- Preparation and chromatic performance of black ceramic tiles from chromium slag, copper slag and silicon manganese slag
Yanglai Houa, Jiajie Yua, Dingli Zhengb,c,*, Ju Xub,c, Guojun Mab,c, Shokhrukh Khojievd,e and Nodir Kadirovd,e
aCollege of Science, Wuhan University of Science and Technology, Wuhan 430081, Hubei, China
bKey Laboratory for Ferrous Metallurgy and Resources Utilization of Ministry of Education, Wuhan University of Science and Technology, Wuhan 430081, Hubei, China
cJoint International Research Laboratory of Refractories and Metallurgy, Ministry of Education, Wuhan University of Science and Technology, Wuhan 430081, China
d“Magistr” private scientific and production enterprise, Tashkent 100106, Republic of Uzbekistan
eDepartment of Metallurgy, Almalyk branch of Tashkent State Technical University, Tashkent 110100, Republic of UzbekistanThis article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Chromium slag, copper slag and silicon manganese slag are solid waste generated in industrial production. The recovery of valuable elements (Fe, Cr, Mn, etc.) in the waste slag to produce black ceramic tiles can enhance the utilization value of these solid waste and solve the problem of environmental resource constraints. In this study, the industrial waste of chromium slag, copper slag and silicon manganese slag were used as raw materials to prepare black ceramic tiles. The results showed that the best color of ceramic tiles appeared at the molar ratio of Fe/Cr/Mn=3:2:1, sintering temperature of 1150 oC, holding time of 45 min and cooling in the furnace. The valuesof L*, a*, b* and C* are 26.2, 1.0, 0.2, and 1.02, respectively. Meanwhile, the compressive strength and Cr6+ leaching concentration of the ceramic tiles at the optimal process parameters were 162.61 MPa and 0.97 mg/L, respectively, which met the national standards. The chromaticity coordinates of prepared black ceramic tiles are very near to the point with a saturation of 0, indicating that it is feasible to use chromium slag, copper slag and silicon manganese slag to prepare black ceramic tiles.
Keywords: Chromium slag, Copper slag, Silicon manganese slag, Black ceramic tiles.
Chromium slag is a type of solid waste generated during producing chromium salts, with a high content of Cr (VI) which is harmful to animals, plants and humans. Cr (VI) is a highly toxic potential carcinogen [1-4]. The chemical industry in China annually emits 0.2 to 0.3 million tons of chromium slag, with a total accumulated storage volume exceeding 6 million tons [5, 6]. Most chromium slag is stored for treatment, and Cr (VI) in the chromium slag continuously immerses into the surrounding soil and groundwater over time, causing continuous pollution to the surrounding ecological environment [7, 8]. Simultaneously, chromium slag contains large amount of valuable metal elements and has a high recovery value [9, 10]. Meanwhile, copper slag is a byproduct generated during the copper extraction process [11], which contains small amounts of harmful elements, such as Pb, Cd and As. It is classified as hazardous waste [12]. According to statistics, it generates 2.2 tons of copper slag per ton copper produced, with an annual slag production of 21.1 million tons. Untreated copper slag not only occupies a large amount of land, but also accelerates the migration rate of harmful elements to the environment and human health [13-15]. Silicon manganese slag is a type of slag with high manganese content emitted by ferroalloy factories for the production of silicon manganese alloy. Its main components are SiO2, MnO and CaO, which can be recycled as valuable components [16-18]. Typically, producing 1 ton of silicon manganese alloy produce 1.2 to 1.3 tons of silicon manganese slag. At present, the accumulation amount of silicon manganese slag has reached hundreds of millions of tons [19, 20]. With the development of industrialization and the continuous consumption of primary resources, making full use of industrial waste and simultaneously exploring various secondary resources treatment processes are effective ways to improve ecological environment governance capabilities and promote the achievement of the dual carbon goal.
Black ceramic tiles are widely used in the fields of architecture and decoration. Traditional black ceramic tiles use transition metal elements such as Fe, Co, and Cr as colorants. Due to the high cost of Co, much attention is paid to the development of low-cost Co-free black pigments [21, 22]. The preparation of black ceramic pigments from industrial solid waste can effectively reduce production costs and promote economic circulation. Costa et al. [23] prepared black pigments with (Ni, Fe)(Fe, Cr)2O4 as the main phase using electroplating sludge rich in Cr and Ni and galvanized sludge rich in Fe, and the color of the pigment was comparable to that of commercial pigments. After mixing 35%-55% leather sludge with other metal oxides, Chen et al. [24] burn at 1100 ˚C to obtain black pigments with NiFe2O4, CuCr2O4, and NiCr2O4 as the main phases. Vilarinho et al. [25] used Cr and Ni from electroplating sludge as colorants and mixed them with stone slurry to obtain a uniformly colored brown stone slurry. In summary, developing efficient and clean comprehensive utilization methods for secondary resources can effectively solve the problem of enterprise waste discharge [26, 27].
This study develops a one-step sintering method to prepare Fe, Cr and Mn black ceramic tiles. CaO, SiO2 and Al2O3 in chromium slag, copper slag and silicon manganese slag were used as the main components of ceramic matrix, as well as transition metal elements Fe, Cr and Mn in the slag as the coloring elements of tiles. The raw materials reacted at elevated temperatures to generate a stable black spinel phase. The aim of the present work is to explore the effects of molar ratio of Fe/Cr/Mn, sintering temperature, holding time, cooling method and atmosphere on the phase and color of ceramic tiles, which provides a guidance for the industrial production of black ceramic tiles.
Preparation of black ceramic tiles
The raw materials of chromium slag, copper slag, and silicon manganese slag were from a domestic chromium salt factory, copper smelter and ferroalloy factory, respectively. The chemical compositions of the raw materials are listed in Table 1. Firstly, the raw materials were dried, crushed and proportioned according to the designed molar ratio of Fe/Cr/Mn. Then, the raw materials were mixed for 30 min using a turnover shaker (DR-MIX). About 5 g of the mixed raw materials were pressed in a cylindrical mold with a diameter of 15mm at the molding pressure of 10MPa and the holding time of 60 s. Subsequently, the formed cylindrical samples were heated in a muffle furnace (SX2-10 13) with the heating rate of 7 ˚C/min in air or Ar atmosphere and then held at various target temperatures (1120, 1130, 1140, 1150 and 1160 ˚C) for various holding time (15, 30, 45, 60 and 90 min). Table 2 shows the mass fractions of various raw materials. Table 3 lists the process parameters to produce black ceramic tiles.
Compressive strength and leaching tests of ceramic tiles
For each type of produced ceramic tiles, a hydraulic universal test machine (WE-30, Tai Tian machinery Jiangsu Co., Ltd, China) was employed to measure the compressive strength of five produced ceramic tiles. The average of measured five values was used to determine the compressive strength values.
Theleaching tests of Cr6+ in raw materials and ceramic tiles were conducted according to the Chinese environmental protection industry standard HJ/T299-2007 [28]. The extraction agent with the pH of 3.21 was prepared by using the raw materials of deionized water, concentrated sulfuric acid and concentrated nitric acid. The mass ratio of concentrated sulfuric acid to concentrated nitric acid was 2:1. About 100 g powdery sample were added in prepared extraction agent which was quantitative in a 1000 ml volumetric flask. Then, the mixed agents were loaded in a flip shaker and shaked at a speed of 30 r/min at the temperature of 24 ˚C for 20 h. The concentration of Cr6+ in the leaching solution was tested by ultraviolet visible spectrophotometry (UV-6100S ultraviolet visible spectrophotometer, Shanghai Yuanxi Instrument Co., Ltd., China) in triplicate.
Analytical methods
The types of phases in the raw materials and ceramic tiles were identified by X-ray diffractometer (XRD) (PANalytical X'Pert PRO MPD, Netherlands) with the Cu Kα radiation. The morphology of phases in the ceramic tiles were observed by a field-emission scanning electron microscope (SEM) (FEI, Nova NanoSEM400) coupled with an energy-dispersive X-ray spectrometry (EDS) (Oxford, INCA PentaFET-x3).
The diffuse reflectance spectra of the sample in the range of 200-800 nm were measured using a UV visible spectrophotometer (UV-2600, Shimadzu). A colorimeter (3nh, TS7010) was used to measure the chromaticity values (L*, a* and b*) of ceramic tiles. L*, a*, and b* are color models developed by the International Commission on Illumination (CIE). L* value is the brightness of the color, with a scale from white (L*=100) to black (L*=0). a* value and b* value represent the scales of green (-a*) to red (+a*), blue (-b*) to yellow (+b*), respectively. In addition, C* value represents the color saturation value of ceramic tiles, and its calculation formula is shown in equation (1). The sample exhibits black performance if its values of L*, a*, b* and C* are near to 0.

|
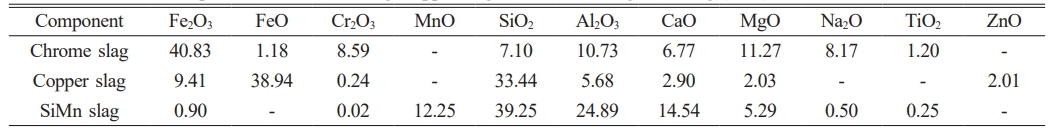
Table 1 Chemical compositions of chrome slag, copper slag and silicon manganese slag (wt%). |
Characteristics of raw materials
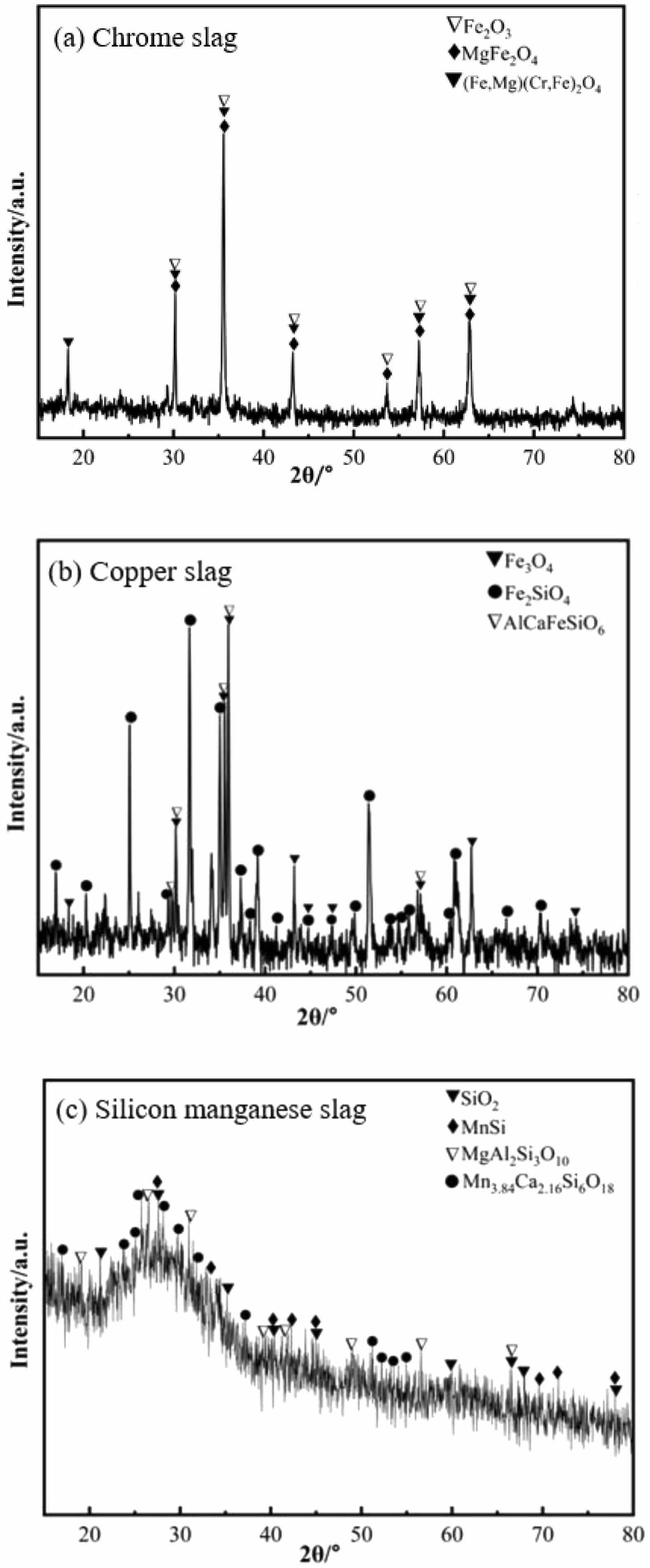
It can be seen from Table 1 that chromium slag and copper slag contain a large amount of iron oxides (42.01 wt%, 48.35 wt%) and a small amount of Cr2O3 (8.59 wt%, 0.24 wt%), while silicon manganese slag contains 12.25 wt% of MnO. All the aforementioned slag contains some amount of SiO2 (7.10 wt%, 33.44 wt%, 39.25 wt%) and Al2O3 (10.73 wt%, 5.68 wt%, 24.89 wt%). It should be noted that SiO2 and Al2O3 are the main components of the ceramic matrix. Fig. 1 presents the XRD results of chromium slag, copper slag and silicon manganese slag. The main phases in chromium slag are Fe2O3, MgFe2O4 and (Fe, Mg)(Cr, Fe)2O4. The main phases in copper slag are Fe2SiO4. Silicon manganese slag is mainly composed of glass and contains some amount of manganese silicon alloy (MnSi) and silicate.
Effect of Fe/Cr/Mn molar ratio on the chromatic performance of ceramic tiles
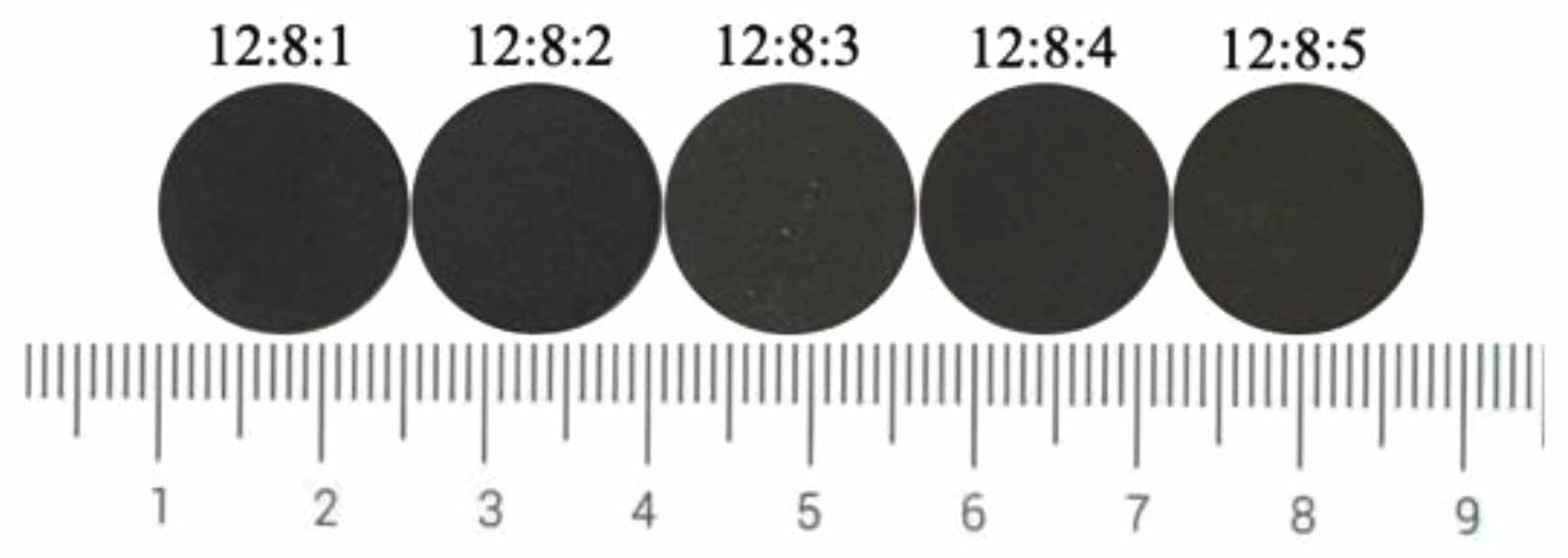
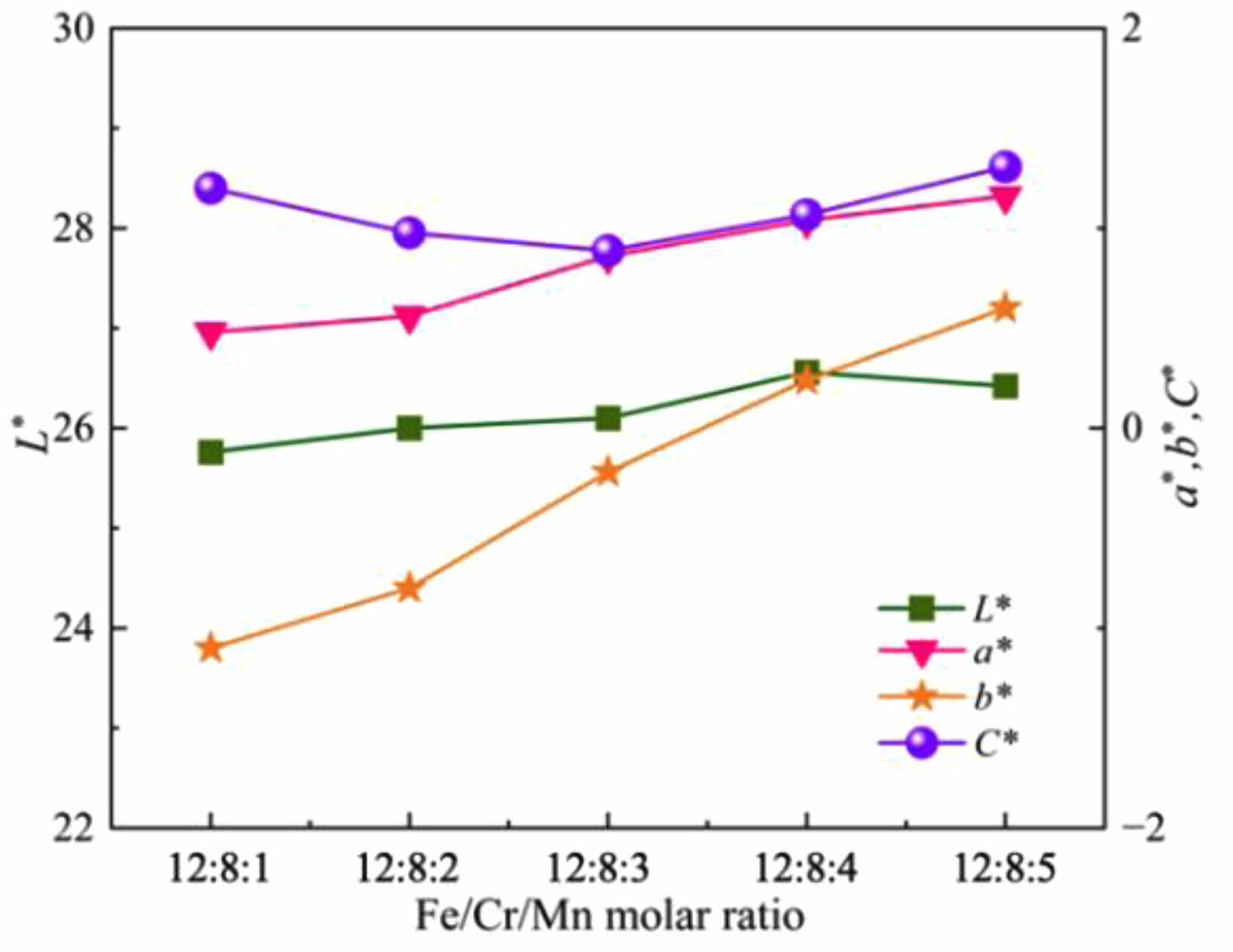
Fig. 2 shows the photos of ceramic tile with different Fe/Cr/Mn molar ratios. It can be seen from Fig. 2 that the overall appearance of the ceramic tiles is black, smooth and flat. Fig. 3 presents the effects of Fe/Cr/Mn molar ratios on the coloration of ceramic tiles prepared with the mixture of chromium slag, copper slag and silicon manganese slag sintered at 1150 ˚C for 30 min by furnace cooling. As the Mn content increases, the L* value of ceramic tiles shows a trend of first increasing and then decreasing, with little fluctuation. When Fe/Cr/Mn molar ratio is 12:8:4, L* reaches the maximum value of 26.56. The value of C* fluctuates around 1. The chromaticity value of ceramic tile is determined by the values of L*, a*, b*, and C*. To achieve the purpose of comprehensive utilization of multiple slags, this study selects Fe/Cr/Mn=12:8:4 to investigate the influence of other factors on the coloration of ceramic tile.
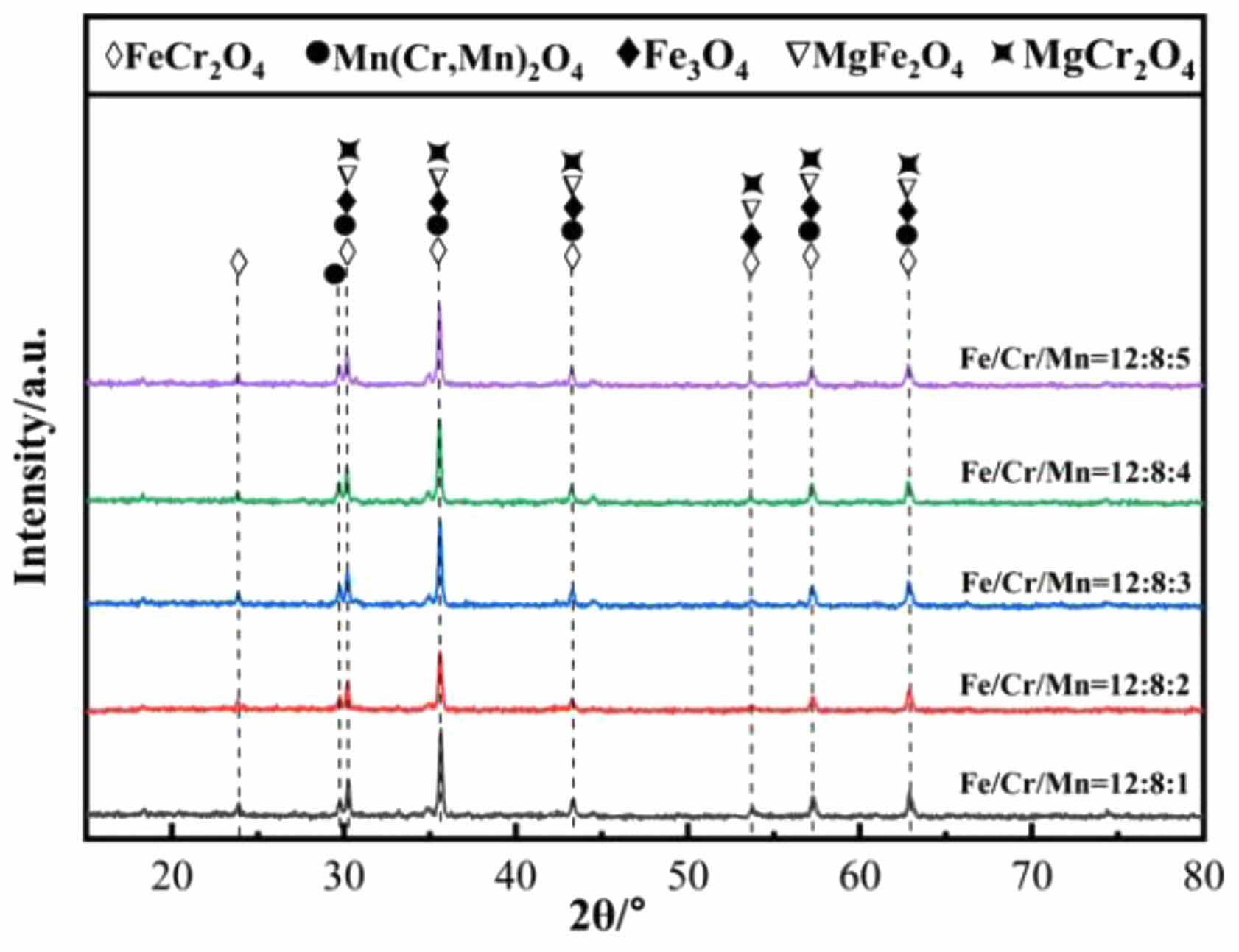
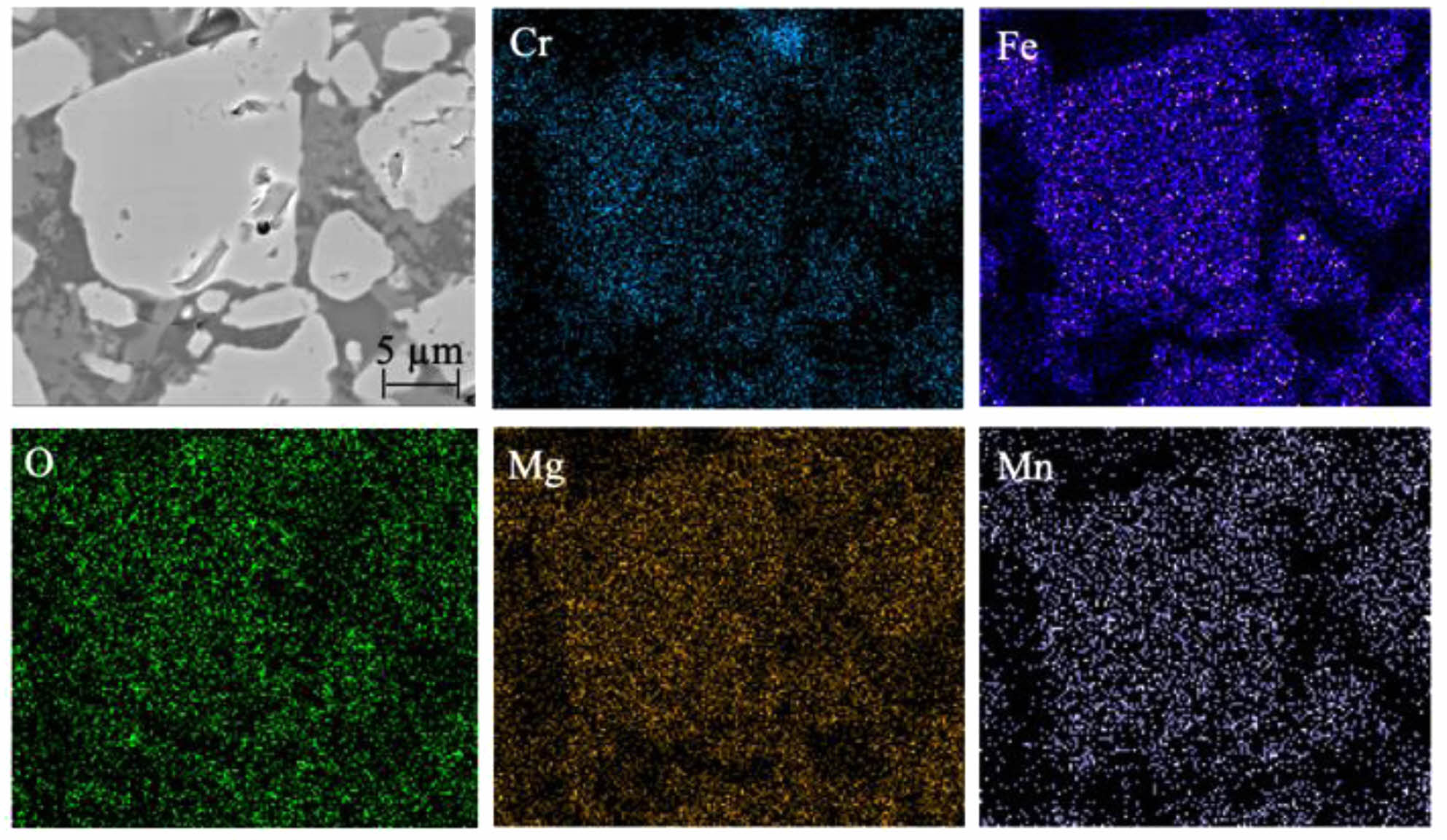
Fig. 4 shows the XRD patterns of ceramic tiles with different Fe/Cr/Mn molar ratios sintered at 1150 ˚C. It can be seen from Fig. 4 that the main phases in ceramic tiles are FeCr2O4, Mn(Cr, Mn)2O4, Fe3O4, MgFe2O4 and MgCr2O4. The black appearance of ceramic tiles is mainly due to the formation of FeCr2O4 originating from the solid solution reaction between FeO and Cr2O3 in the raw material, as well as the generation of Fe3O4 resulting from the solid solution reaction between FeO and Fe2O3. MgFe2O4 originated from the solid solution reaction between MgO and Fe2O3. At the presented sintering temperature, the reaction between MnO and Cr2O3 could take place and generated MnCr2O4. In the study by Liu et al. [29], it has been confirmed that this reaction can occur at 1100 ˚C~1200 ˚C. In addition, MnO in the raw material was oxidized to Mn3+ at elevated temperature and participated in MnCr2O4 to generate Mn(Cr, Mn)2O4 [30]. Fig. 5 exhibits that the spinel phase in ceramic tiles with Fe/Cr/Mn molar ratio of 12:8:4 are irregular polygons and enriched in elements Cr, Fe and Mg. Mn element clustered on the spinel phase.
Effect of sintering temperature on the chromatic performance of ceramic tiles
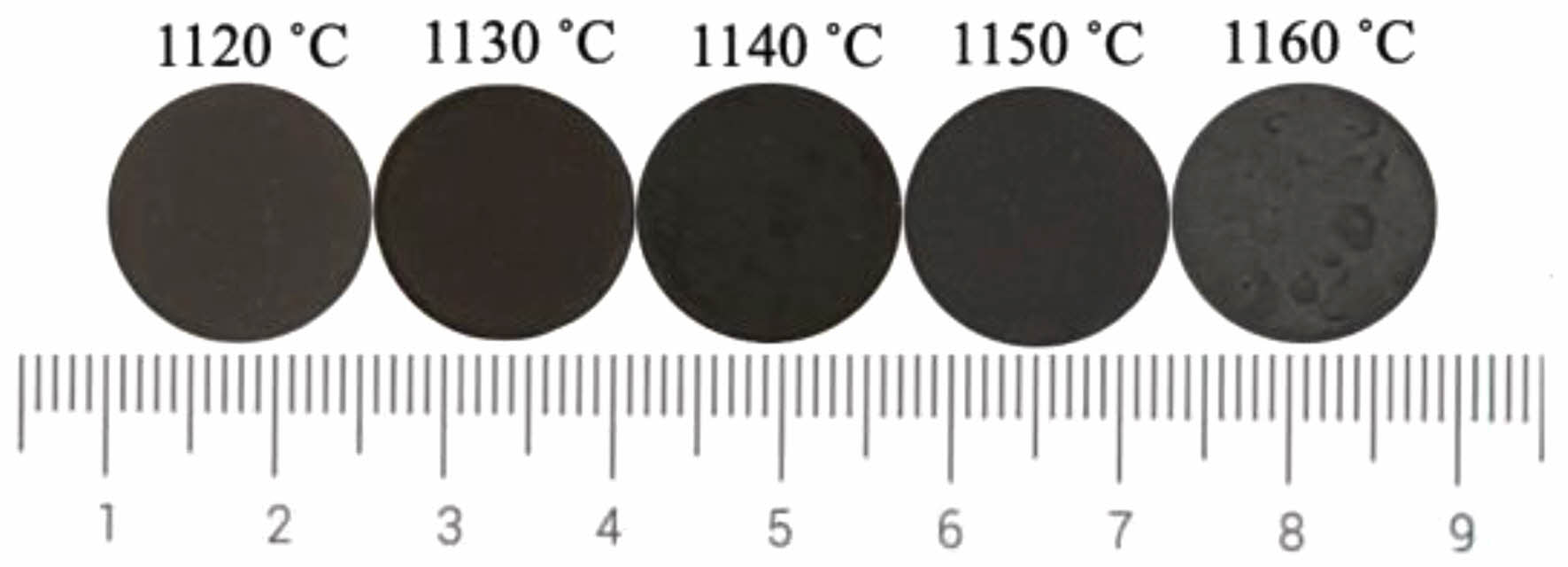
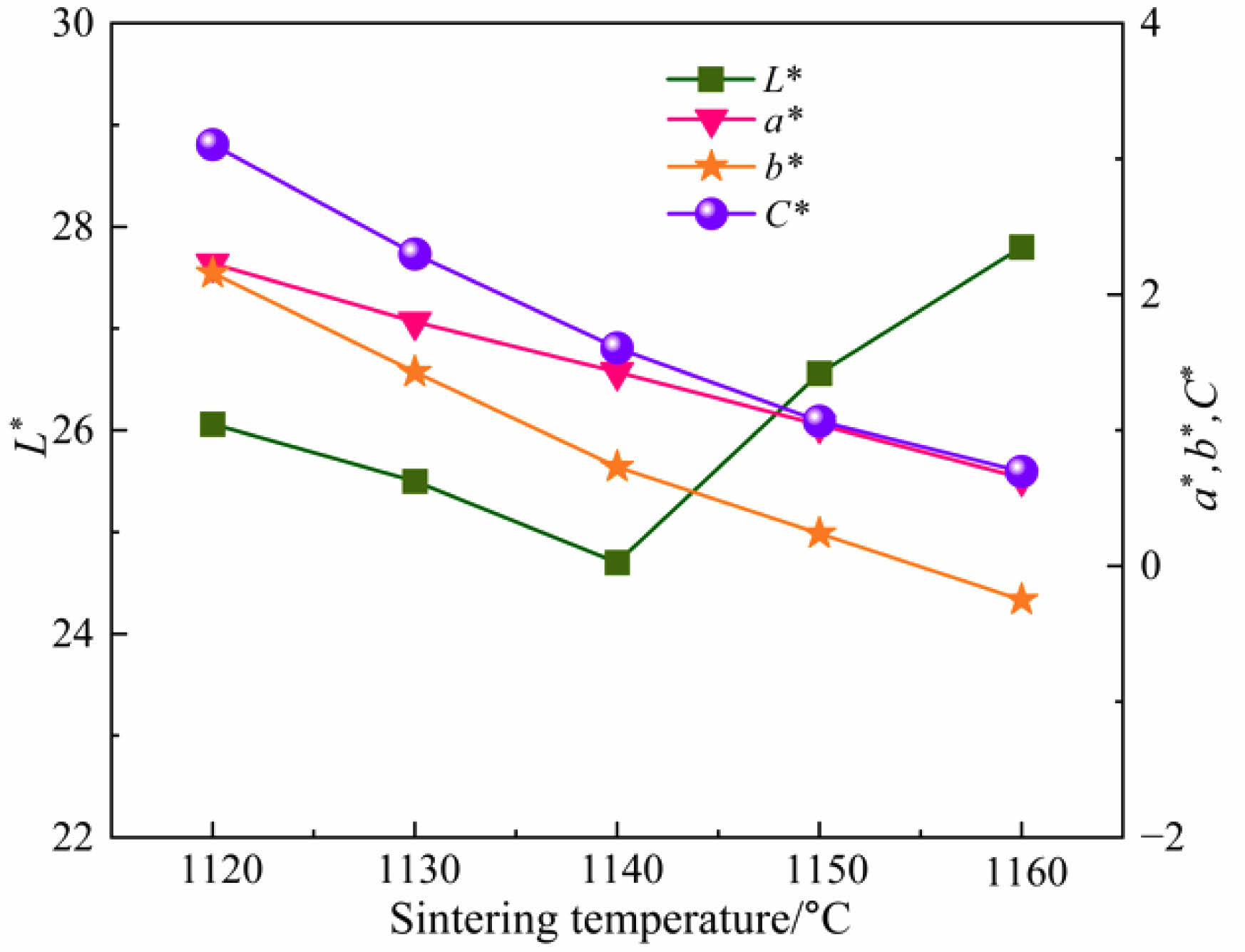
Fig. 6 presents the photos of ceramic tile sintered at different sintering temperature. It is clear that when the sintering temperature is 1160 ˚C, the surface of the ceramic tile is uneven. The relatively elevated temperature leads to an increase in the amount of the liquid phase in the ceramic tiles and obvious bubbles on the surface of ceramic tile. It can be seen from Fig. 7 that as the temperature increases, the values of a*, b* and C* become near to 0. When the temperature rises to the point where a large amount of liquid phase generated in the ceramic tile, the L* value of the ceramic tile increases. Therefore, the optimal sintering temperature is 1150 ˚C.
In the raw materials containing Fe2O3, Cr2O3, MnO, the following chemical reactions (2)-(5) may take place:
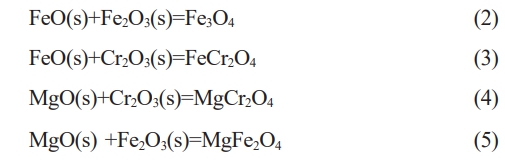
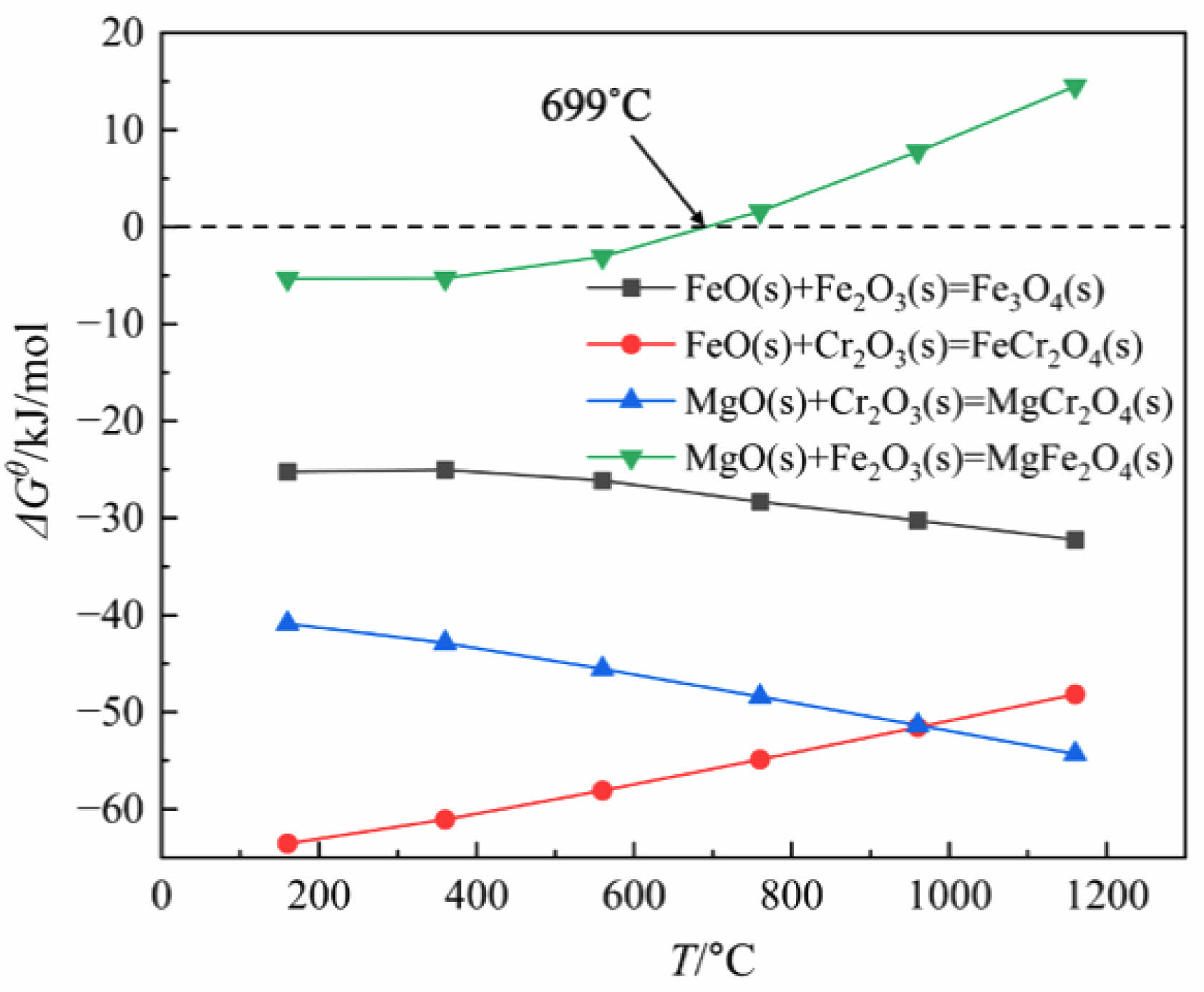
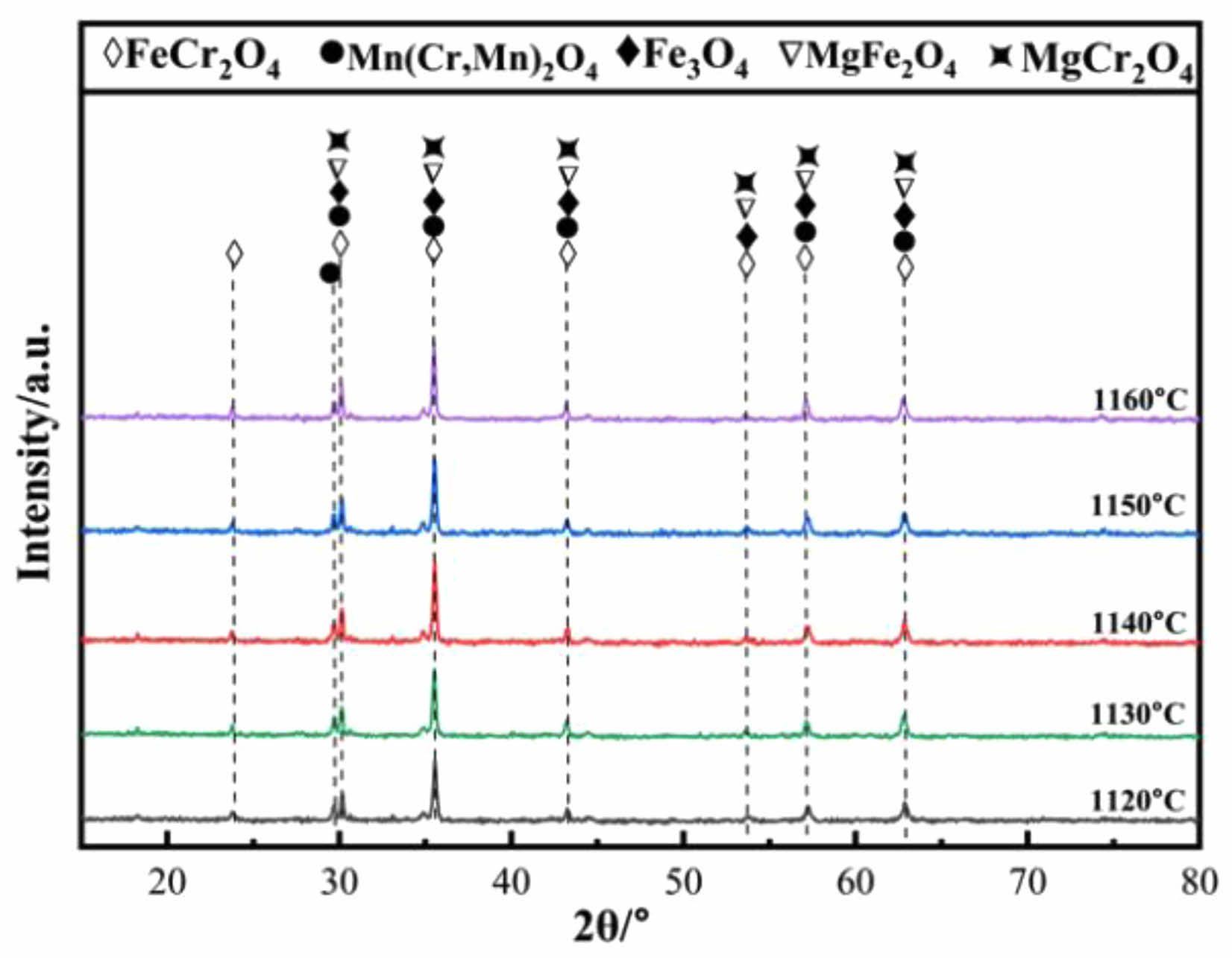
The standard Gibbs free energy change for these above reactions were calculated using FactSage 8.1, and the results are presented in Fig. 8. The ∆Gθ values for reactions (3) and (5) gradually increase with increasing reaction temperature. The ∆Gθ values for reactions (2) and (4) gradually decreases with increasing reaction temperature. Reaction (5) take place at the temperature below 699 ˚C which corresponding to negative ∆Gθ values. Fig. 9 shows the XRD patterns of ceramic tiles at different sintering temperatures. The main phase in ceramic tiles are FeCr2O4, Mn(Cr, Mn)2O4, Fe3O4, MgFe2O4, and MgCr2O4. In the study by Bipra et al. [31], when the temperature is 374 ˚C~989 ˚C, the decomposition reaction of Fe2SiO4 can occur. As the temperature increases, Fe2+ contained in the Fe2SiO4 in the raw material is oxidized and decomposes into Fe2O3 and SiO2, which provide sufficient Fe2O3 for the formation of Fe3O4 and FeCr2O4 in the ceramic tile.
Effect of holding time on the chromatic performance of ceramic tiles
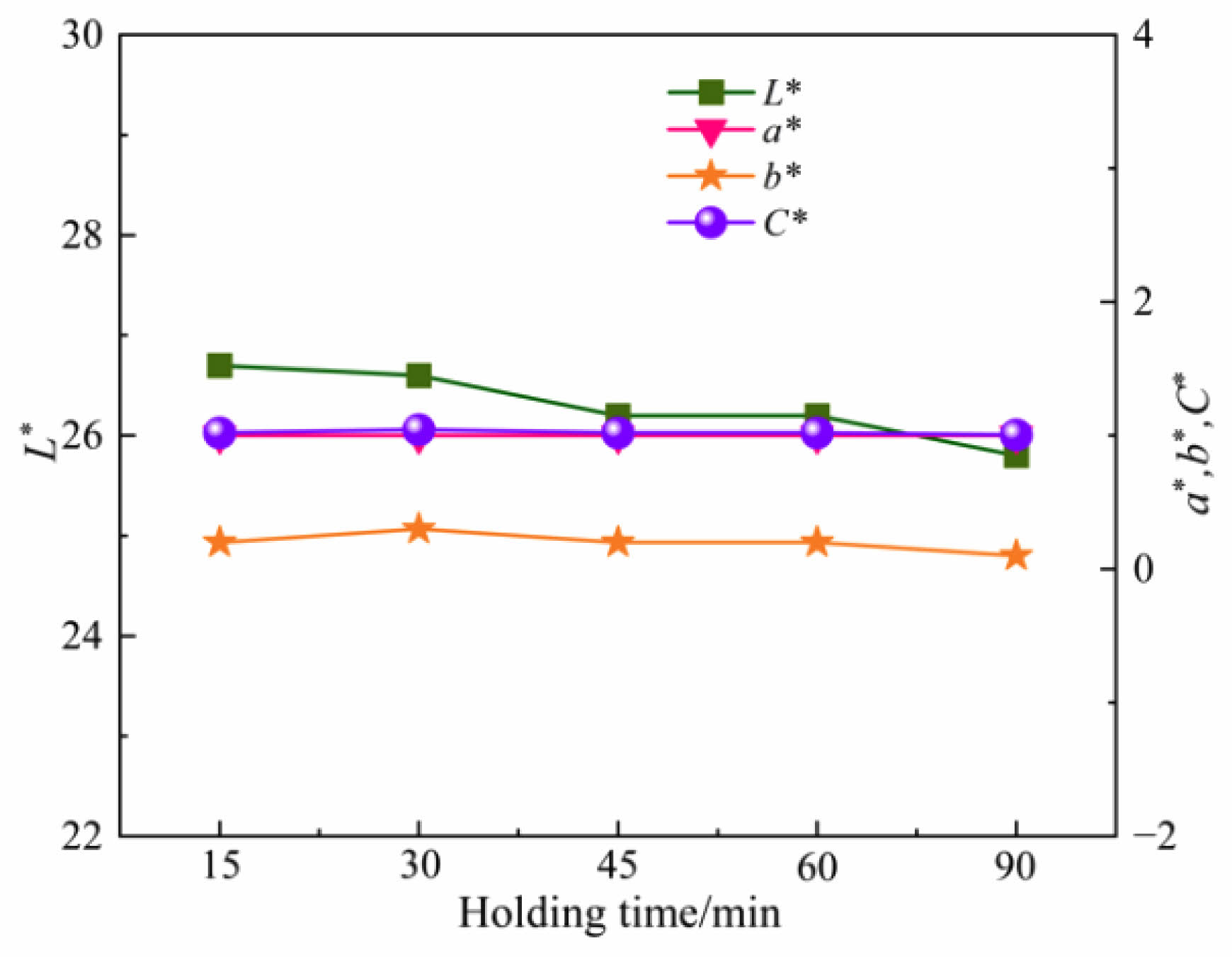
Fig. 10 and 11 show the photos and chromaticity values of ceramic tiles with the Fe/Cr/Mn molar ratio of 12:8:4 and sintered at 1150 ˚C for various holding times followed by furnace cooling. It can be seen that the overall color of the ceramic tiles appears black. As the holding time increases, the L* value gradually decreases. When the holding time increases to 60 min, the L* value remains unchanged. When the holding time increases to 90 min, the L* value significantly decreases. The a* value remains unchanged at about 1, while the b* value fluctuates near to 0 and the C* value remains unchanged. After comprehensive consideration, the most suitable holding time is 45 min.
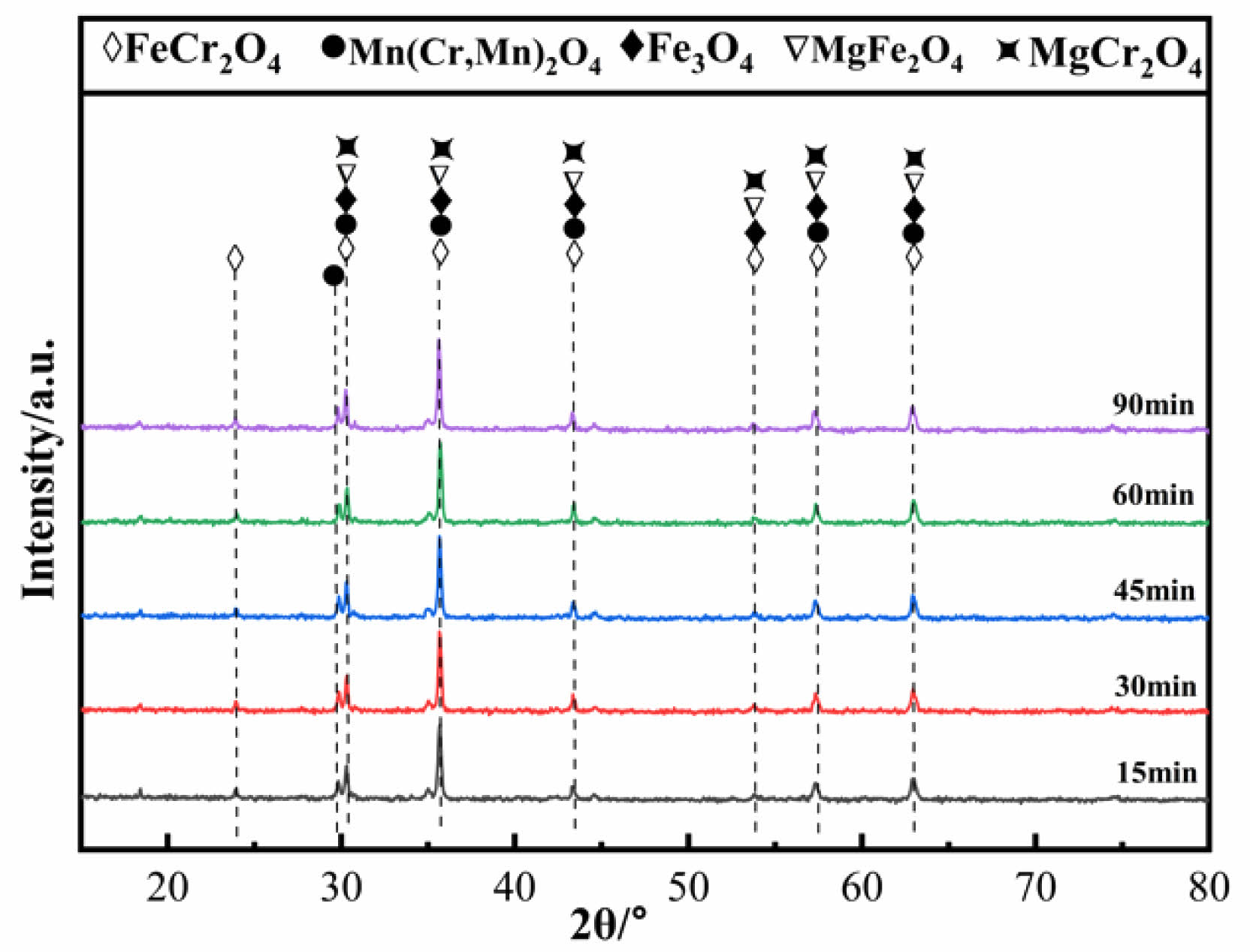
Fig. 12 shows the XRD patterns of ceramic tiles at different holding times. The main phases in ceramic tiles are FeCr2O4,Mn(Cr, Mn)2O4,Fe3O4,MgFe2O4 and MgCr2O4. Extending the holding time can encourage more colored ions in the raw material to participate in the reactions of generating spinel. Appropriately extending the holding time is more conducive to improving the crystallinity of the grains.
Effect of cooling methods on the chromatic performance of ceramic tiles
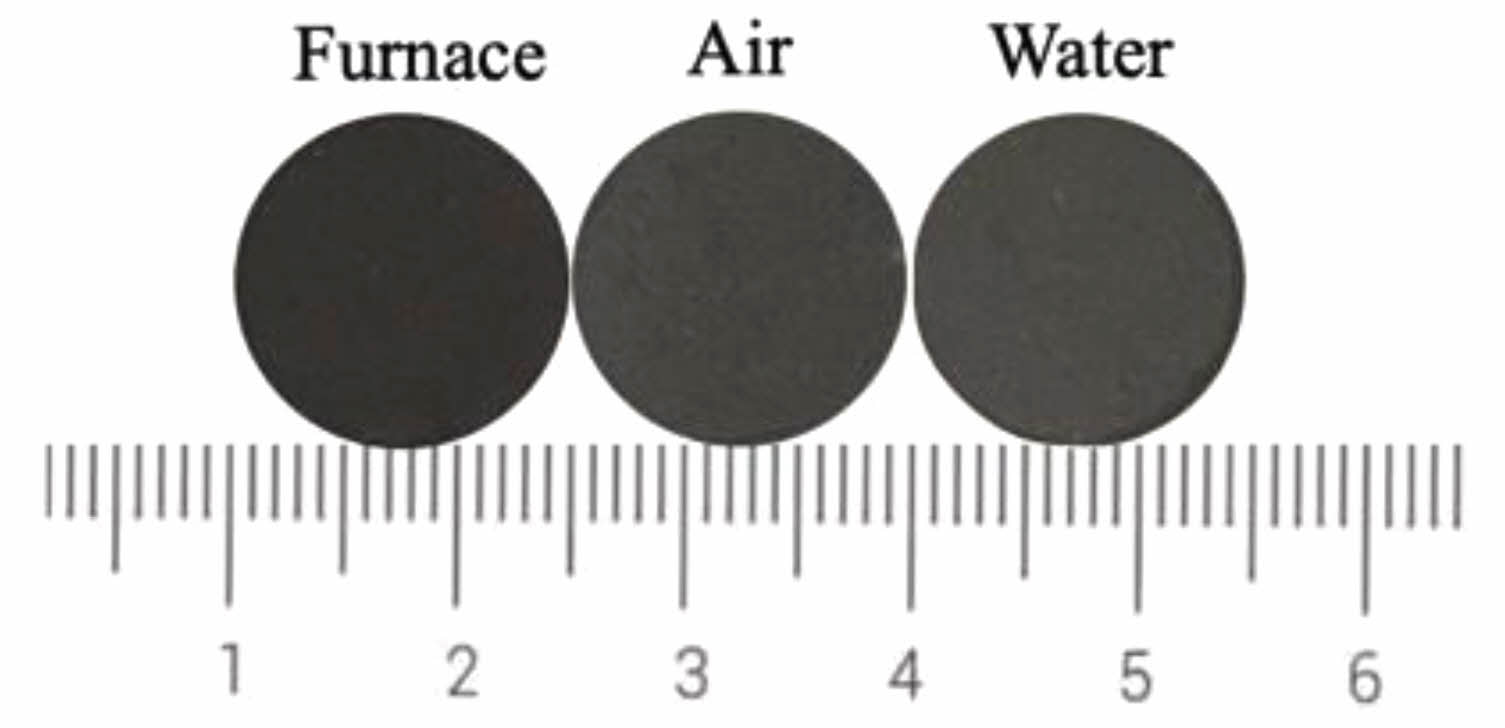
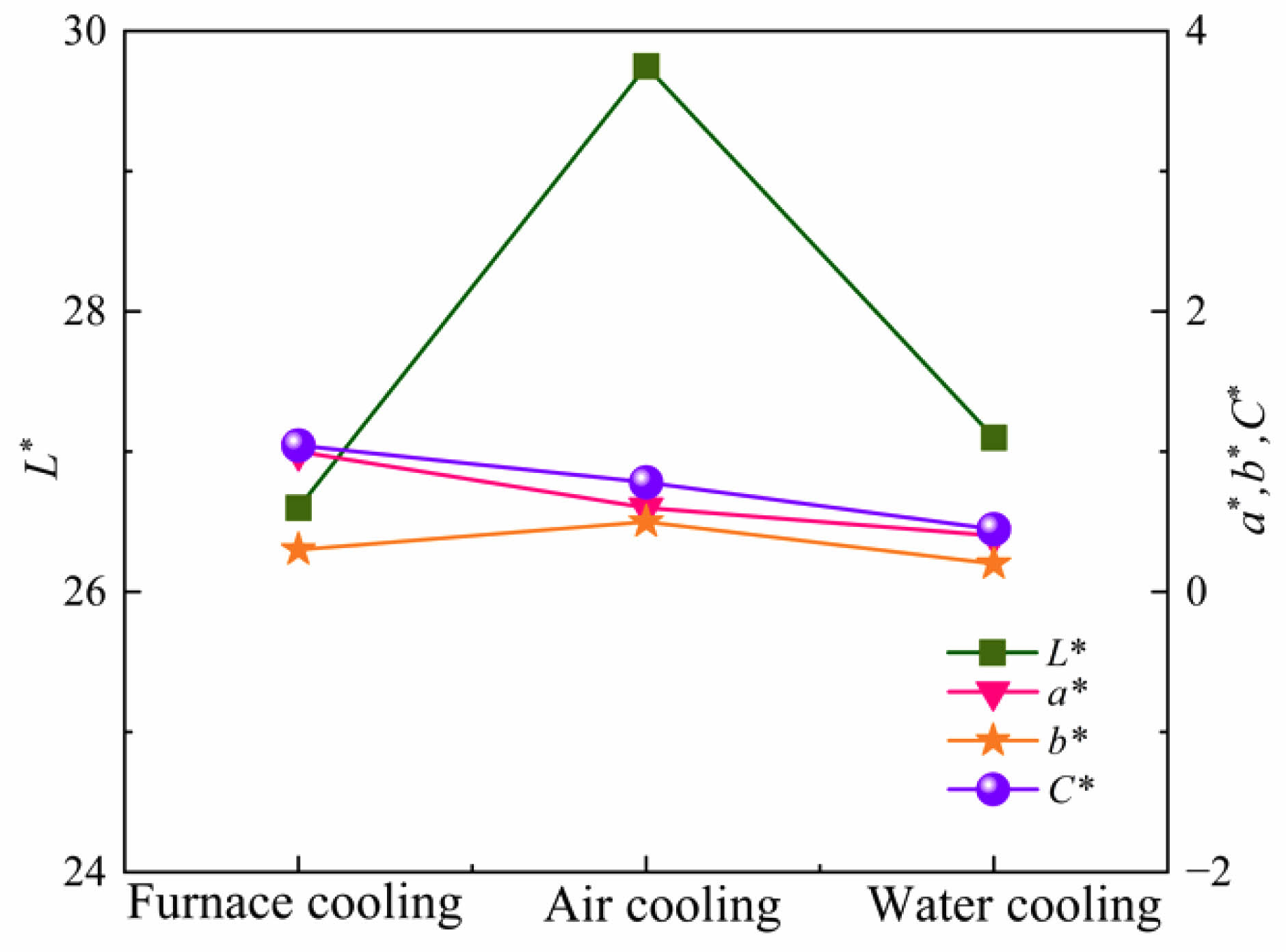
Fig. 13 shows the photos of ceramic tiles with the Fe/Cr/Mn molar ratio of 12:8:4 and sintered at 1150 ˚C for 45 min followed by furnace cooling, air cooling and water cooling, respectively. Ceramic tiles cooled by water and air are fragile. Fig. 14 presents that the ceramic tiles cooled in the furnace has the lowest L * value. Cooling rate affects the crystal growth of black phase in ceramic tiles. Irrespective of the cooling methods, the changes in a*, b* and C* values are slight.
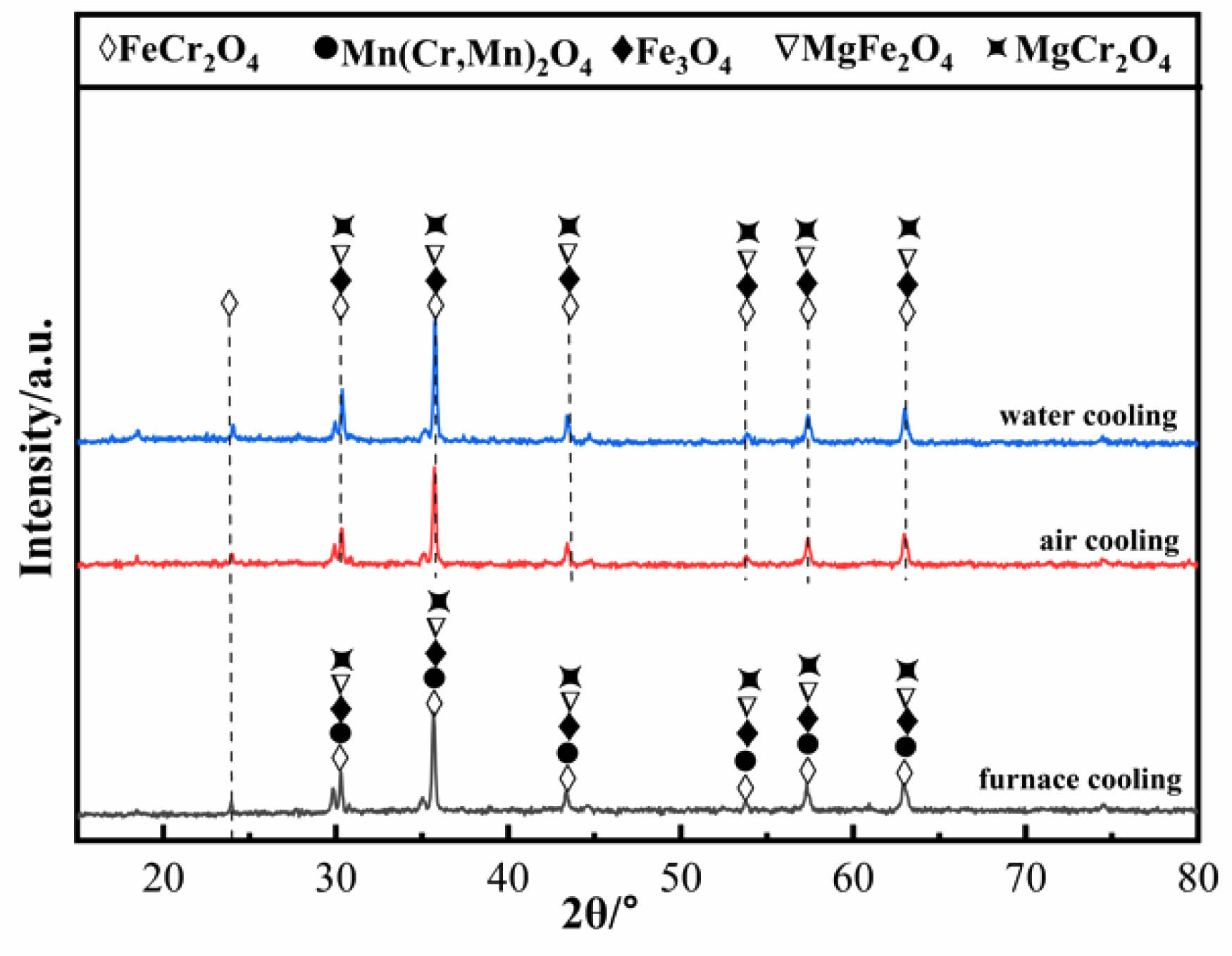
Fig. 15 shows the XRD patterns of ceramic tiles with different cooling methods. Mn(Cr, Mn)2O4 spinel was not detected in the ceramic tiles cooled by air cooling and water quenched, indicating that the growth conditions for Mn(Cr, Mn)2O4 spinel are only met during furnace cooling, which is because that the lower cooling rate improve the growth of Mn (Cr, Mn)2O4 crystals.
Effect of sintering atmosphere on the chromatic performance of ceramic tiles
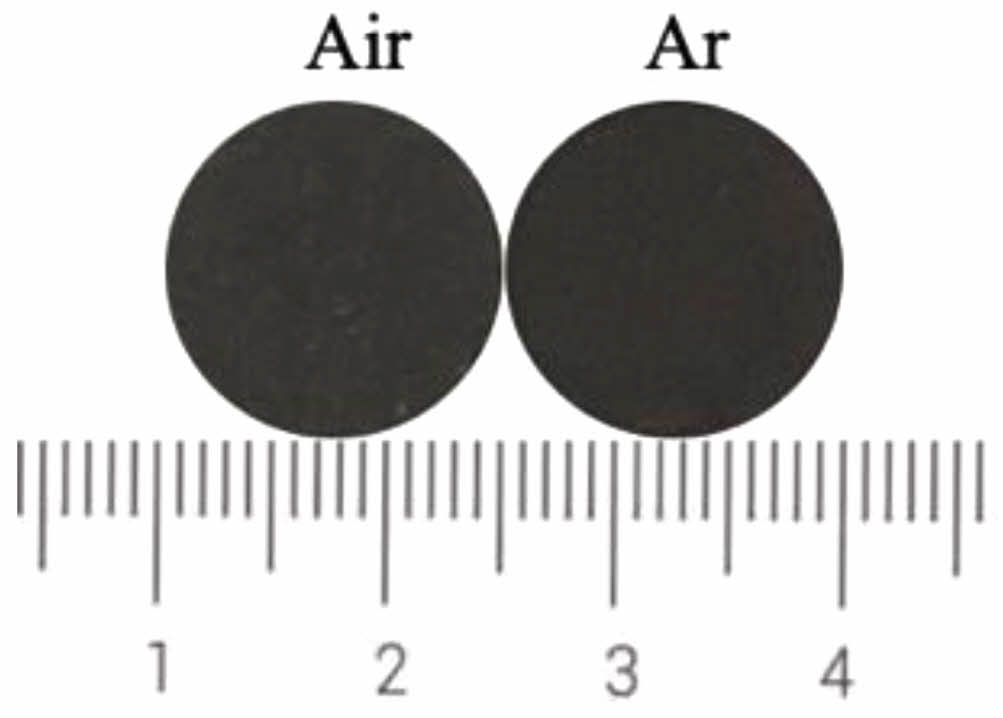
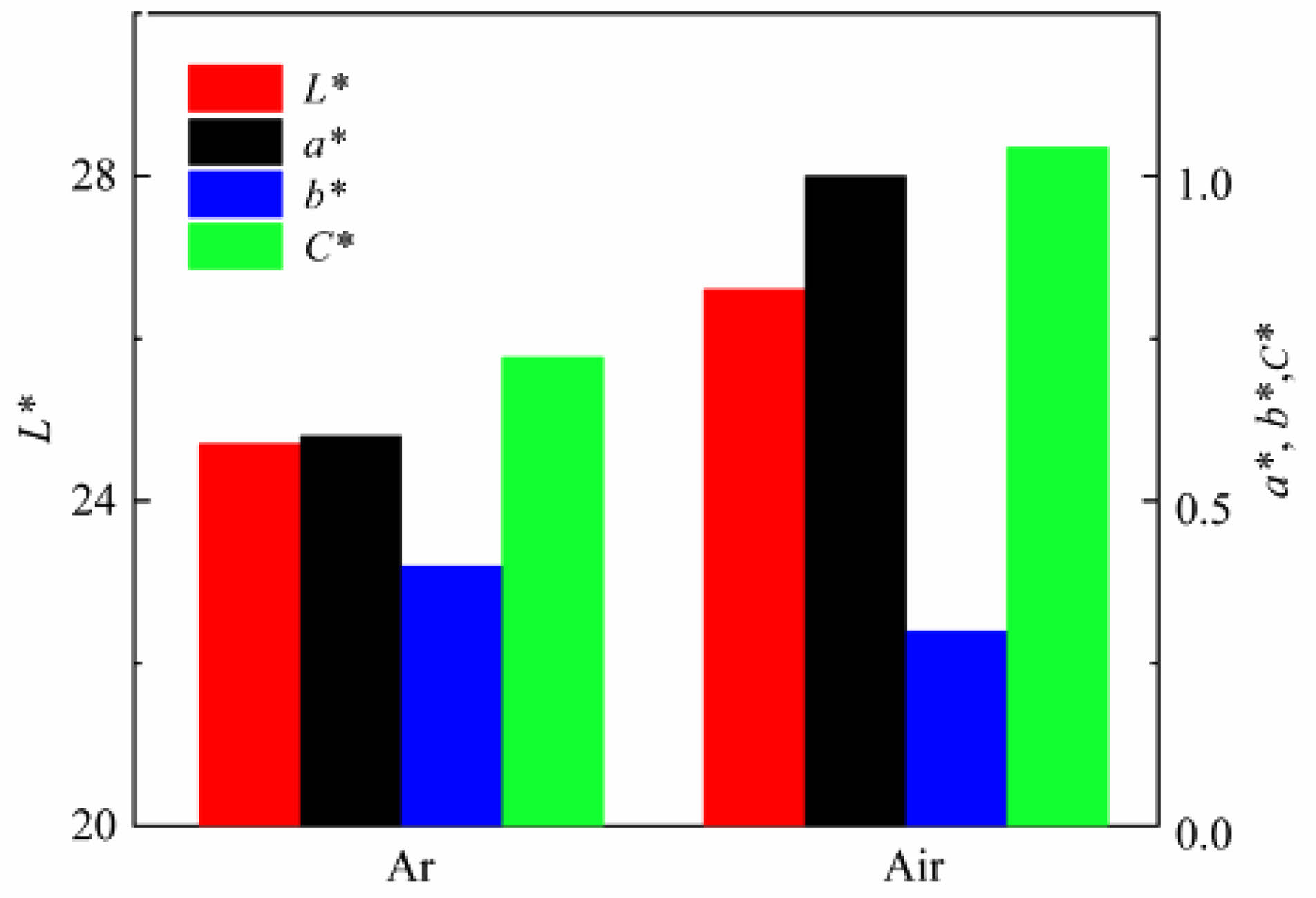
Fig. 16 and 17 show the photos and chromaticity values of black ceramic tiles sintered in argon and air, respectively. From Fig. 16, the surface of ceramic tile sintered in argon and air atmospheres are both smooth and appears black. The values of L*, a*, b* and C* in argon atmosphere are 24.7, 0.6, 0.4, and 0.26, respectively. The values of L*, a*, b* and C* in air atmosphere are 26.6, 1.0, 0.3, and 0.54, respectively. It can be seen from Fig. 17 that the values of L*, a* and C* of ceramic tiles sintered in argon atmosphere are significantly smaller than those of sintered in air atmosphere. Sintering atmosphere has little effect on the b* value of ceramic tiles.
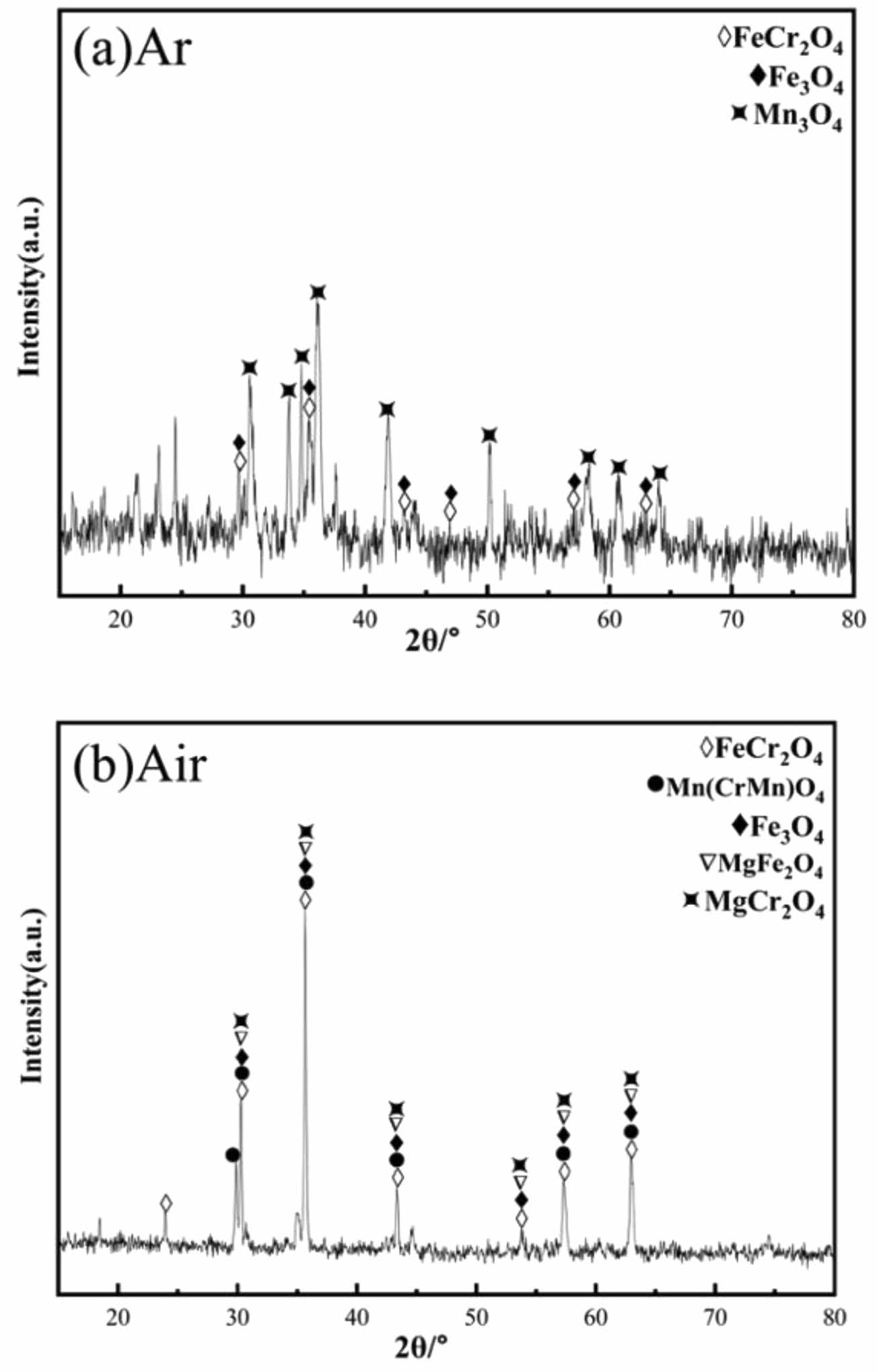
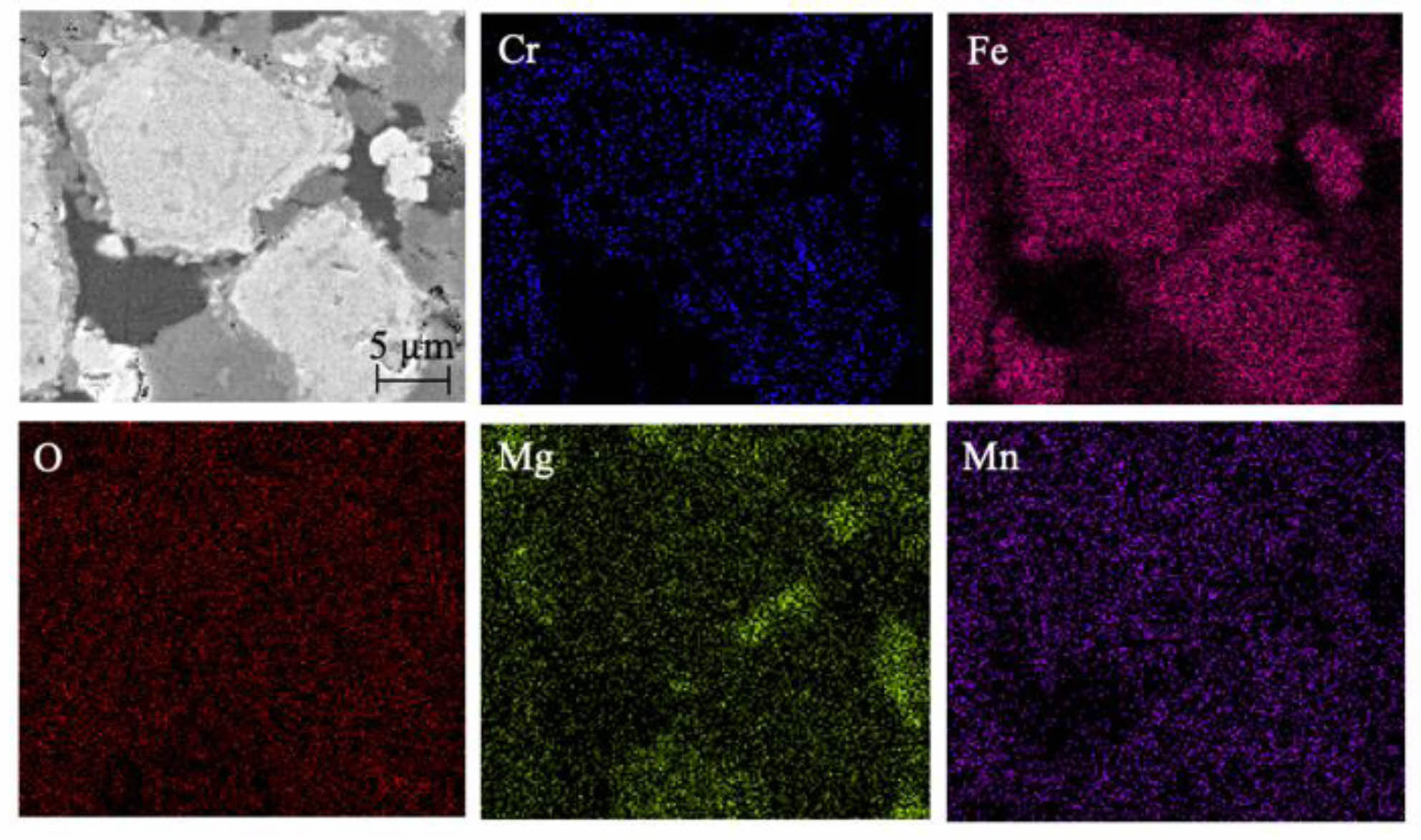
Fig. 18 and 19 show the XRD patterns and SEM images of ceramic tiles sintered in different atmospheres, respectively. From Fig. 18, the main phases of ceramic tiles in argon atmosphere are FeCr2O4, Fe3O4 and Mn3O4. It can be seen From Fig. 19 that in argon atmosphere, the distribution of enrichment region for Fe and Cr elements is the same. In addition, Mg2+ didn’t participate in the reaction of forming MgFe2O4 and MgCr2O4 spinel, which was because that Fe2+ was not oxidized in argon atmosphere and then replacing the position of Mg2+ in MgFe2O4 and MgCr2O4 spinel. Therefore, Fe and Cr elements are tightly aggregated and embedded in the ceramic matrix.
Compressive strength, Leaching tests and diffuse reflectance spectra of Ceramic tiles
To investigate whether the properties of prepared ceramic tiles meet national standards and provide guidance for industrial production, the presented work tested the compressive strength, leaching concentration of Cr6+ and diffuse reflectance spectra of the prepared ceramic tiles with Fe/Cr/Mn molar ratio of 12:8:4, sintering in air atmosphere, sintering temperature of 1150 ˚C, holding time of 45 min and cooled in the furnace. The tested results are listed in Table 4. The compressive strength is 162.61 MPa, which is larger than the critical value of 27 MPa in the national standard for polished tiles (GB/T4100-2006) [32]. The leaching concentration of Cr6+ in the mixed raw material and prepared ceramic tiles are 11.59 mg/L and 0.97 mg/L, respectively. The concentration of Cr6+ in the prepared ceramic tiles can meet the threshold value of 5mg/L specified in the national standard (GB 5085.3-2007) [33] and the standard of U.S. Environmental Protection Agency (EPA). Therefore, the black ceramic tiles produced in the present work can achieve value-added purposes.
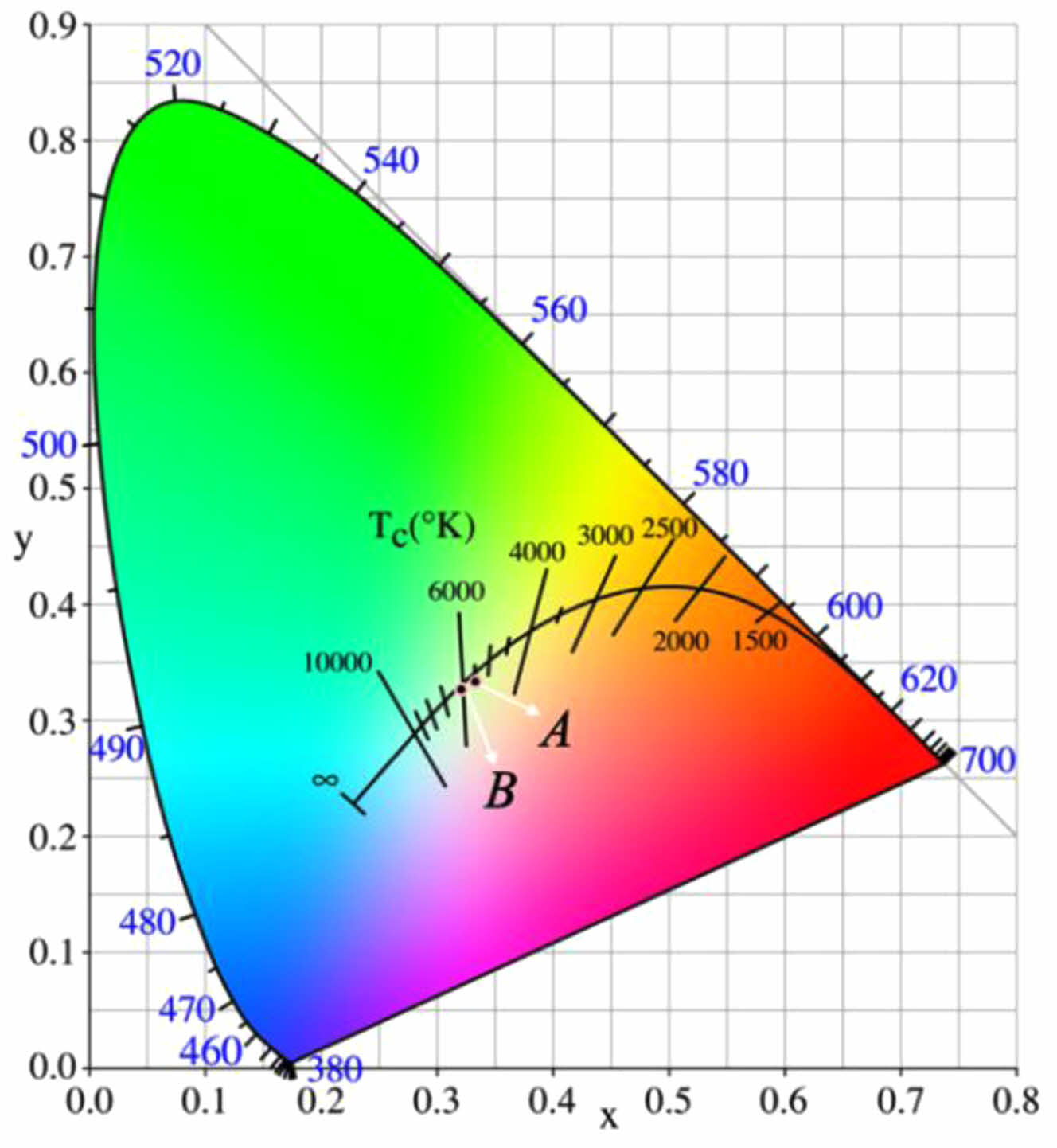
To further verify the color purity of the prepared black ceramic tiles, the chromaticity coordinates of the prepared black ceramic tiles was exhibited in the chromaticity diagram, and the results are presented in Fig. 20.
The point B in Fig. 20 represents the corresponding point of the prepared black ceramic tile, which is with the chromaticity coordinates of x=0.3219, y=0.3271. Point A in Fig. 20 is with a saturation of 0, which can be black or white in color. The corresponding CIE chromaticity coordinates are x=0.3333, y=0.3333. For black ceramic tiles, the closer the color coordinate near to point A, the higher the purity of black color. The coordinates of point B is very close to point A, indicating that the color saturation of point B is close to 0. Therefore, it is feasible to use chromium slag, copper slag and silicon manganese slag to prepare black ceramic tiles.
|
Fig. 1 XRD patterns of chrome slag, copper slag and silicon manganese slag. |
|
Fig. 2 Ceramic tile photos with different Fe/Cr/Mn molar ratios. |
|
Fig. 3 Effects of Fe/Cr/Mn molar ratios on the coloration of ceramic tiles. |
|
Fig. 4 XRD patterns of ceramic tiles with different Fe/Cr/Mn molar ratios. |
|
Fig. 5 SEM images and elemental mapping of the ceramic tiles with Fe/Cr/Mn molar ratio of 12:8:4. |
|
Fig. 6 Photos of ceramic tile with different sintering temperature. |
|
Fig. 7 Chromaticity values of ceramic tiles at different sintering temperature. |
|
Fig. 8 Dependence of reaction standard Gibbs free energy on reaction temperature. |
|
Fig. 9 XRD patterns of ceramic tiles with different sintering temperature. |
|
Fig. 10 Photo of ceramic tile with different holding time. |
|
Fig. 11 Chromaticity values of ceramic tiles at different holding time. |
|
Fig. 12 XRD patterns of ceramic tiles with different holding time. |
|
Fig. 13 Ceramic tile photos with different cooling methods. |
|
Fig. 14 Chromaticity values of ceramic tiles at different cooling methods. |
|
Fig. 15 XRD patterns of ceramic tiles with different cooling methods. |
|
Fig. 16 Ceramic tile photos with different atmosphere. |
|
Fig. 17 Chromaticity values of ceramic tiles in different atmosphere. |
|
Fig. 18 XRD patterns of ceramic tiles in argon atmosphere. |
|
Fig. 19 SEM images and mapping of the black ceramic tiles in argon atmosphere. |
|
Fig. 20 Chromaticity diagram of ceramic tiles. |
|
Table 4 Leaching concentration of Cr6+ in mixed raw materials and ceramic tile (mg/L), GB 5085.3-2007 and U.S. EPA. |

(1) The optimal parameters of preparing black ceramic tiles as the raw materials of chromium slag, copper slag and silicon manganese slag are with the sintering temperature of 1150 ˚C, the holding time of 45 min, cooling in furnace in air atmosphere. The corresponding values of L*, a*, b* and C* are 26.2, 1.0, 0.2 and 1.02, respectively. The main black phases in the ceramic tiles are FeCr2O4 and Fe3O4.
(2) Among the process parameters, sintering temperature and cooling method have the greatest impact on ceramic tiles. The sintering temperature higher than 1160 ˚C leads to the formation of liquid phase in the tiles matrix, resulting in uneven surface of the tiles. The cooling method has the greatest impact on its compressive strength, and ceramic tiles cooled by water and air are fragile.
(3) Under the optimal process parameters, the compressive strength and Cr6+ leaching concentration of the ceramic tiles were 162.61 MPa and 0.97 mg/L, respectively, which met the national standards.
(4) The chromaticity coordinates of prepared black ceramic tiles are very near to the point with a saturation of 0 in the chromaticity diagram, indicating that it is feasible to use chromium slag, copper slag and silicon manganese slag to prepare black ceramic tile.
This work was support by the Hubei Provincial Key Laboratory for New Processes of Ironmaking and Steelmaking (Grant No. KF-20-3).
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
- 1. Y.J. Tian, C.Q. Yan, Z.L. Cheng, X.J. Quan, and G. Li, Inorg. Chem. Ind. 53[12] (2021) 129-134.
- 2. Z.F. Tong, C.C. Xu, J.X. Wang, and Z.H. Jia, J. Ceram. Process. Res. 24[1] (2023) 17-28.
-

- 3. Z.B. Liu, J.Y. Zheng, W.Z. Liu, X.M. Liu, Y.X. Chen, X.Q. Ren, P. Ning, and Z. Lin, Sci. Total. Environ. 703(2020) 135075.
-

- 4. C.L. Wu, H. Zhang, P.J. He, and L.M. Shao, J. Environ. Sci. 22[7] (2010) 1110-1115.
-

- 5. X.J. Quan, H.Q. Tan, Y.C. Zhao, and Y. Hu, J. Hazard. Mat. 137[2] (2006) 836-841.
-

- 6. Q. Qi, L. Li, L.Y. Wei, B.M. Hu, Z. Liu, and X.Q. Liu, Main. Group. Chem. 20[3] (2021) 317-329.
-

- 7. X. Yan, J.L. Wang, M.J. Zhang, and X.Y. Liu, Chin. J. Biotechno. 37[10] (2021) 3591-3603.
-

- 8. S.H. Huang, B. Peng, Z.H. Yang, L.Y. Chai, and L.C. Zhou, T. Nonferr. Metal. Soc. 19[1] (2009) 241-248.
-

- 9. W.D. Xue, J. Xie, Y. Li, and J.L. Sun, Rare. Metal. Mat. Eng. 38[z2] (2009) 1226-1228.
- 10. T. Sun, L.L. Liu, S.Y. Xu, L.L. Wang W. Li, and H. Hao, Micro. Nano. Lett. 8[9] (2013) 487-490.
-

- 11. C.W. Hong, J. Lee, and J.H. Ryu, J. Ceram. Process. Res. 18[4] (2017) 324-328.
-

- 12. T. Kundu, S. Senapati, S.K. Das, S.I. Angadi, and S.S. Rath, Powder Technol.426(2023) 118693.
-

- 13. Y.L. Ye, L.Q. Luo, R.S. Chen, M.X. Wang, C. Liu, and Y.M. Lei, Bull. Chin. Ceram. Soc. 42[5] (2023) 1740-1749.
- 14. X.Y. Zhao, T. Yang, J.C. Yang, and Y. Li, Nonferr. Metal. Sci. Eng.14[1] (2023) 99-106.
- 15. H.Y. Tian, Z.Q. Guo, J. Pan, D.Q. Zhu, C.C. Yang, Y.X. Xue, S.W. Li, and D.Z. Wang, Resour. Conserv. Recy. 168(2021) 105366.
-

- 16. X.Y. Li, Y. Tang, W.D. Pan, H.M. Shen, X. Chen, Z.Y. Lu, and J. Li, Bull. Chin. Ceram. Soc. 42[5] (2023) 1794-1803.
- 17. X.W. Miao, Z.T. Bai, G.H. Lu, L. Liu, M. Gou, F.Q. Cheng, and M. Zhang, Chin. J. Eng. 42[6] (2020) 663-679.
- 18. Z.Q. Wang, C. Ma, and C.T. Han, Glass Enamel. 29[6] (2001) 16-18.
- 19. L.R. Dou, China's Manganese Industry. 35[4] (2017) 136-138.
- 20. X. Xin, Z.X. Tan, J.X. Zhao, A.L. Hu, Z. Wang, Y. Kang, H. Wang, and Z.K. Zhang, J. Chin. Ceram. Soc. 50[6] (2022) 1677-1684.
- 21. B. Tanisan and S. Turan, J. Ceram. Process. Res. 12[4] (2011) 462-467.
-

- 22. X. Zhang, Z.Q. Li, G.J. Ma, Q. Wang, and M.K. Liu, Bull. Chin. Ceram. Soc.40[4] (2021) 1318-1329.
- 23. G. Costa, V. Della, M. Ribeiro, A. Oliveira, G. Monros, and J. Labrincha, Dyes. Pigments.77[1] (2008) 137-144.
-

- 24. Z.T. Chen, Y. Du, Z.F. Li, D.D. Sun, and C.F. Zhu, Ceram. Int. 41[8] (2015) 9455-9460.
-

- 25. I. S. Vilarinho, J. Carneiro, C. Pinto, J.A. Labrincha, and M.P. Seabra, Sustainability. 13[4] (2021) 1-13.
-

- 26. W. Qin, Z.C. Xu, and H.X. Tian, Foshan. Ceramics,18[2] (2008) 17-20.
- 27. K. Chen, C.M. Ke, and J.H. Zhang, J. Wuhan. University. Sci. Techno. 38[5] (2015) 346-350.
- 28. H.Q. Ji, P.C. Yan, Discussion on the leaching method of the (Solid Waste-Extraction Procedure for Leaching Toxicity-Sulfuric Acid & Nitric Acid Method). Pollution Control Technology. 2017.
- 29. M.K. Liu, G.J. Ma, X. Zhang, D.L. Zheng, and Z.Q. Li, Mater. Today. Commun. 33(2022) 104609.
-

- 30. W.L. Zhang, China Ceramics. 33[5] (1997) 12-14.
- 31. B. Gorai and R.K. Jana, Resour. Conserv. Recy. 39[4] (2003) 299-313.
-

- 32. Ceramic Tiles, GB/T4100-2006. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2006.
- 33. Identification Standards for Hazardous Wastes-Identification for Extraction Toxicity. GB 5085.3-2007. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing. China, 2007.
 This Article
This Article
-
2025; 26(1): 139-147
Published on Feb 28, 2025
- 10.36410/jcpr.2025.26.1.139
- Received on Jul 24, 2024
- Revised on Sep 30, 2024
- Accepted on Oct 24, 2024
 Services
Services
- Abstract
introduction
experimental
results and discussion
conclusions
- Acknowledgements
- Conflict of Interest
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Dingli Zheng
-
bKey Laboratory for Ferrous Metallurgy and Resources Utilization of Ministry of Education, Wuhan University of Science and Technology, Wuhan 430081, Hubei, China
cJoint International Research Laboratory of Refractories and Metallurgy, Ministry of Education, Wuhan University of Science and Technology, Wuhan 430081, China
Tel : +86-17801024415 Fax: +86-02768862529 - E-mail: dinglizheng@wust.edu.cn







 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.