technol. 214[3] (2011) 300-305.
-

- 41. H.B. Jin, J.T. Li, M.S. Cao, and S. Agathopoulos, Adv. Powder. Technol. 196[2] (2009) 229-232.
-

- 42. A. Najafi, F. Golestani-Fard, H.R. Rezaie, and N. Ehsani, J. Sol-Gel. Sci. Technol. 59[2] (2011) 205-214.
-

- 43. Y.J. Lin and C.M. Chuang, Ceram. Int. 33[5] (2007) 779-784.
-

- 44. K. Yang, G.H. Liu, and J.T. Li, J. Phys. Chem. C. 112 (2008) 6285-6292.
-

- 45. C.P. Tsang and Y.J. Lin, Ceram. Int. 29 (2003) 69-75.
-

- 46. D.O. Moskovskikh, Y.C. Lin, A.S. Rogachev, P.J. McGinn, and A.S. Mukasyan, J. Eur. Ceram. Soc. 35[2] (2015) 477-486.
-

- 47. X.Z. Guo, L. Zhu, W.Y. Li, and H. Yang, J. Adv. Ceram. 2[2] (2013) 128-134.
-

- 48. J.P. Chen, G. Song, Z. Liu, Q.Q. Kong, S.C. Zhang, and C.M. Chen, J. Alloys. Compd. 833 (2020).
-

- 49. B.J. Zhang, J.W. Ji, X. Liu, C.S. Li, M. Yuan, J.Y. Yu, and Y.Q. Ma, Sci. Total. Environ. 647 (2019) 57-65.
-

- 50. C.C. Hwang, T.Y. Wu, J. Wan, and J.S. Tsai, Mater. Sci. Eng. B-Adv. 111 (2004) 49-56.
-

- 51. K. Aoyagi, R. Sivakumar, X. Yi, T. Watanabe, and T. Akiyama, J. Ceram. Soc. Jpn. 117[6] (2009) 777-779.
-

- 52. K. Morsi, J. Mater. Sci. 47 (2012) 68-92.
-

- 53. A. Abdulkadir, A.V. Rayer, D. Viet Quang, N.E. Hadri, A. Dindi, P.H.M. Feronb, and M.R.M. Abu-Zahra, Energy Procedia. 63 (2014) 2070-2081.
-

 This Article
This Article
-
2025; 26(2): 219-230
Published on Apr 30, 2025
- 10.36410/jcpr.2025.26.2.219
- Received on Nov 5, 2024
- Revised on Feb 15, 2025
- Accepted on Mar 1, 2025
 Services
Services
- Abstract
introduction
experimental
results
analysis and discussion
conclusion
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Faqi Zhan and Peiqing La
-
State Key Laboratory of Advanced Processing and Recycling of Nonferrous Metals, School of Materials Science & Engineering, Lanzhou University of Technology, Lanzhou, 730050, China
Tel : +86 15209310025 Fax: +86 15209310025 - E-mail: zhanfaqi@lut.edu.cn, pqla@lut.edu.cn






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.