- Structural and magnetic properties of W-type hexa-ferrite Ba1-xSmxMg2Fe15.6Co0.4O27 ferrites
Jinsong Lia, Siyuan Lia, Xiubin Zhaoa and Ailin Xiaa,*
Advanced Ceramics Research Center, School of Materials Science and Engineering, Anhui University of Technology, Maanshan, 243032, China
This article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Sm3+-substituted W-type Ba1-xSmxMg2.Fe15.6Co0.4O27 (x=0.00 to 0.30 with steps of 0.06) ferrites were prepared by a solid-state method. The XRD results indicated that the single W-type ferrite structure can be obtained for the specimens with x≤0.18, while the impurity phases appeared for x≤0.18. The lattice parametersa and c, the cell volume (Vcell) and the average grain size (Da) decrease with the increase of x. From the FE-SEM images, it can be found that all the samples exhibited a hexagonal plate shape. The saturation magnetization and the initial magnetic permeability first increased with x from 0.0 to 0.12, but then decreased withx>0.12. The coercivity kept increasing with the increase of x gradually.
Keywords: W-type ferrite, Solid-state method, Magnetic properties, Permeability.
Hexagonal ferrite has attracted increasing attention in many technical fields due to its excellent performance. It is widely used in the fields of communication, new energy, automobiles, cloud computing, lighting, IT, home appliances, industrial automation, medical, military and so on [1-6].
Generally, magnetic ferrites can be divided into M, W, Y, X, Z and U types according to their crystalline structure [4], in which M and W types are widely studied and used. W-type hexagonal ferrite has a chemical formula of AMe2Fe16O27 (A=Ba, Sr, Me=Fe, Mg, Zn, Co, Cu, Mn and so on). Their crystal structure can be seen as an R block and an S block stacked along the c-axis of hexagonal crystal system in the order of RSSR*S*S* [7]. R block contains three oxygen ion layers, with an A2+ layer in the middle layer, which is the mirror plane of crystal, and S block is another oxygen ion layers without A2+, stacked as spinels. In the W-type hexaferrites, the Fe3+ ions exist at seven different positions, namely 12k, 4fVI, 6g, 4f, 4e, 4fIV and 2d [8]. The space group of W-type hexaferrites is P63/mmc (194). The lattice parameters a of W-type and M-type hexagonal ferrite is about 0.588 nm, while the lattice parameters c is different [9].
For a long time, many attempts have been made to improve the magnetic properties of W type ferrites in hexagonal crystal system, in which ion substitution is the most widely studied. Rare earth elements may contribute to the modification of magnetic interactions and thus improve magnetic properties [10]. Wang et al. [11] prepared Co2W type ferrite with a chemical formula Ba1-xSmxCo2Fe16O27 (x=0.00, 0.05, 0.10, 0.15 and 0.20) via a solid-state reaction method. With the increasing doping amount of Sm3+ ions, the real (ε' ) and imaginary parts (ε" ) of the dielectric constant also gradually increases, and the maximum value of ε" moves towards the low frequency band. When x=0.15, the magnetic complex permeability (imaginary part) µ" improved significantly, and the peak value was about 1.75 at 18 GHz. I. Sadiq et al. [12] prepared W type ferrite with a chemical formula Sr3-xCexFe16O27 (x=0.00, 0.02, 0.04, 0.06, 0.08 and 0.10) by a sol-gel method. All the samples obtained are single-phase W-type structure. With the doping of rare earth Ce3+ ions, both the coercivity (Hc) and the electrical resistivity increase first and then decrease, while the saturation magnetization (Ms) increases in a wavy manner. Yang et al. [13] prepared Sr1-xPrxZn0.8Co1.2Fe16O27 (0.00 ≤ x ≤ 0.40) W-type hexagonal ferrite using a traditional ceramic technology. With the increase of x, the Ms, the residual magnetization (Mr) and the magneton number (nB) first increase for x from 0.00 to 0.16, and then decrease for x higher than 0.16. F. Aen et al. [14] synthesized Co2W type ferrite Ba1-xHoxCo2Fe16O27 by a sol-gel method. They found that the grain size decreased from 44 nm to 34 nm with increasing Ho3+ concentration, accompanying with the decrease of dielectric constant and loss angle.
In summary, the substitution of rare earth elements significantly improves the magnetic properties. The substitution of Sm3+ is expected to reduce the dielectric
constant and enhance the magnetic permeability. Therefore, in this study, the W-type Ba1-xSmxMg2Fe15.6Co0.4O27 (x=0.00, 0.06, 0.12, 0.18, 0.24 and 0.30) magnetic powders were prepared by a solid-state method. The effects of the Sm3+ content (x) on their structure and the magnetic properties are systematically studied.
Materials
The raw materials used in this experiment were as follows: Fe2O3 (99.2% purity), BaCO3 (99.5% purity), Sm2O3 (99.9% purity), MgO (99% purity) and CoO (99% purity) powders etc. All selected materials were analytical purity, no further purification treatment was required.
Preparation of W-type ferrites Ba1-xSmxMg2.Fe15.6Co0.4O27 powders
The solid-state method was used for preparation, and the approximate process was as follows: the ingredients are prepared according to the molar percentage of the main formula, and the ball: material: water ratio is 4:1:1 by mass, and milled for 2 hours using a ball mill; After ball milling, the slurry was taken out and dried in a vacuum drying oven, crushed into small pieces, and then calcined in air at 1300 ℃ for 2 hours in a muffle furnace. Then, used a vibrator to crush the calcined block and prepare sample powder through a 100 mesh sieve. Add 10% mass fraction PVA solution for granulation and pass through 60 mesh and 150 mesh sieves respectively to obtain qualified materials with particle sizes ranging from 150 to 60 mesh. Pressed into a circular sample (inner diameter 22 mm, outer diameter 7 mm, thickness 5 mm) under a pressure of 100 MPa. Finally, the ring sample was sintered in air at 1200 ℃ for 2 hours to obtain the final test sample.
Characterization
A Philips X'Pert MPD diffractometer was used to identify the phase composition of specimens (Cu Kα radiation, λ=1.5406 Ǻ). The morphology of all samples was obtained by a field emission scanning electron microscope (FESEM, Hitachi S-4800). A vibrating sample Magnetometer (VSM, MicroSense EZ7) was used to measure the room-temperature (RT) magnetic properties, and the maximum external electric field is 20000 Oe. An Agilent 4284A LCR meter was used to measure the magnetic permeability of specimens at a frequency of 1MHz at room temperature.
Structure and morphology
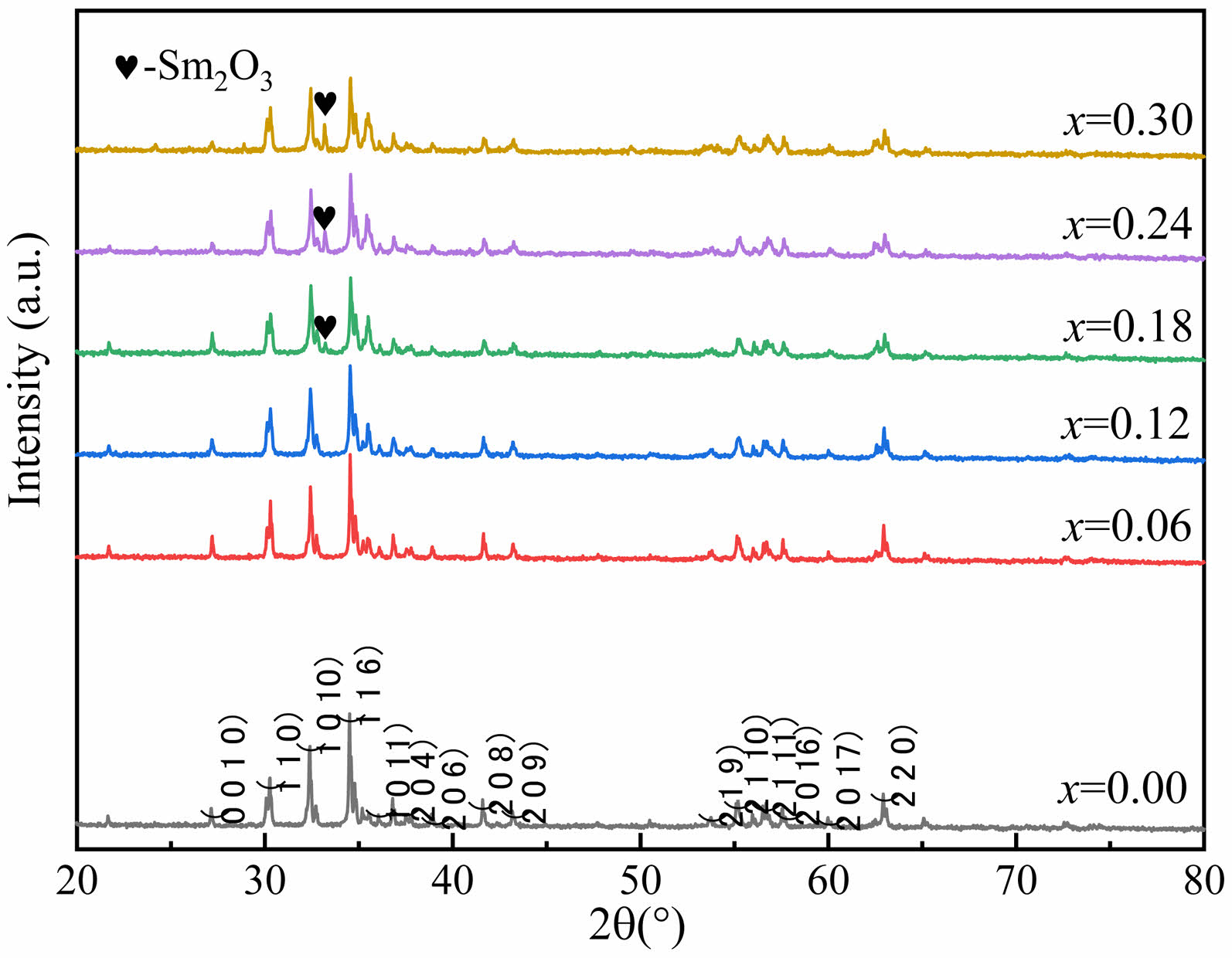
Fig. 1 shows the XRD patterns of W-type ferrites
Ba1-xSmxMg2Fe15.6Co0.4O27 (x=0.00, 0.06, 0.12, 0.18, 0.24 and 0.30) powders. Comparing all the peaks in Fig. 1 with standard card (JCPDS PDF#75-0406), impurity peaks from Sm2O3 emerged for the specimens with x≥0.18. When x<0.18, no impurity peak can be found in the XRD patterns, indicating the single W-type hexagonal structure of specimens. Therefore, Sm3+ ions were completely dissolved in the crystalline lattice for the specimens with x<0.18. The magnetic properties of the specimen may be affected by the second phase Sm2O3.
The lattice parameters a and c of specimens were calculated according to the characteristic peaks (116) and (1010) by the following formula [15, 16]:
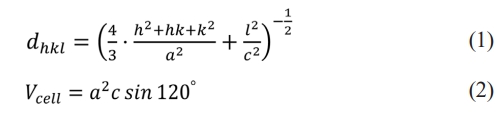
where (h, k, l), dhkl and Vcell are the Miller indices, the crystal face spacing and the cell volume, respectively. The X-ray density (dX-ray) and the average grain size (D) are obtained by the following two equations [17, 18]:


Here, M, NA, K, λ, β and θ are the molar mass, 6.02×1023, 0.89, 1.5406 Ǻ, the full-width at half-maximum and the Bragg diffraction angle, respectively. All the values calculated using the above four formulas are listed in Table 1.
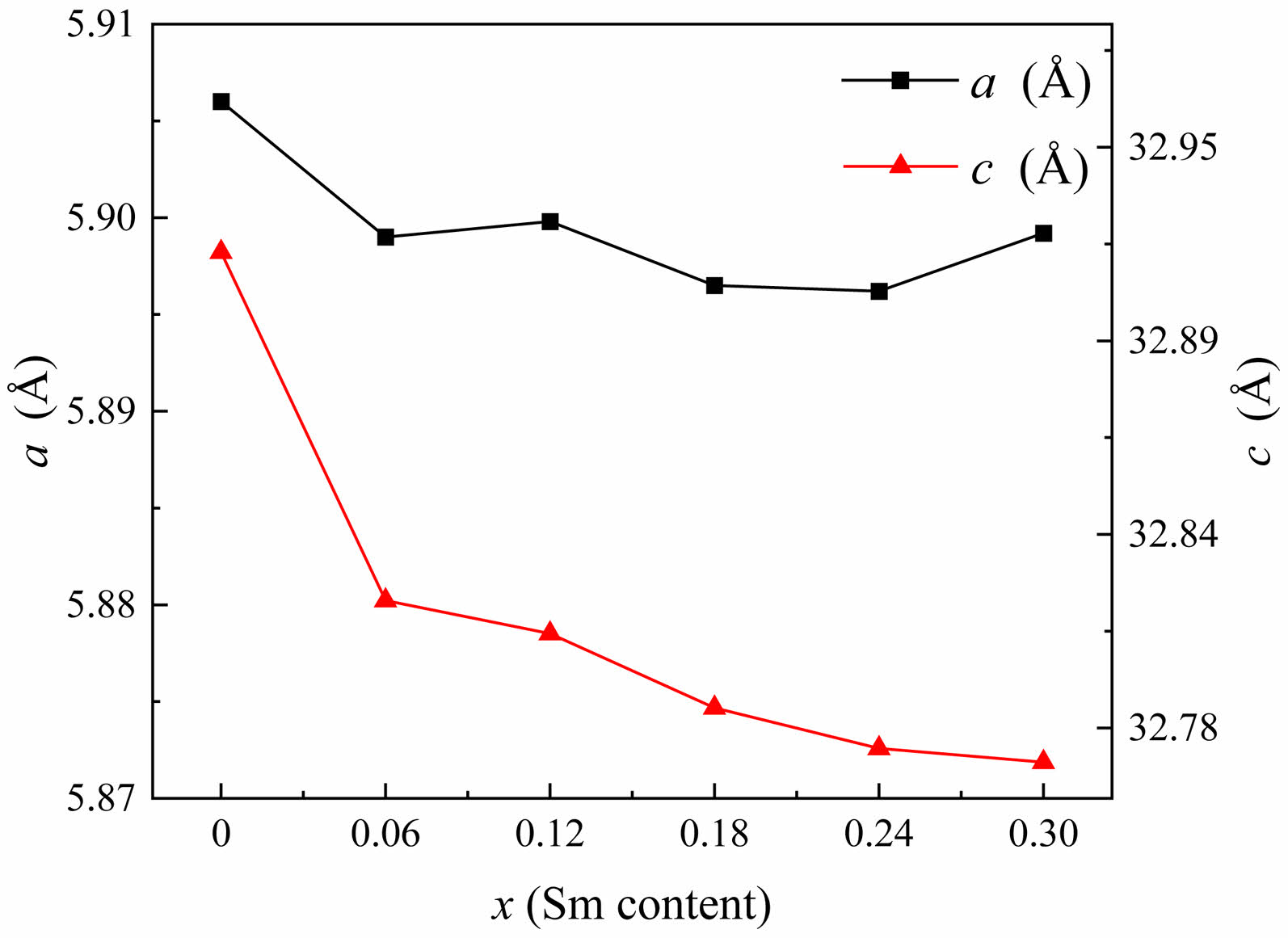
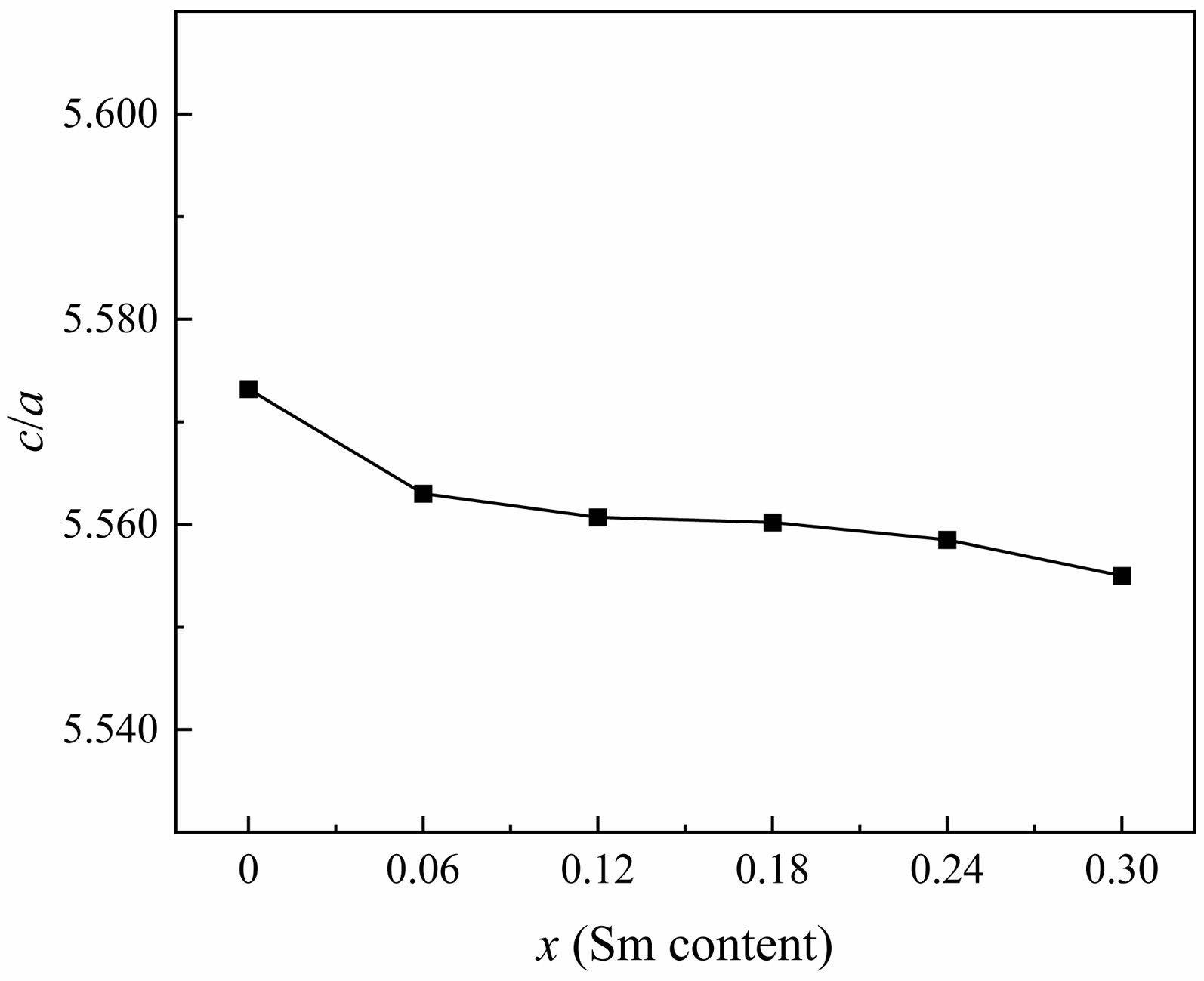
Fig. 2 shows the change of lattice constants a and c of specimens. The a changes slightly. However, the c decreases significantly from 32.9152 Å (x=0.00) to 32.7703 Å (x=0.30). As is known, the ionic radius of Ba2+ and Sm3+ are 1.35 Å and 1.05 Å [13, 19], respectively. Therefore, when Sm3+ replaces Ba2+, the distance between crystal planes decreases and the lattice volume shrinks. It is consistent with the changes in lattice parameters caused by ion substitution reported in early literature [20-23]. The c has a significant change compared to the a because the c-axis is the easy magnetized axis in W-type hexagonal ferrite [19]. The anomalous increase in Vcel when x is 0.3 may be due to the excessive addition of Sm3+, which leads to an increase in the lattice constant a. The ratio c/a listed in Table 1 is between 5.5572 and 5.5630. As shown in Fig. 3, with the increase of x, the change in c/a ratio is not significant.
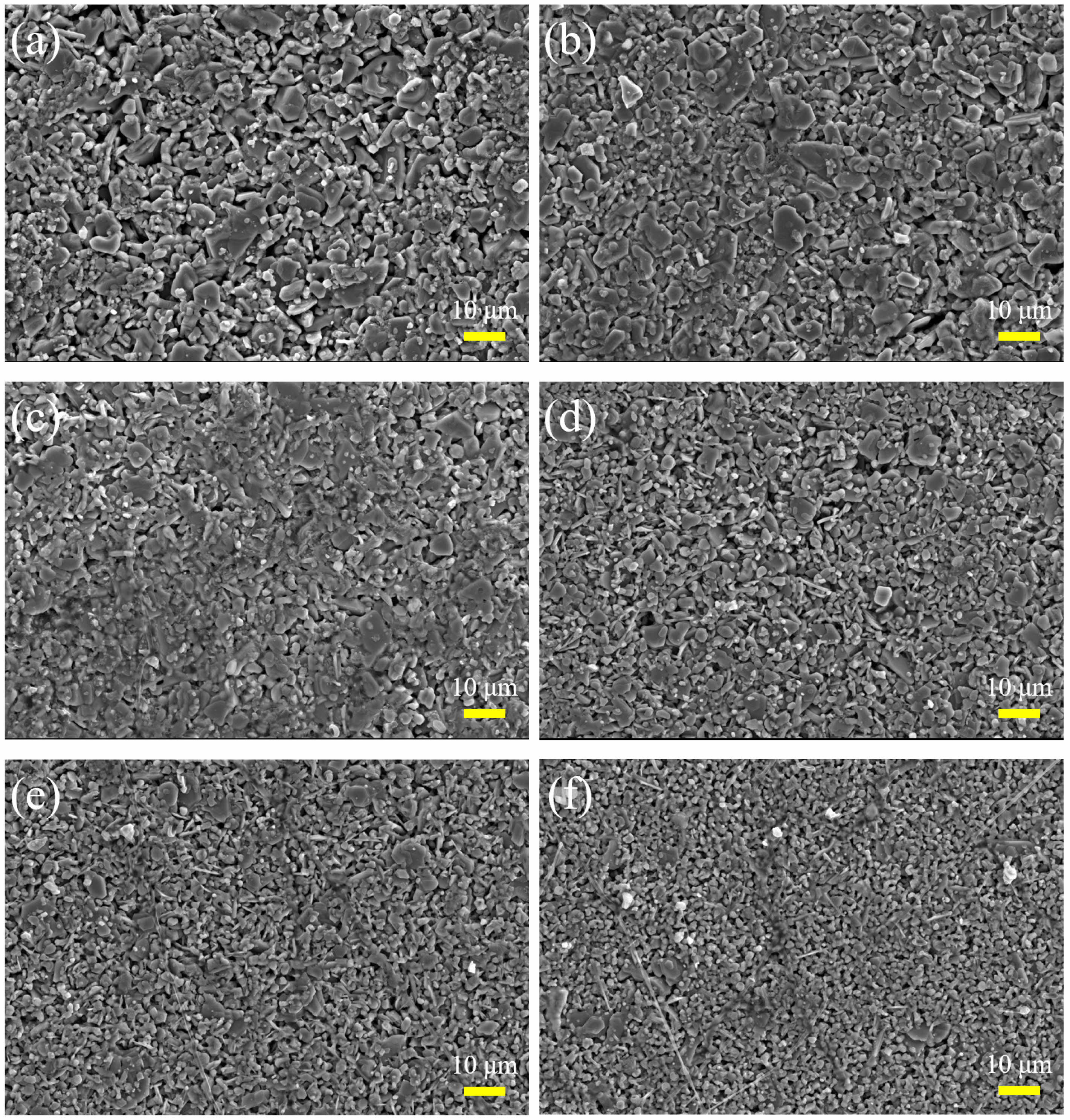
The typical SEM images of Ba1-xSmxMg2Fe15.6Co0.4O27 (x=0.00, 0.06, 0.12, 0.18, 0.24 and 0.30) are shown in Fig. 4. All the specimens exhibit a hexagonal plate shape.The particle size decreases with the increase of x, which may be attributed to the deposition of Sm3+ on the grain boundaries [11].
Magnetic properties
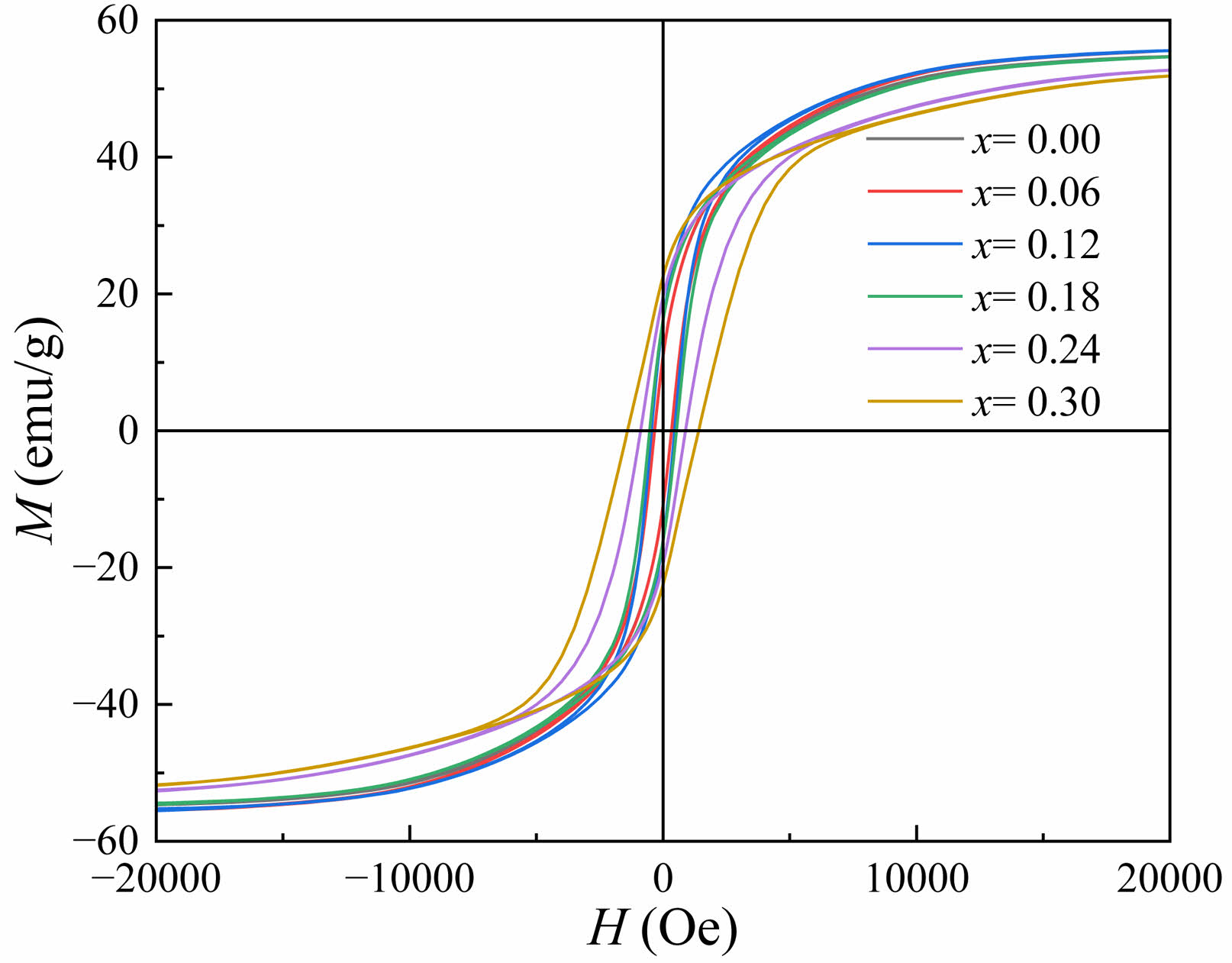
Figure 5 shows the RT magnetic hysteresis loops of specimens. Under a magnetic field of 20 kOe, the hysteresis loops of the specimens approach saturation. The values of Ms, Hc, remanent magnetization (Mr) and square ratio (Mr/Ms) were obtained through the RT hysteresis loops and listed in Table 2.
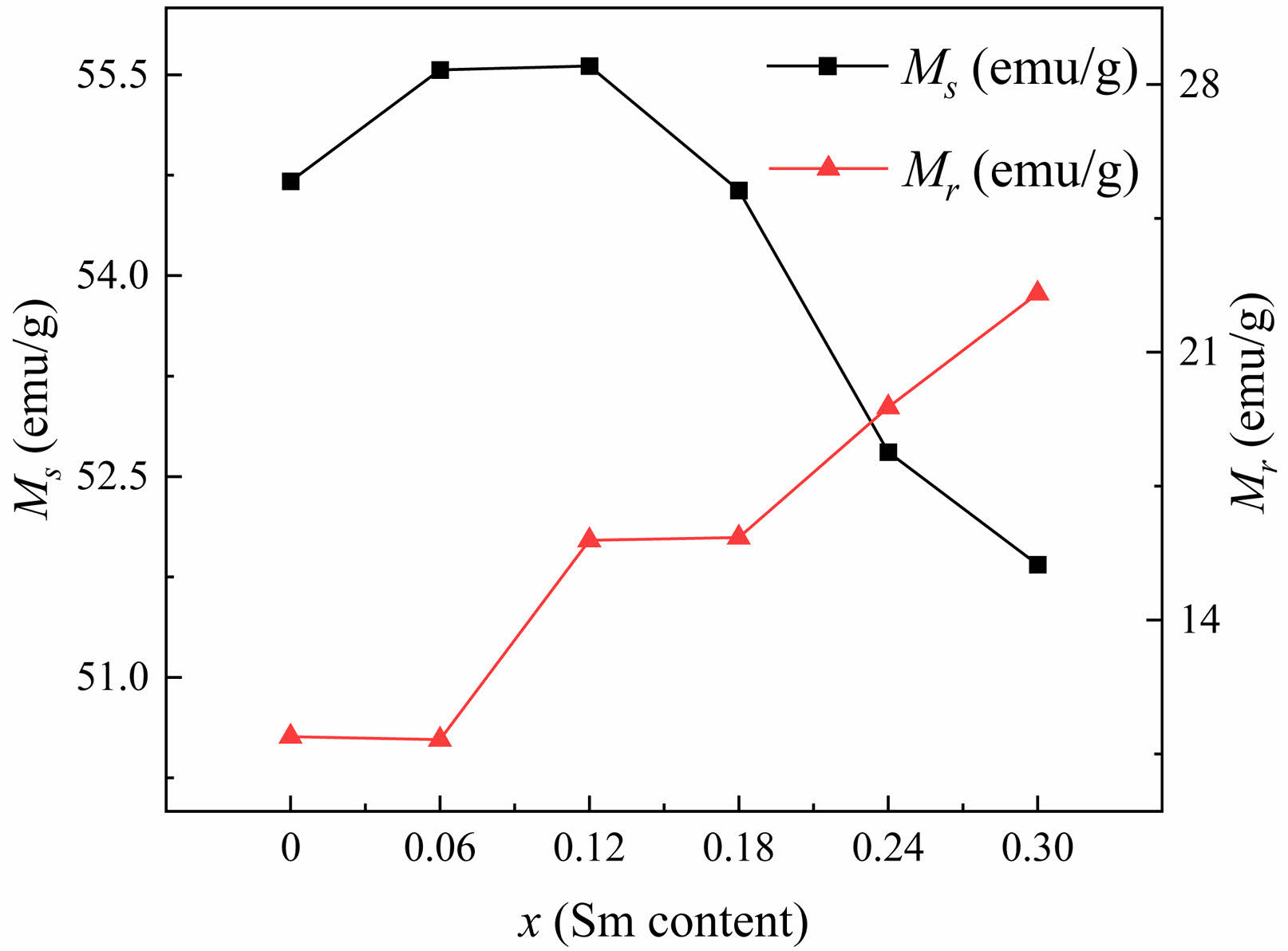
Typical hysteresis loops of W-type ferrite Ba1-xSmxMg2Fe15.6Co0.4O27 specimens (x=0.0~0.30) are shown in Fig. 5. The saturation magnetization (Ms) and coercivity (Hc) of magnetic powders with different Sm3+ concentrations (x) can be determined from the hysteresis loops, as shown in Table 2. From Fig. 6, it can be seen that when x=0.12, the peak value of Ms reaches 55.566 emu/g, which enhanced the Ms compared with the unsubstituted specimen (x=0). However, when x>0.12, the Ms decreases with the increase of x. The Ms of ferrite is mainly influenced by the chemical composition and the crystal structure. When x≤0.12, the Ms increases with the increase of x since the trivalent Sm3+ replaces the divalent Ba2+. In order to achieve the electronic balance, some Fe3+ ions in the ferrite structure will be converted into Fe2+ ions [24]. The migration of electrons enhances the electron leaps between the iron ions at the octahedral positions in the lattice, resulting in an enhanced hyperfinement field at the 12k position in the lattice, which strengthens the superexchange interaction between Fe3+-O-Fe3+ [25]. As a result, the superexchange between Fe3+-O-Fe3+ decreases, which leads to the increase of the Ms . When x>0.12, the Ms decreases with x mainly because too much Sm3+ entered into the crystalline lattice, which can cause the decrease of Ms due to the weakening the superexchange between Fe3+-O-Fe3+ resulting from the formation of a spin canting structure [26].
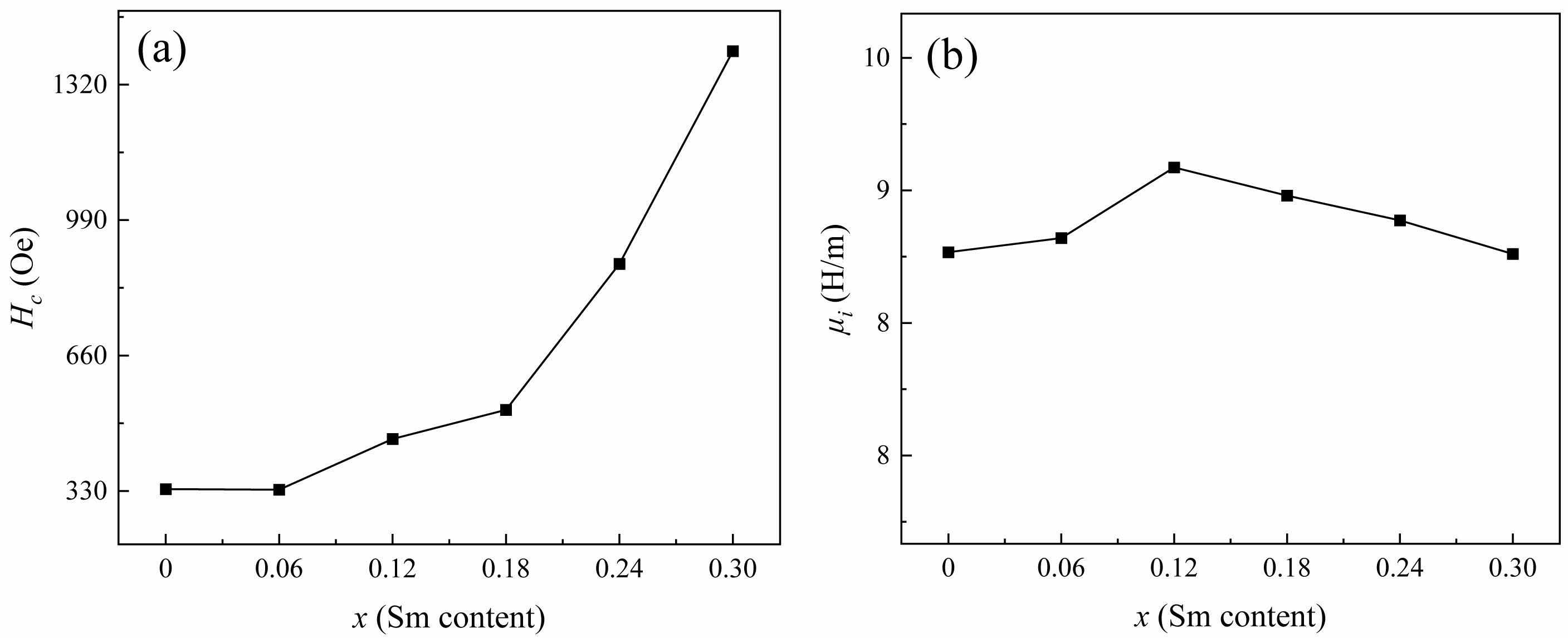
As shown in Fig. 7(a), the increase of Hc in specimens after the substitution of rare earth ion Sm can be ascribed to the following reasons. On the one hand, the greater magnetocrystalline anisotropy of specimens caused the greater energy required for the rotation of magnetic domains, that is, the greater Hc. When Sm3+ replaces Ba2+, there will be a transition of Fe3+ to Fe2+ in the ferrite, and the appearance of strong Fe2+ ions anisotropy will lead to the increase of magnetocrystalline anisotropy. Therefore, the Hc will increase after the replacement of rare earth ions [27]. On the other hand, the larger grains are conducive to the formation of domain walls. In large grains, more domain walls move during magnetization, and the energy consumed by domain wall movement is less than that of domain rotation, resulting in a smaller amount of energy required for magnetization. Therefore, the Hc in specimens with larger grains is smaller, that is, the grain size is inversely proportional to the Hc. According to the microstructure in Fig. 4, the specimens after the replacement of Sm3+ have smaller grains, resulting in their high Hc [28].
Fig. 7(b) illustrates the initial magnetic permeability (ui) of specimens with different x. As the x increases from 0.00 to 0.12, the ui increases from 8.65 to 9.13. When x>0.12, the ui decreases from 9.13 to 8.64. The main influencing factors on the ui of ferrite are the Ms and the magnetic crystal anisotropy constant (K1), as shown in the following equation [29]:
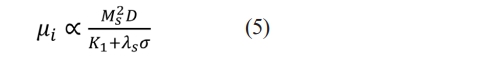
Where λS and σ are the magnetostriction coefficient and the internal stress, respectively. Generally, the internal stress in ferrite materials is so low that the λS and σ can be ignored [30]. As described above, the replacing of Ba2+ by Sm3+ will result in the transition of Fe3+ to Fe2+. The ion magnetic moment of Fe2+ (4μB) is smaller than that of Fe3+ (5μB) [13]. Therefore, this transition reduces the total ion magnetic moment in specimens, which reduces their ui correspondingly.
|
Fig. 1 The XRD results of W-type Ba1-xSmxMg2Fe15.6Co0.4O27 ferrites. |
|
Fig. 2 The change of lattice constants a and c of specimens with different x from 0.00 to 0.30. |
|
Fig. 3 The change c/a of specimens with different x from 0.00 to 0.30 |
|
Fig. 4 Typical SEM images of Ba1-xSmxMg2Fe15.6Co0.4O27 specimens with different x. (a-f): x is from 0.00 to 0.30 with steps of 0.06, respectively. |
|
Fig. 5 RT magnetic hysteresis loops of Ba1-xSmxMg2Fe15.6Co0.4O27 specimens with different x. |
|
Fig. 6 The variation of Ms and Mr of Ba1-xSmxMg2Fe15.6Co0.4O27 specimens with different x. |
|
Fig. 7 The Hc (a) and the ui (b) of specimens with different x from 0.0 to 0.3. |
Sm3+ substituted W-type barium ferrites Ba1-xSmxMg2Fe15.6Co0.4O27 (x=0.00 to 0.30 with steps of 0.06) were obtained by a solid-state method. The XRD results indicate that the specimens with x<0.18 were single W-type hexagonal crystal structure. When x≥0.18, the impurities Sm2O3 phases emerged. As the x increases, both the lattice parameters a and c decrease, but c changes more significantly than a. When x≤0.12, the Ms increases with the increase of x, while when x>0.12, it begins to decrease. However, the Hc keeps increasing with the increase of x. The initial magnetic permeability of specimens exhibits the same variation as Ms. When x=0.12, it reaches a maximum.
Jinsong Li: Investigation, Methodology, Writing - original draft, Writing - review & editing, Funding acquisition. Siyuan Li: Writing - review & editing, Investigation, Formal analysis. Xiubin Zhao: Writing - review & editing, Investigation, Formal analysis. Ailin Xia: Funding acquisition, Methodology, Project administration, Supervision.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data will be made available on request.
- 1. A.L. Xia, J.J. Ji, X.S. Zhu, C.C. Cao, S.B. Su, H.Y. Zhang, H.L. Li, Z.Y. Liu, and C.G. Jin, J. Mater. Sci.: Mater. Electron. 32 (2021) 12725-12731.
-

- 2. M. Etier and H. Aimomani, J. Ceram. Process. Res. 21[5] (2020) 565-570.
-

- 3. X.B. Zhao and A.L. Xia, J. Ceram. Process. Res. 24[4] (2023) 634-639.
-

- 4. Y. M. Zhang, Y.J. Yang, D.Y. Chen, C.L. Chen, and Y.T. Meng, J. Ceram. Process. Res. 24[2] (2023) 342-347.
-

- 5. X.B. Zhao, S. Zhang, J.S. Li, A.L. Xia, and Y.J. Yang, J. Ceram. Process. Res. 24[1] (2023) 98-102.
-

- 6. J.S. Li, X.B. Zhao, and A.L. Xia, J. Ceram. Process. Res. 25[2] (2024) 316-321.
-

- 7. M. Ahmad, I. Ali, and R. Grössinger, J. Alloys Compd. 579 (2013) 57-64.
-

- 8. P.S. Sawadh and D.K. Kulkarni, J. Mater. Chem. Phys. 63 (2000) 170-173.
-

- 9. Y.J. Yang, C.L. Chen, and D.Y. Chen, Magnetochemistry 8 (2022) 75.
-

- 10. J.S. Li, X.B. Zhao, and A.L. Xia, J. Ceram. Process. Res. 24[5] (2023) 894-898.
-

- 11. L.X. Wang, J. Song, Q.T. Zhang, X.G. Huang, and N.C. Xu, J. Alloys Compd. 481 (2009) 863-866.
-

- 12. I. Sadiq, I. Khan, F. Aen, M.U. Islam, and M.U. Rana, Phys. B 407 (2012) 1256-1261.
-

- 13. Y.J. Yang, X.S. Liu, and S.J. Feng, J. Ceram. Process. Res. 21[3] (2020) 378-385.
-

- 14. F. Aen, M.F. Wasiq, M.U. Rana, H.M. Khan, and M.A. Khan, J. Ceram. Int. 42[14] (2016) 16077-16083.
-

- 15. X. Niu, X. Liu, S. Feng, F. Lv, F. Huang, X. Huang, Y. Ma, and K. Huang, J. Optik 126 (2015) 5513-5516.
-

- 16. M.N. Akhtar, K. Ali, A. Umer, T. Ahmad, and M.A. Khan, Mater. Res. Bull. 101 (2018) 48-55.
-

- 17. M.J. Iqbal and S. Farooq, J. Alloys Compd. 505 (2010) 560-567.
-

- 18. Y.F. Wu, Y. Huang, and L. Niu, J. Magn. Magn. Mater. 324 (2012) 616-621.
-

- 19. F.R. Lv, X.S. Liu, and S.J. Feng, Mater. Lett. 157 (2015) 277-280.
-

- 20. I. Khan, M.N. Ashiq, I. Sadiq, A.M. Qureshi, and M.U. Rana, J. Chem. Soc. Pak. 34 (2012) 579-583.
-

- 21. M.J. Iqbal, R.A. Khan, S. Mizukami, and T. Miyazaki, Ceram. Int. 38 (2012) 4097-4103.
-

- 22. F. Leccabue, R. Panizzieri, G. Albanese, G. Leo, and N.S. Almodovar, Mater. Res. Bull. 23[2] (1988) 263-275.
-

- 23. F.K. Lotgering, P.H.G.M. Vromans, and M.A.H. Huyberts, J. Appl. Phys. 51[11] (1980) 5913-5918.
-

- 24. Y.B. Han, J. Sha, L.N. Sun, Q. Tang, Q. Lu, H.X. Jin, D.F. Jin, H. Bo, H.L. Ge, and X.Q. Wang, J. Alloys Compd. 486 (2009) 348-351.
- 25. J. Tang, D. Li, H. He, Y.M. Li, J.S. Zeng, and C. Liu, J. Appl. Phys. A. 126 (2020) 277.
-

- 26. M.J. Iqbal and S. Farooq, J. Mater. Res. Bull. 44 (2009) 2050-2055.
-

- 27. S. Ounnunkad, J. Solid. State. Comm. 138 (2006) 472-475.
-

- 28. D.M. Hemeda, A.A. Sharif, and O.M. Hemeda, J. Magn. Magn. Mater. 315 (2007) L1-L7.
-

- 29. H. Su, H. Zhang, X. Tang, B. Liu, and Z. Zhong, J. Alloys Compd. 475[1] (2009) 683-685.
-

- 30. Y. Peng, X.H. Wu, Z.Y. Chen, W.H. Liu, F. Wang, Z.K. Feng, Y.J. Chen, and V.C. Harris, J. Alloys Compd. 630 (2015) 48-53.
-

 This Article
This Article
-
2025; 26(1): 76-81
Published on Feb 28, 2025
- 10.36410/jcpr.2025.26.1.76
- Received on Sep 26, 2024
- Revised on Nov 24, 2024
- Accepted on Dec 19, 2024
 Services
Services
- Abstract
introduction
experimental procedures
results and discussion
conclusions
- Author Contributions
- Conflict of Interest
- Data availability statement
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Ailin Xia
-
Advanced Ceramics Research Center, School of Materials Science and Engineering, Anhui University of Technology, Maanshan, 243032, China
Tel/Fax: +86 05552311570 - E-mail: alxia@126.com






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.