- Geopolymer and recycled glass ceramics for sustainable materials and environmental protection
Li Shanga, Jun Songa and Qiancheng Fangb,*
aSchool of Energy Engineering, Huanghuai University, Zhumadian, 463000, Henan, China
bInstitute of Architecture Engineering, Huanghuai University, Zhumadian 463000, Henan, ChinaThis article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
The growing emphasis on sustainable development has catalyzed advancements in the design and application of ceramic materials, shifting focus toward environmental protection and green design principles. Conventional ceramics, known for their robust mechanical, thermal, and chemical properties, face criticism due to energy-intensive production and reliance on non-renewable raw materials. This research explores innovative approaches to ceramic material design that align with sustainability objectives, including reduced carbon emissions, resource optimization, and waste reutilization. Special attention is given to two categories of eco-friendly ceramics: geopolymer ceramics and recycled glass ceramics. Geopolymer ceramics, synthesized from industrial by-products like fly ash and slag, demonstrate low-energy manufacturing processes and reduced CO₂ emissions, making them ideal for applications in construction and insulation. Recycled glass ceramics, created by transforming waste glass into functional materials, mitigate glass waste while offering superior mechanical and aesthetic properties for architectural uses. This study highlights the synthesis methods and properties of these materials, emphasizing their potential to address pressing environmental challenges while maintaining functional performance.
Keywords: Geopolymer ceramics, Recycled glass ceramics.
The pressing need for sustainable development has driven significant innovation in materials science, with ceramic materials emerging as a crucial focus area. Traditional ceramics, while offering exceptional mechanical, thermal, and chemical stability, are often associated with energy-intensive manufacturing processes and the use of non-renewable raw materials. These challenges underscore the importance of designing ceramic materials that align with principles of environmental protection and green design. The transition toward sustainable ceramics not only addresses environmental concerns but also enables the development of materials tailored for applications in renewable energy, environmental remediation, and sustainable construction.
Ceramic material design for green applications emphasizes reducing carbon emissions, utilizing waste streams, and optimizing energy efficiency during production. This approach encompasses strategies such as recycling industrial by-products, incorporating renewable resources, and engineering materials with extended life cycles to minimize environmental impact. Furthermore, the integration of advanced manufacturing techniques, such as additive manufacturing and low-temperature sintering, enhances resource efficiency and broadens the applicability of eco-friendly ceramics.
In this context, two categories of ceramics stand out for their potential to meet the dual goals of environmental sustainability and functional performance:
(i) Geopolymer Ceramics: Derived from industrial by-products like fly ash and slag, geopolymer ceramics are synthesized through low-temperature processes, significantly reducing energy consumption and CO₂ emissions compared to conventional ceramics. Their unique chemical composition and structural integrity make them suitable for construction, insulation, and other applications.
(ii) Glass Ceramics (recycled): By repurposing waste glass into functional materials, recycled glass ceramics address the growing issue of glass waste while reducing the need for virgin raw materials. These ceramics exhibit excellent mechanical properties and aesthetic versatility, making them ideal for tiles, countertops, and architectural elements.
In this work, we focus on exploring the materials aspects and potential of these two categories, delving into their synthesis methods and properties.
Geopolymer
Geopolymers are a class of advanced inorganic materials created through a chemical process known as geopolymerization, which involves the activation of silica and alumina-rich materials in an alkaline environment. This process was discovered by French scientist Joseph Davidovits in 1978, who identified that alternative binders could be synthesized from naturally occurring minerals or industrial by-products [1]. The key feature of geopolymers is their formation at low temperatures and under atmospheric pressure, which distinguishes them from traditional cementitious materials like Ordinary Portland Cement, which require high temperatures and substantial energy (Table 1).
Geopolymers are formed by dissolving alumina-silicate materials, such as fly ash, slag, meta-kaolin, volcanic ash, or even agricultural and glass wastes, in alkaline solutions like sodium hydroxide or potassium hydroxide (Fig. 1). The reaction produces a three-dimensional network structure that binds the material into a durable, rock-like substance. This structure gives geopolymers their strength and similar properties to natural rocks, while being much more environmentally friendly. One of the main advantages of geopolymers is their significant reduction in carbon footprint. Unlike ordinary portland cement production, which releases large amounts of CO₂ and consumes a lot of energy due to the high temperatures required, geopolymerization emits up to 9 times less CO₂ and uses up to 60% less energy. This makes geopolymers an attractive, low-energy alternative for applications that traditionally rely on concrete. Additionally, geopolymers are highly reactive and can be produced from waste materials, such as fly ash, which is a byproduct of coal-fired power plants. Fly ash, which is abundant, fine, and rich in alumina and silica, is particularly well-suited for geopolymerization and provides an eco-friendly means of recycling industrial waste.
The potential of geopolymers extends beyond their environmental benefits. They are cost-effective due to the relatively low cost of their raw materials and the simplified production process. Geopolymers have found uses in various fields such as construction, where they can replace traditional concrete for applications like building materials, road construction, and even in fire-resistant applications. As research continues to explore their properties, geopolymers are gaining attention as an effective and sustainable alternative to conventional materials, offering a greener solution for industries looking to reduce their environmental impact.
Fly Ash-Based Geopolymer Ceramics
Synthesis Methods: In recent years, significant efforts have been directed towards the efficient use of abundant fly ash, particularly within the context of green engineering. Fly ash-based geopolymers have emerged as an alternative to traditional Portland cement, with the added benefit of reducing CO₂ emissions by up to 9-fold compared to the Ordinary Portland Cement system [2, 3]. These geopolymers are gaining attention due to their comparable mechanical strength and durability to hydrated Portland cement, positioning them as a sustainable inorganic cement solution. Additionally, fly ash, when used as a precursor or supplementary cementing material (SCM), enhances concrete’s workability, mitigates early-stage hydration heat, and reduces thermal cracking while improving long-term mechanical and durability performance. The production of fly ash-based geopolymers involves a complex, exothermic chemical process called geopolymerization, which was introduced by Davidovits. This process includes several stages: dissolution, precipitation, restructuring, gelation, and poly-condensation [4]. Fly ash’s chemical transformation is initiated by alkaline activation, which dissolves the alumino-silicate components at low temperatures and atmospheric pressure, forming monomers of aluminate and silicate. These ions then condense to form a gel network, which eventually leads to the creation of an alumino-silicate polymer with a 3D network structure. However, the exact reaction kinetics remain complex and difficult to fully comprehend. The alkali activators, such as Na2SiO3, NaOH, KOH, and K2SiO3, trigger the breaking of Si-O-Si and Si-O-Al bonds, releasing Si4+ and Al3+ ions [5]. This process leads to the formation of a cross-linked amorphous network of SiO4 and AlO4 tetrahedra [6].
The microstructure and properties of fly ash-based geopolymers are heavily influenced by the Si/Al ratio. This ratio plays a crucial role in controlling the porosity and, consequently, the mechanical strength of the resulting geopolymer. Additionally, the type of alkaline activator used (e.g., NaOH or KOH) also affects the leaching and dissolution of Si4+ and Al3+ ions. A higher concentration of NaOH, for instance, enhances the dissolution of these ions, promoting faster polymerization. Furthermore, the addition of Na2SiO3 to the alkali solution can increase the Si/Al ratio, resulting in a finer pore structure and lower porosity in the geopolymer matrix [7, 8]. The setting time of fly ash-based geopolymers is another important factor, affecting the workability of the final product. The setting time is typically between 1 to 2 hours at room temperature, although the presence of higher CaO content or calcium additives, such as CaCl2, can accelerate this process. Calcium ions from these additives contribute to the formation of calcium-silicate-hydrate, calcium-aluminate-hydrate, or calcium-alumino-silicate-hydrate gels, which speed up the setting time [9]. However, excessive acceleration may hinder the full development of the geopolymer network. Higher NaOH concentrations can slow down the setting time, allowing for more complete geopolymerization.
Curing is essential to ensure the completion of the geopolymerization process and the development of a dense microstructure. Curing at temperatures between 30 °C and 50 °C enhances the reactivity of fly ash and accelerates the formation of the geopolymer network. To further improve the properties of fly ash-based geopolymers, supplementary materials such as blast furnace slag, fiber, chitosan, red mud, and rice husk–bark ash (RHBA) are often incorporated. BFS, for example, can improve fly ash reactivity during geopolymerization, while RHBA, which is rich in SiO2, increases the pozzolanic content and enhances the Si-O-Si bonding in the geopolymer gel. The addition of red mud, which contains Fe, Al, and Si, can help control the Si/Al ratio and reduce the need for alkaline activators.
One of the remarkable attributes of fly ash-based geopolymers is their ability to immobilize heavy metals, making them useful for environmental applications, such as waste stabilization. This makes fly ash-based geopolymers not only a sustainable alternative to traditional cements but also a promising material for environmental protection.
Properties: Fly ash-based geopolymer ceramics exhibit high compressive strength (ranging from 20 MPa to 80 MPa), low thermal conductivity, and excellent resistance to chemical attacks. Their inherent fire resistance and thermal stability make them suitable for high-temperature applications. The compressive strength of fly ash-based geopolymers is influenced by multiple factors, including the Si/Al ratio, the composition and concentration of alkaline activator solutions, calcium content, additives, and curing conditions such as temperature and duration. Among these, the nature and concentration of the alkali activator play a pivotal role in releasing Si4+ and Al3+ ions from fly ash, which are essential for geopolymerization. While higher concentrations of alkaline activators generally enhance compressive strength, there is an optimal threshold beyond which performance may plateau or decline. These findings highlight that achieving optimal compressive strength in fly ash-based geopolymers requires careful balancing of activator concentration, sequential processing, and curing protocols. The strategic use of sodium silicate alongside sodium hydroxide and the adoption of tailored activation methods can significantly enhance the performance of these sustainable materials [10, 11].
Fly ash-based geopolymers exhibit excellent compressive strength, yet their tensile performance—often governed by crack initiation and propagation—merits closer investigation. Under tensile loads, performance varies significantly depending on mix design and additives. Lee et al. studied the tensile behavior of geopolymeric concrete, observing that increasing the sand-to-fly-ash ratio resulted in a gradual decline in tensile strength.
Additive incorporation also plays a vital role. Adak et al. reported a 5-6% nano silica supplement significantly enhanced tensile strength when subjected to standard curing. Similarly, Al-Majidi et al. found that adding ground granulated blast furnace slag (GGBFS) to geopolymer concrete resulted in a tensile strength improvement of up to 30 %, demonstrating the beneficial role of slag in direct tensile performance. Studies comparing geopolymer and Ordinary Portland Cement concrete reveal that fly ash-based geopolymer concrete consistently surpasses Ordinary Portland Cement concrete in tensile attributes when both possess equivalent compressive strengths [12-14]. This body of research highlights that the tensile and flexural strength of fly ash-based geopolymers can be tailored through mix design adjustments and strategic use of additives, making them a superior alternative to conventional Ordinary Portland Cement systems in many applications.
Glass Ceramics
Glass is widely used in everyday life, from consumer goods such as bottles and sheet glass to industrial applications like vacuum tubes. However, the disposal and recycling of glass remain critical environmental challenges. In 2015, the United States generated 10.4 million tonnes of waste glass, of which only 26% was recycled, while 60% ended up in landfills. Globally, annual waste glass production exceeds 100 million tonnes, with major contributors being China, the United States, and the European Union, producing approximately 33, 32, and 20 million tonnes, respectively. The low recycling rates and high volumes of waste have motivated researchers to explore the use of waste glass as a sustainable component in concrete production, where it can replace traditional materials like Portland cement and natural fine aggregates.
Waste glass particles (Fig. 2), when processed into sizes below 100 μm, can act as a substitute for Portland cement, while coarser particles below 4.75 mm can replace fine aggregates This substitution provides several benefits, including reducing greenhouse gas emissions by up to 19% and energy consumption by 17%, while also achieving significant cost savings. The incorporation of waste glass into concrete not only offers environmental advantages but also preserves natural resources by reducing the demand for virgin materials. These benefits make it an attractive option for sustainable construction practices.
Studies have demonstrated the positive impact of waste glass on the mechanical properties and durability of concrete. For instance, replacing 10-30% of fine aggregates with recycled fine glass (RFG) has been shown to achieve optimal performance. Research by Wang (2009) indicated that replacing 20% of FA with liquid crystal display (LCD) glass significantly improved the mechanical performance of concrete across different mix designs, with compressive strength enhancements noted. Additionally, replacing fine aggregates with waste glass has been found to reduce thermal conductivity and water absorption, further improving the durability of the concrete. In self-compacting concrete (SCC), Ali et al. demonstrated that fine-particle recycled waste glass could function effectively as fine aggregates without compromising its properties, showcasing its compatibility with advanced concrete applications.
Waste glass not only contributes to improved performance but also addresses waste management challenges by providing a sustainable solution for recycling large volumes of glass. This aligns with circular economy practices, where waste materials are repurposed to minimize environmental impact. However, challenges such as variability in glass composition and potential alkali-silica reaction (ASR) must be addressed to fully realize its potential. Mitigating ASR and ensuring consistent particle quality are critical areas for future research, alongside scaling the application of recycled glass concrete (RGC) for widespread use in construction projects. In conclusion, waste glass offers a promising alternative to traditional concrete components, combining environmental, economic, and performance benefits. Its incorporation into concrete represents a step toward more sustainable construction practices, reducing the environmental footprint of the industry while addressing global waste management concerns.
The traditional approach to producing glass-ceramics involves a two-step heat treatment process. Initially, the glass undergoes a low-temperature heat treatment at a temperature that promotes a high nucleation rate. This stage, typically conducted near the nucleation temperature, generates a high density of nuclei uniformly distributed throughout the glass matrix. Achieving a high density of nuclei is critical, as it fosters the formation of a microstructure characterized by numerous fine crystals. Subsequently, the material undergoes a second, higher-temperature heat treatment near the growth temperature, enabling controlled growth of the nuclei at an optimal rate.
Before the crystallization process, the parent glass can be shaped using traditional techniques such as casting and forming or specialized methods like extrusion, depending on the desired application. However, the production of glass and the associated heat treatment steps are inherently energy-intensive, contributing to higher costs. This underscores the importance of optimizing the process to balance energy consumption and production efficiency.
Powder Methods
The fabrication of glass-ceramics using powder methods typically involves shaping through cold compaction followed by high-temperature heat treatment to achieve sintering. This technique, commonly employed for ceramics, has also been adapted for glass-ceramic production. However, limitations on the size and shape of components, as well as the costs associated with powder production, restrict its use to cases where specific benefits are evident. In most scenarios, compacting and sintering a glass-ceramic powder offers little advantage due to the requirement for high sintering temperatures and the similar properties of the final product compared to other fabrication methods.
Sintering parent glass powders is often more appealing, as it occurs through a viscous flow mechanism at relatively lower temperatures. However, achieving a balance between the rates of viscous flow sintering and crystallization is crucial. Excessively rapid crystallization can lead to high crystallinity, which inhibits low-temperature sintering, resulting in undesirable porosity. Conversely, if sintering is completed before crystallization, the properties of the final product are comparable to those produced by conventional techniques. In certain cases, it is possible to optimize the process so that densification and crystallization occur simultaneously at the same temperature, enabling the production of dense glass-ceramics. Adjusting the composition and sintering temperature can yield distinct microstructures and crystalline phases, leading to different material properties compared to conventional methods. Advanced techniques such as hot pressing and hot isostatic pressing can achieve near-full densification and improved product quality but are more costly, making them less suitable for processing waste materials into monolithic glass-ceramics.
Powder technology also facilitates the creation of dispersion-reinforced glass-ceramic matrix composites. The process involves mixing powdered parent glass with reinforcement materials in specific proportions, followed by shaping, sintering, and crystallization. While rigid inclusions used for reinforcement can impede sintering, these composites enhance the material’s mechanical properties. The production of continuous fiber-reinforced glass-ceramics, though more complex and equipment-intensive, offers additional versatility. For both particulate and fiber reinforced composites, densification is typically achieved through hot pressing, with a subsequent heat treatment to crystallize the glass matrix and finalize the desired properties.
Properties
Glass-ceramics, derived from a variety of waste materials such as ashes, blast furnace residues, and steel residues, exhibit diverse compositions and predominant crystal phases. These cost-effective materials are renowned for their strength, hardness, and chemical resistance, making them ideal for applications in heavy-duty industries. Common uses include abrasion-resistant components, chemically resistant parts, and floor and wall tiles for construction and mechanical settings. A notable product in this category is Neopari’s, a crystallized glass material with a marble-like appearance. It serves as a superior alternative to stone for interior and exterior walls, floors, countertops, and tabletops. Available in large flat or curved panels, it combines aesthetic appeal with functional durability.
Lithium aluminosilicate glass-ceramics are particularly significant due to their extremely low coefficient of thermal expansion (CTE), measuring 0.00 ± 0.02 × 10⁻⁶/K between 0 °C and 50 °C. In certain temperature ranges, the CTE can even be zero or slightly negative. These materials are characterized by remarkable homogeneity and transparency across the 400 to 2,300 nm wavelength range, which facilitates the detection of internal flaws such as bubbles and inclusions. Despite their advantageous properties, many glass-ceramics have limited use temperatures due to the residual glass phase, which causes deformation at temperatures below 700 °C. However, exceptions like the celsian glass-ceramics within the SrO–BaO–Al₂O₃–SiO₂ system demonstrate thermal stability up to 1,450 °C, with CTEs compatible with materials like silicon, silicon carbide (SiC), and silicon nitride (Si₃N₄). These glass-ceramics can also be melted at commercial temperatures, approximately 1650 °C, expanding their applicability in high-temperature environments.
Machinable glass-ceramics rely on mica crystals within their microstructure, offering a high CTE that matches most metals and sealing glasses. These materials are notable for their zero porosity, excellent insulation properties at high voltages and various frequencies, and thermal stability at elevated temperatures. These attributes make them indispensable in specialized applications requiring precision and resilience.
High-strength glass-ceramics based on chain silicates, such as enstatite (MgSiO₃), potassium fluorrichterite (KNaCaMg₅Si₈O₂₂F₂), and fluor-canasite (K₂Na₄Ca₅Si₁₂O₃₀F₄), exhibit impressive fracture strength and toughness. Strategies to further enhance their mechanical performance include fiber reinforcement, chemical strengthening through ion-exchange techniques, and the development of a thin compressive surface layer with a lower thermal expansion coefficient than the material’s interior. These innovations not only improve the strength and toughness but also extend the range of potential applications for glass-ceramics in advanced engineering and industrial contexts.
Bioactive Glass-Ceramics
The heat treatment of MgO–CaO–SiO₂–P₂O₅ glass results in a bioactive glass-ceramic containing crystalline apatite (Ca₁₀(PO₄)₆O,F₂) and beta-wollastonite (CaO, SiO₂) within an MgO–CaO–SiO₂ glassy matrix. This material demonstrates significant bioactivity and maintains fairly high mechanical strength, even under load-bearing conditions within the body, making it suitable for clinical applications such as artificial vertebrae and iliac bones. The bioactivity of this glass-ceramic is primarily attributed to the formation of an apatite layer on its surface when implanted in the body. The dissolution of calcium and silicate ions from the glass-ceramic plays a crucial role in the development of this surface apatite layer.
Additionally, novel bioactive materials have been developed using CaO–SiO₂-based glasses. These materials enable the formation of bone-like apatite coatings on ceramics, metals, and organic polymers when placed near such glasses in simulated body fluid environments. A bioactive bone cement has also been formulated by combining CaO–SiO₂-based glass powder with a neutral ammonium phosphate solution, achieving a compressive strength of approximately 80 MPa.
Moreover, advancements have been made in creating a bioactive and ferromagnetic glass-ceramic through the heat treatment of Fe₂O₃–CaO·SiO₂–B₂O₃–P₂O₅ glass. This material contains crystalline magnetite (Fe₃O₄) embedded in a matrix of CaO–SiO₂-based glassy and crystalline phases. Its unique properties make it valuable as thermoseeds for hyperthermia treatments in cancer therapy. These innovations highlight the diverse applications and potential of bioactive glass-ceramics in both medical and therapeutic fields.
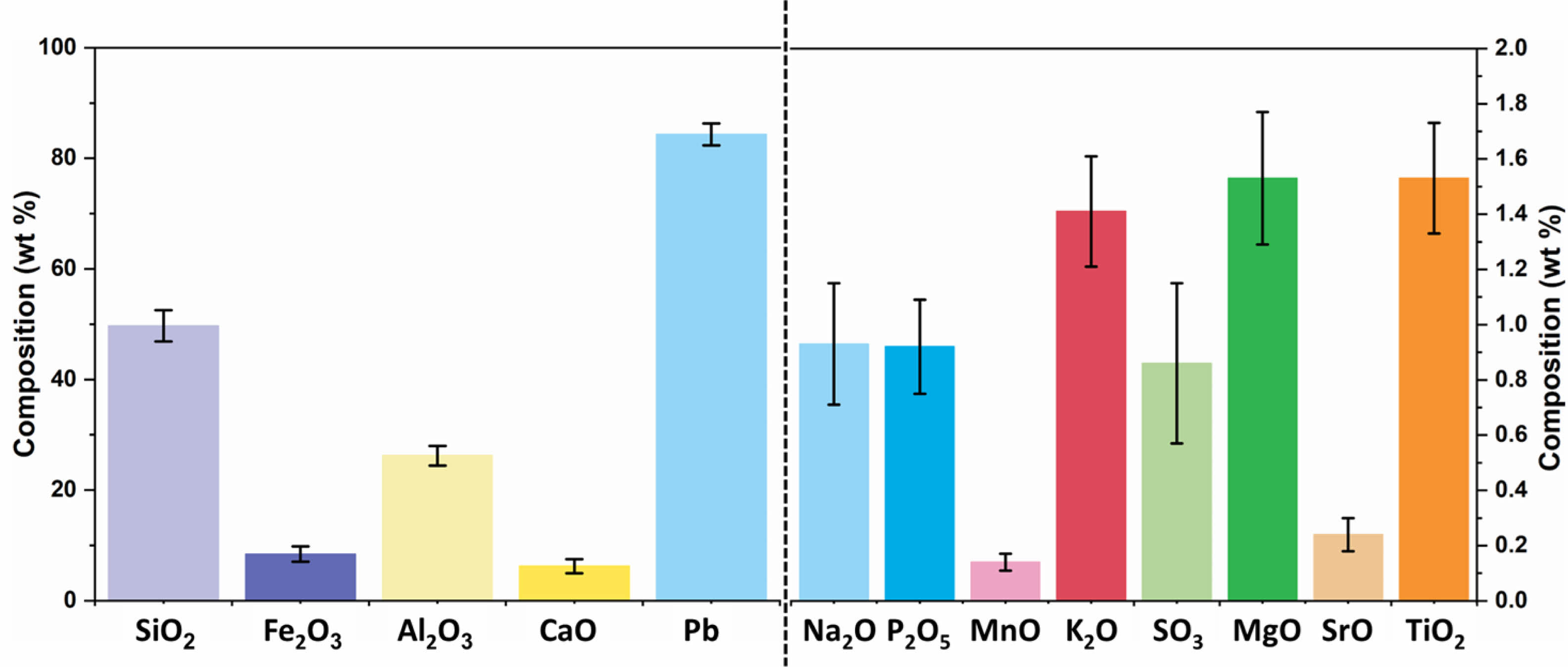
|
Fig. 1 Oxide composition of Fly-ash geopolymer ceramics. |
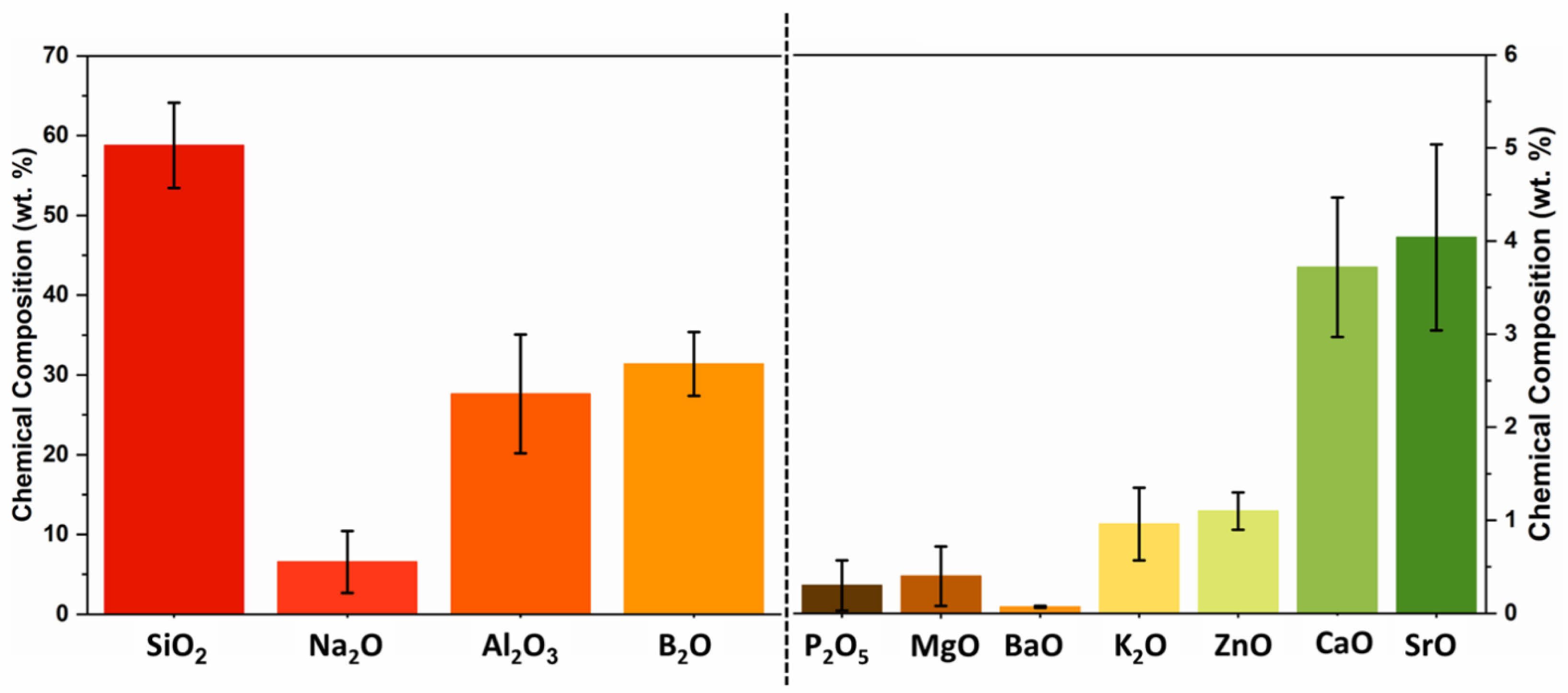
|
Fig. 2 Composition of the Glass-Ceramic Represented in wt.% of Corresponding Oxides. |
|
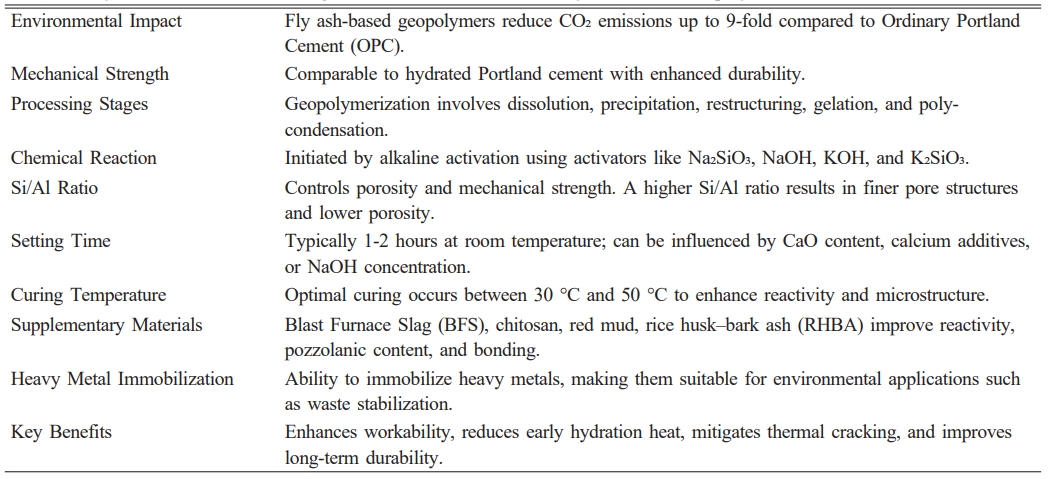
Table 1 Key Characteristics, Processing Parameters, and Benefits of Fly Ash-Based Geopolymers |
The pursuit of sustainable ceramic materials represents a transformative approach to addressing the environmental and resource challenges of traditional ceramics. Geopolymer ceramics, derived from industrial waste through energy-efficient processes, and recycled glass ceramics, repurposing glass waste into high-performance materials, exemplify the potential for eco-friendly innovation in materials science. These ceramics not only reduce carbon emissions and energy consumption but also open avenues for applications in construction, renewable energy, and environmental remediation. By integrating advanced manufacturing techniques and optimizing material life cycles, sustainable ceramics can significantly contribute to a greener future. This work underscores the importance of continued research in material design and highlights the potential for ceramics to play a pivotal role in achieving environmental sustainability without compromising functional performance.
This work was supported by the Zhumadian key R & D special funding (ZMDSZDZX2023006) and the the Science and Technology Key Project of Henan Province (242102230157).
- 1. S.K. Nath, S. Mukherjee, S. Maitra, and S. Kumar, J. Therm. Anal. 127 (2016) 1953-1961.
-

- 2. S. Luhar, T.W. Cheng, D. Nicolaides, I. Luhar, D. Panias, and K. Sakkas, Constr. Build. Mater. 220 (2019) 547-564.
-

- 3. Davidovits, Worlds Resour. Rev. 6 (1994) 263-278.
- 4. P. Steins, A. Poulesquen, O. Diat, and F. Frizon, Langmuir 28 (2012) 8502-8510.
-

- 5. M.A. Khan, S.A. Memon, F. Farooq, M.F. Javed, F. Aslam, and R. Alyousef, Adv. Civ. Eng. 2021 (2021) 6618407.
-

- 6. P. Duxson, J.L. Provis, G.C. Lukey, S.W. Mallicoat, W.M. Kriven, and J.S.J. van Deventer, Colloids Surf. A Physicochem. Eng. Asp. 269 (2005) 47-58.
-

- 7. Y. Ma, J. Hu, and G. Ye, Fuel 104 (2013) 771-780.
-

- 8. U. Rattanasak, K. Pankhet, and P. Chindaprasirt, Int. J. Miner. Met. Mater. 18 (2011) 364-369.
-

- 9. E. Diaz, E. Allouche, and S. Eklund, Fuel 89 (2010) 992-996.
-

- 10. A.I.I. Helmy, Constr. Build. Mater. 110 (2016) 54-64.
-

- 11. U. Rattanasak and P. Chindaprasirt, Miner. Eng. 22 (2009) 1073-1078.
-

- 12. M.H. Al-Majidi, A. Lampropoulos, A. Cundy, and S. Meikle, Constr. Build. Mater. 120 (2016) 198-211.
-

- 13. P. Nath and P. K Sarker, Constr. Build. Mater. 130 (2017) 22-31.
- 14. W. Yang and J. Chen, J. Ceram. Process. Res. 25[5] (2024) 727-736.
-

 This Article
This Article
-
2025; 26(1): 122-128
Published on Feb 28, 2025
- 10.36410/jcpr.2025.26.1.122
- Received on Dec 17, 2024
- Revised on Feb 1, 2025
- Accepted on Feb 5, 2025
 Services
Services
Shared
 Correspondence to
Correspondence to
- Qiancheng Fang
-
Institute of Architecture Engineering, Huanghuai University, Zhumadian 463000, Henan, China
Tel : 03962812853 Fax: 03962853410 - E-mail: fangqiancheng314@126.com






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.