- Synthesis of nano-scale silicon powder by magnesiothermic reduction for anodes in lithium-ion battery
Ju-Chan Kwona and Sang-Jin Leea,b,*
aDepartment of Advanced Materials Science and Engineering, Mokpo National University, Muan 58554, Republic of Korea
bResearch Institute of Ceramic Industry and Technology, Mokpo National University, Muan 58554, Republic of KoreaThis article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Pure Si powder used as an anode material can enhance the capacity of lithium ion batteries. However, repeated charging and discharging can cause significant volume expansion, thereby reducing the durability and battery life cycles. Using round-shape, nanoscale Si powder, which can be fabricated by a reduction process of spherical SiO2 powder, can relieve this problem. This reduction method results in the formation of SiOx including Si structure. In particular, approaches such as using nano powder as a starting material or employing a high-energy milling process to produce nano powder have been extensively explored in recent research. In the present study, nano-sized SiOx powder containing Si was fabricated by using 100 nm synthetic, spherical SiO2 powder and commercial Si powder as the starting material. The materials were mixed by a high-energy milling process using an attrition mill and then subjected to magnesiothermic reduction heat treatment. In particular, NaCl was used during the heat treatment to inhibit the formation of Mg2SiO4 reactant. The crystallinity and the Si-O-Si bonding state of the final SiOx powder, obtained after acid treatment and DI water washing, were examined by characterization using X-ray diffraction, Fourier transform infrared spectroscopy, and X-ray photoelectron spectroscopy.
Keywords: Spherical silica, Nano silicon, Attrition milling, Magnesiothermic reduction, SiOx powder.
Increasing the capacity of Li-ion batteries has recently become a significant issue owing to their growing use in mobile phones and hybrid and battery electric vehicles (EVs) [1]. Graphite is commonly used as the anode, where each carbon atom is bonded to two other carbon atoms in a regular hexagonal pattern, thereby forming thin layer plates [2]. This layered structure facilitates the Li ion transport, thus making graphite a suitable anode material. With the sharp increase in the demand for EV batteries, the use of Si as an anode is also gaining momentum. In particular, Si anodes increase the capacity of the battery by tenfold per gram, thereby extending the driving range of EVs. However, Si and graphite absorb Li by different mechanisms, and this results in significant volume expansion in the case of Si [3-5]. Specifically, Si anodes undergo a significant volume expansion owing to repeated charging and discharging, resulting in reduced durability of the active material and life cycle of the battery.
This limitation of Si anodes can be addressed by mixing a specific quantity of nano Si with graphite to minimize volume expansion. However, the high cost of nano Si remains a crucial problem. Researchers recently have focused on preventing the volume expansion of Si by synthesizing nano-sized SiOx. Kong et al., coated Si particles with SiOx by using an oxidation process; the SiOx coating thickness was 3.4~9.6 nm, which helped alleviate the sudden volume expansion of Si and facilitated the dispersion of Li ions [6]. Chen et al., investigated the production of SiOx, which is primarily composed of Si, through magnesiothermic reduction using SiO2 as the starting material [7]. They observed that the loose structure of multiporous Si could effectively manage volume changes during the charging and discharging processes. Cho et al. [8] investigated the cost reduction in Si fabrication through magnesio-milling of SiO2-Mg mixed powder using a planetary mill and attrition mill equipment.
In this study, the fabrication of Si nano-sized powder containing SiO(0
Fabrication of nano SiOx anode material
A 100 nm amorphous, spherical SiO2 powder synthesized by the Stöber process, and commercial Si powder (99.99%, 10~80 μm, Samchun Chemicals, Korea) were used as the starting material. A sealed planetary mill container (water-cooled, Nano Ceratech, Korea), with, 1,000 zirconia balls having a radius of 3 mm and 500 zirconia balls having a radius of 5 mm was employed to ensure uniform mixing. The synthesized SiO2 and Si powders were added into the container at a mass ratio of 1:1. Subsequently, pulverizing and mixing were conducted by ball milling at 400 rpm for 24 h [10].
Before adding Mg to the planetary milled powder, NaCl powder was mixed (planetary milled powder : NaCl (in wt%) = 1:0.8) to inhibit excessive production of MgO and its by-products. The mixed powder with deionized (DI) water was stirred at 50 °C for 3 h and then vacuum-dried at 80 °C for 24 h. The agglomerated dried powder was pulverized in an alumina mortar. Subsequently, Mg powder (98.5%, 100~150 μm, Daejung Chemicals & Metals, Korea) was added at a SiO2 starting powder : Mg weight ratio of 1:1 and dry mixed at 100 rpm for 5 h using a dry powder mixer. For comparison another reduction material, Mg(NO3)2 was also assessed. It was wet-mixed at a SiO2 starting powder : Mg weight ratio of 1:1, and then dried. The final mixed powders underwent a magnesiothermic reduction process. For the heat treatment process, the temperature was increased to 800 °C at a rate of 5 °C/min in a vacuum atmosphere using a vacuum furnace (electric & vacuum furnace, Pyro Tech, Korea), and held for 3 h. The heat-treated sample was rinsed multiple times with DI water to remove NaCl and subsequently submerged in a 2 M hydrochloric acid solution for 24 h to convert the residual Mg by-products into a highly soluble substance. Furthermore, any remaining SiO2 was eliminated by soaking the sample in a 5% hydrofluoric acid (HF) solution for 1 h. The sample was then washed with ethanol and dried at 105 °C for 4 h to eliminate surface moisture, after rinsing several times with DI water.
Characterization
An X-ray diffraction (XRD) analyzer (X’pert-pro MPD, PANalytical, Netherlands) was employed to examine the variations in the crystalline structure after each process. The particle size of the starting Si powder and SiO2 and Si powder mixtures after planetary milling was measured by laser diffraction analysis (LS13 320, Beckman Coulter, USA). The ultrasonic dispersion of the powder slurry was carried out for 15 min. The microstructure of the powders for each experiment condition was examined using field-emission scanning electron microscopy (FE-SEM, JSM-7100F, Jeol, Japan). The sample was affixed to an Al holder using carbon tape and coated with Au-Pd sputtering. To analyze the atomic bonding structure of the powders at each processing stage, Fourier transform infrared spectroscopy (FT-IR, Vertex 80v, Bruker, USA) was performed within the range of 3,500~500 cm-1. Finally, X-ray photoelectron spectroscopy (XPS, PHI Quantera II, ULVAC-PHI, Japan) was employed to examine the atomic structure of the surface composition of the sample with an Al-Kα (1,186 eV) source under a chamber pressure of 2 × 10−7 Pa.
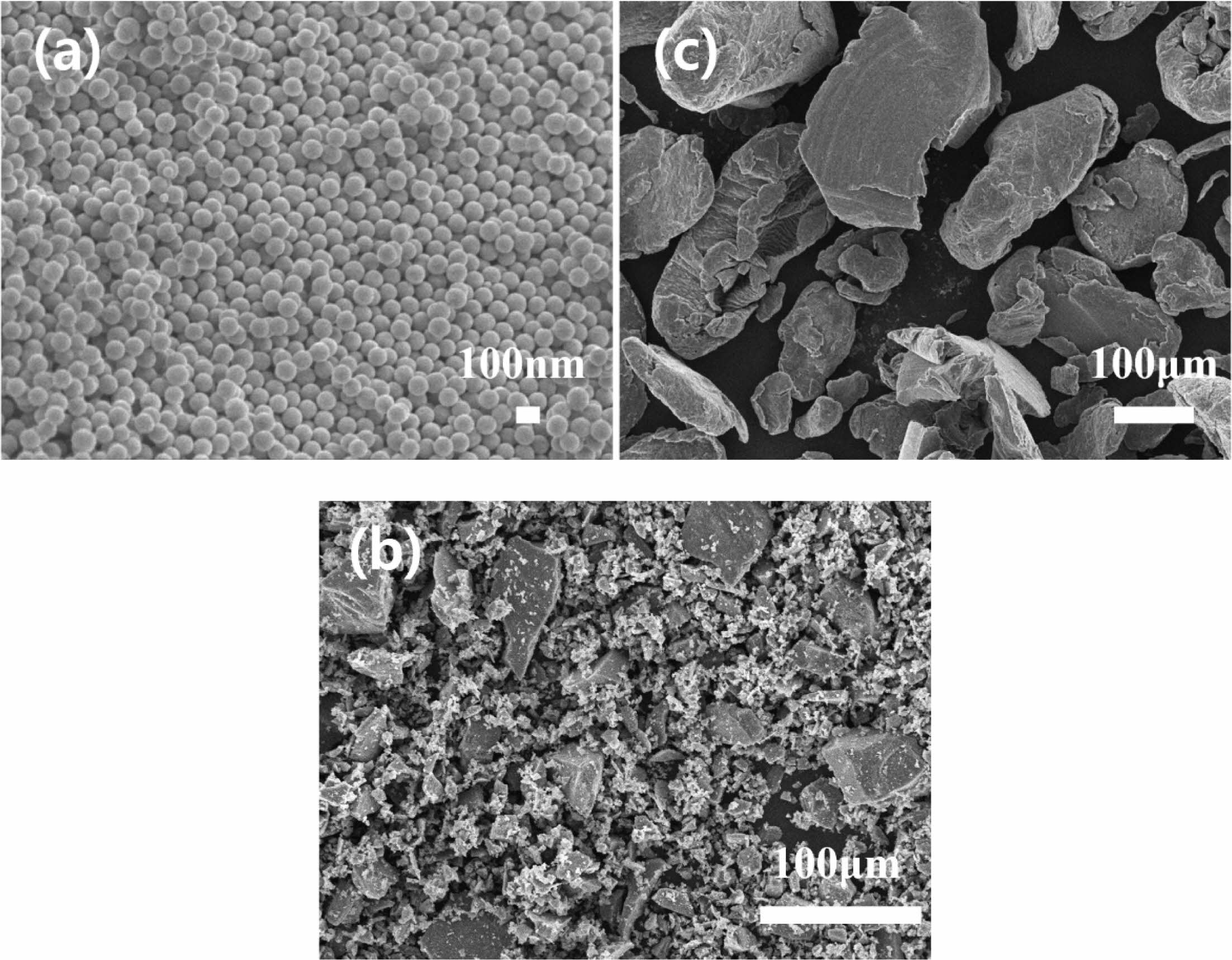
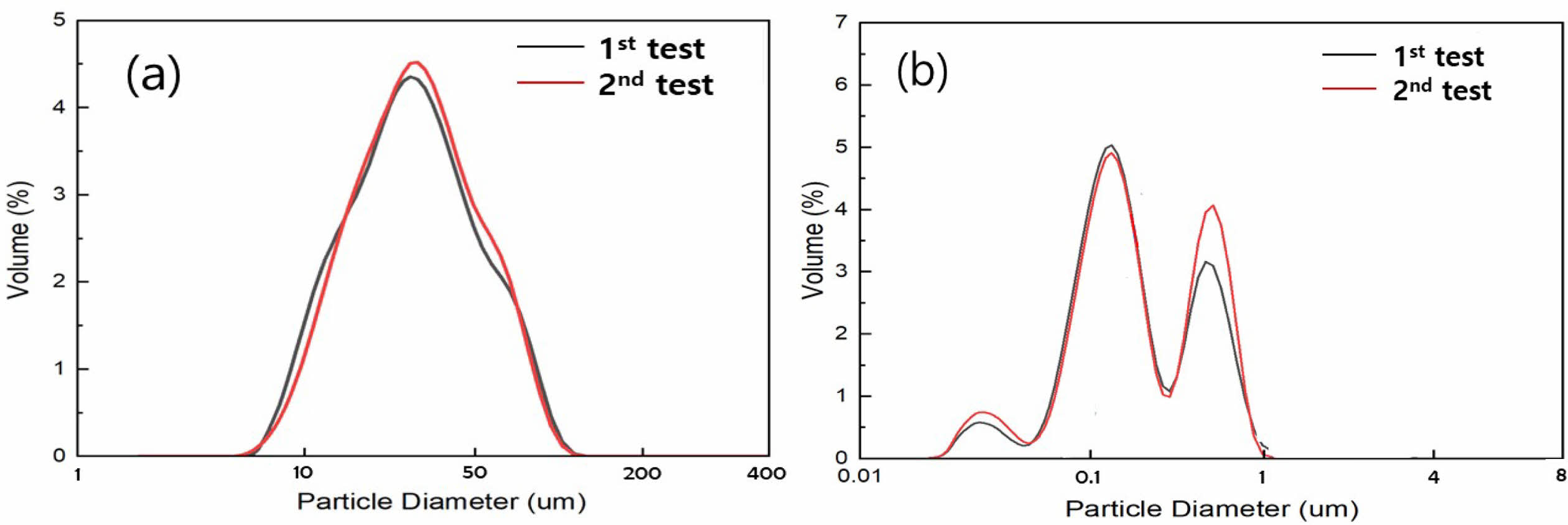
The microstructures of the synthesized SiO2 powder, commercial Si powder, and Mg powder are shown in Fig. 1. The synthesized SiO2 powder exhibits uniformly 100 nm sized spherical shapes, whereas the Si powder displays a broad particle-size distribution. The Si powder was pulverized using high-energy planetary milling, and reacted with O2 in the SiO2 powder [11, 12]. Micro-sized particles were excluded from the Mg powder used as a reducing agent to minimize the formation of by-products resulting from reactions with the planetary ball milled SiOx. Fig. 2 represents the particle size distribution of the Si powder before and after high-energy planetary milling. The starting Si powder exhibited a broad particle size distribution. After planetary milling, the decreasing trend was dramatical showing the size distribution of below 1 μm to nano size.
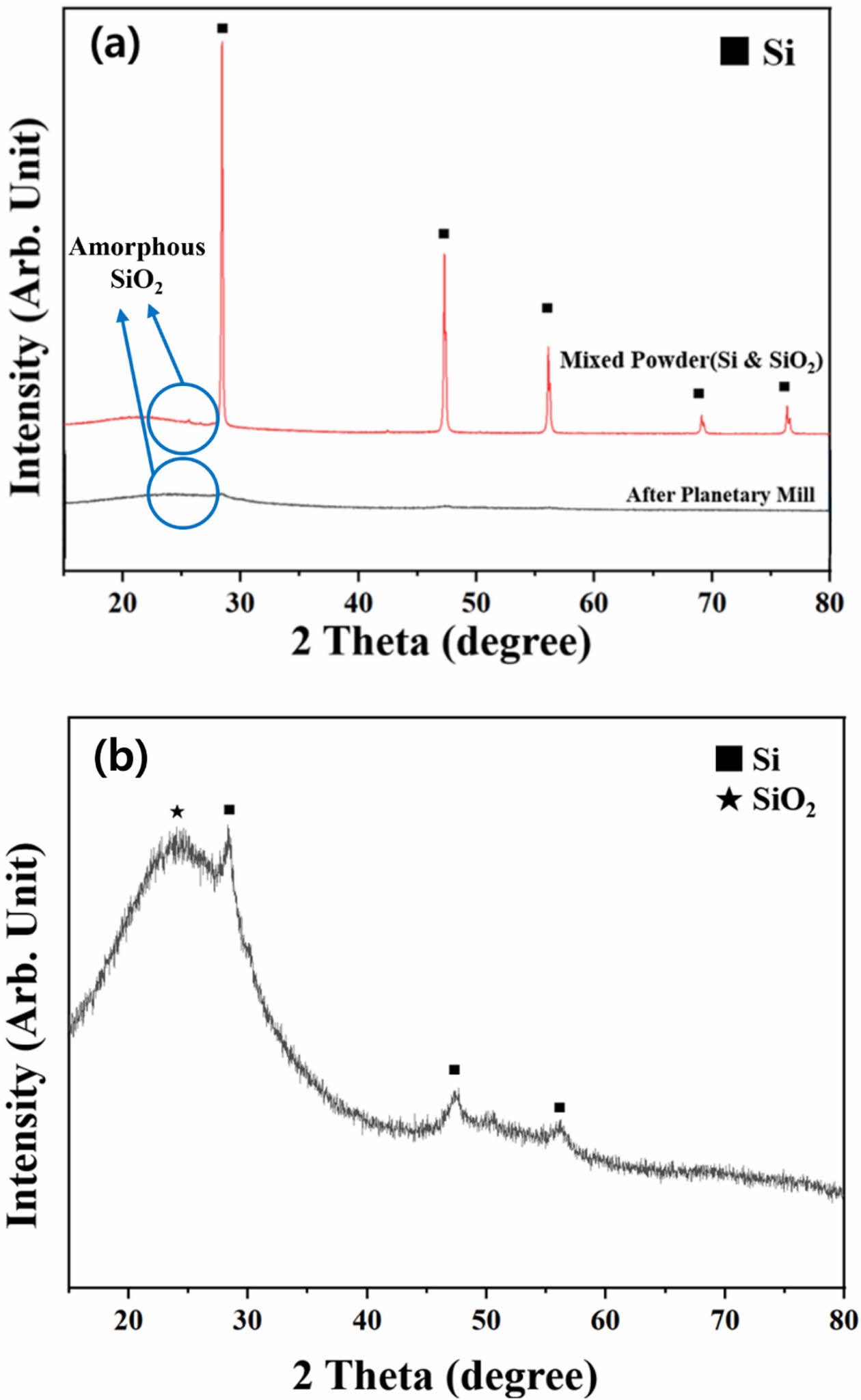
The XRD results of the mixed Si and SiO2 powders before and after planetary milling are presented in Fig. 3. A distinct crystalline Si peak is observed before milling owing to the influence of the metallic Si powder, whereas the amorphous SiO2 peak from the synthesized SiO2 powder is less pronounced. After planetary milling, the crystalline Si peak is not clearly visible, and only the amorphous SiO2 peak is observed at approximately 25° (Fig. 3(a)). The magnified image (Fig. 3(b)) reveals an amorphous phase with a small Si peak, which is likely due to the pulverization of large Si particles into nanoscale sizes through high-energy milling. During this process, Si extensively reacts with O2 in SiO2, forming SiOx (Si, if x = 0).
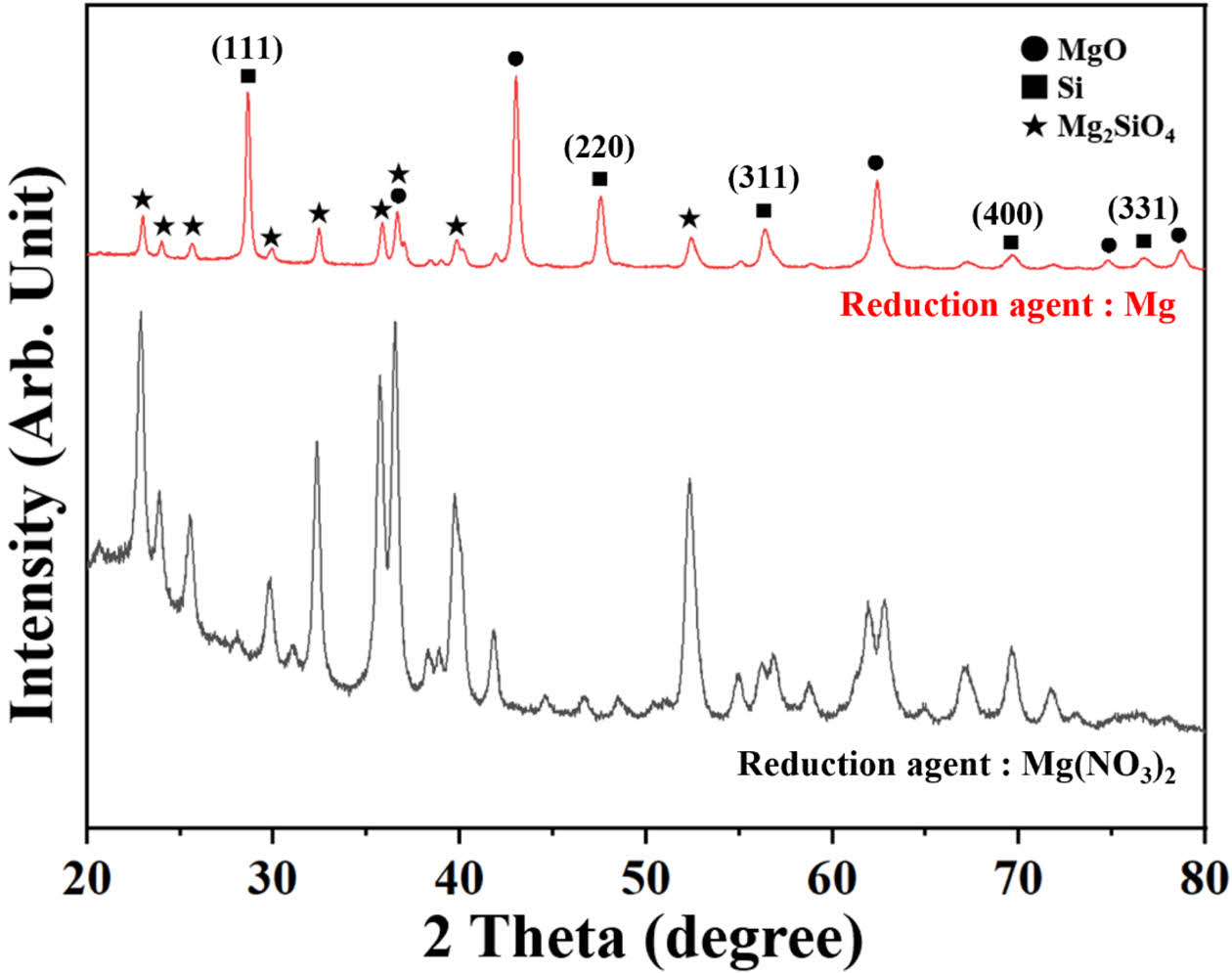
The changes in the crystalline structure of the SiOx powder after the addition of Mg or Mg(NO3)2 as a reducing agent and the vacuum heat treatment are shown in Fig. 4. In both cases, the samples were fabricated without adding NaCl. When the Mg powder was used as the reducing agent, oxidized MgO, reactant Mg2SiO4, and crystalline Si peaks were observed. As an amorphous SiO2 peak was not observed at approximately 25°, most of the amorphous SiOx powder is considered to be reduced through the reduction process or formed a reactant with Mg. Chen et al., synthesized mesoporous Si through magnesiothermic reduction by performing heat treatment at 650 °C in a N2 atmosphere. An the XRD analysis of the synthesized particles revealed Si (111), (220), (311), (400), and (331) peaks, whereas MgO, Mg2Si, and Mg2SiO4 were observed as by-products [7, 13-15]. In this experiment, heat treatment was conducted at 800 °C in a vacuum atmosphere. Si synthesized through reduction displayed clear peaks on five crystalline planes, consistent with the findings of Chen et al. [7]. However, Mg2Si was not observed, suggesting that the reduced Si did not react with Mg in the vacuum atmosphere. In the previous study, for the reduction of spherical monodisperse silica particles, a nitrogen gas atmosphere mixed with 5% hydrogen was applied without Mg powder, and carbothermal reduction method was also tried by adding some carbons to selectively remove oxygen atoms in the silica. However, crystalline Si peak was not observed in the XRD examination [16, 17].
Mg(NO3)2 was wet-mixed and used as a reducing agent to improve the blend homogeneity with the SiOx powder; In this study, a reducing effect was not achieved. In addition to amorphous SiOx peaks at around 2θ 25°, peaks of Mg2SiO4 were observed. The Mg ions had already reacted with O2 during the drying process, resulting in amorphous MgO, and then reacted with SiOx during heat treatment, thereby generating Mg2SiO4.
Fig. 5 reveals the microstructure of the powder, which underwent magnesiothermic reduction with Mg as the reducing agent. Fig. 5(a) shows reduced SiOx powder and the MgO or Mg2SiO4 by-products. Large sized by-products (red circles) were formed when the smaller Mg particles reacted with amorphous SiOx during heat treatment. The magnified image of the nano-sized spherical particles shown in Fig. 5(b) depicts the reduced spherical SiO2 starting powder. The partially fractured and agglomerated particles originate from the SiOx and commercial Si powder obtained through planetary milling with pulverization.
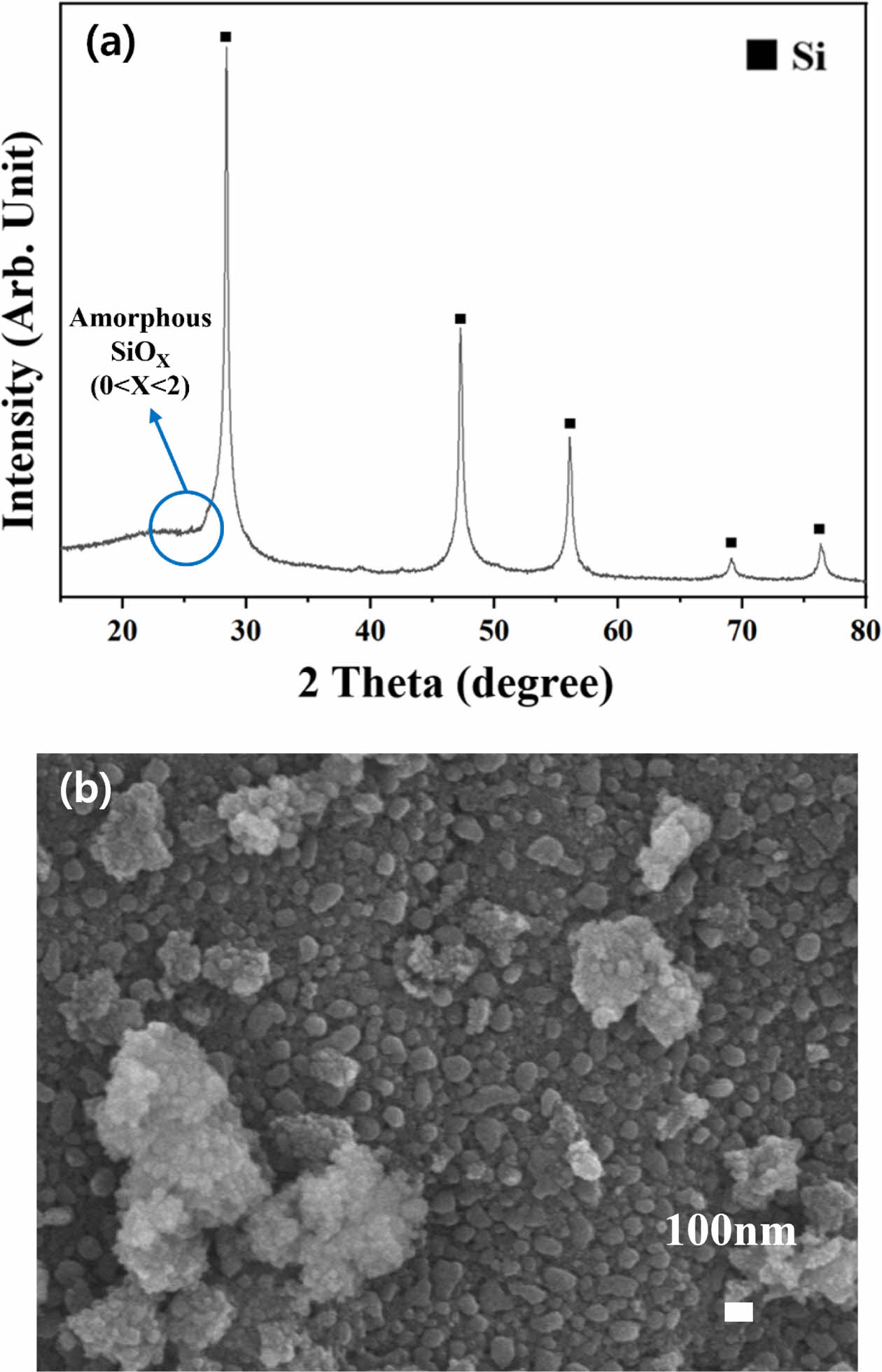
The formation of a by-product of Mg2SiO4 was inhibited by the addition of NaCl during heat treatment, and after heating the NaCl was removed by rinsing with DI water. The XRD and FE-SEM results of the finally obtained powder are represented in Fig. 6. The XRD spectra do not include peaks of Mg, MgO, and Mg2SiO4, and only crystalline Si and amorphous SiOx peaks are observed. The added NaCl inhibits the reaction between SiOx and MgO by absorbing the surrounding heat during melting, which has a high enthalpy level, in a reducing atmosphere [18-20]. Mg is therefore present as MgO rather than as a Mg2SiO4 reactant. Mg and MgO are converted into highly soluble MgCl through HCl treatment and removed after rinsing with water [15]. Furthermore, the residual amorphous SiOx is dissolved through HF acid treatment and eliminated to a certain extent. Amorphous SiOx peaks are still observed at approximately 25°, indicating that the starting SiO2 powder is not entirely reduced to Si (where X = 0 in SiOx) and partially remains in an amorphous SiOx state [21]. The microstructure analysis revealed the agglomeration of some pulverized powder owing to the planetary milling, drying, and heat treatment processes. The complete spherical form was lost during acid treatment, resulting in particle sizes of approximately 100 nm. The agglomerated powders during the several processes are due to the fine particles of nano size, and the powders seem not to have an influence on the capacity of silicon anode [22].
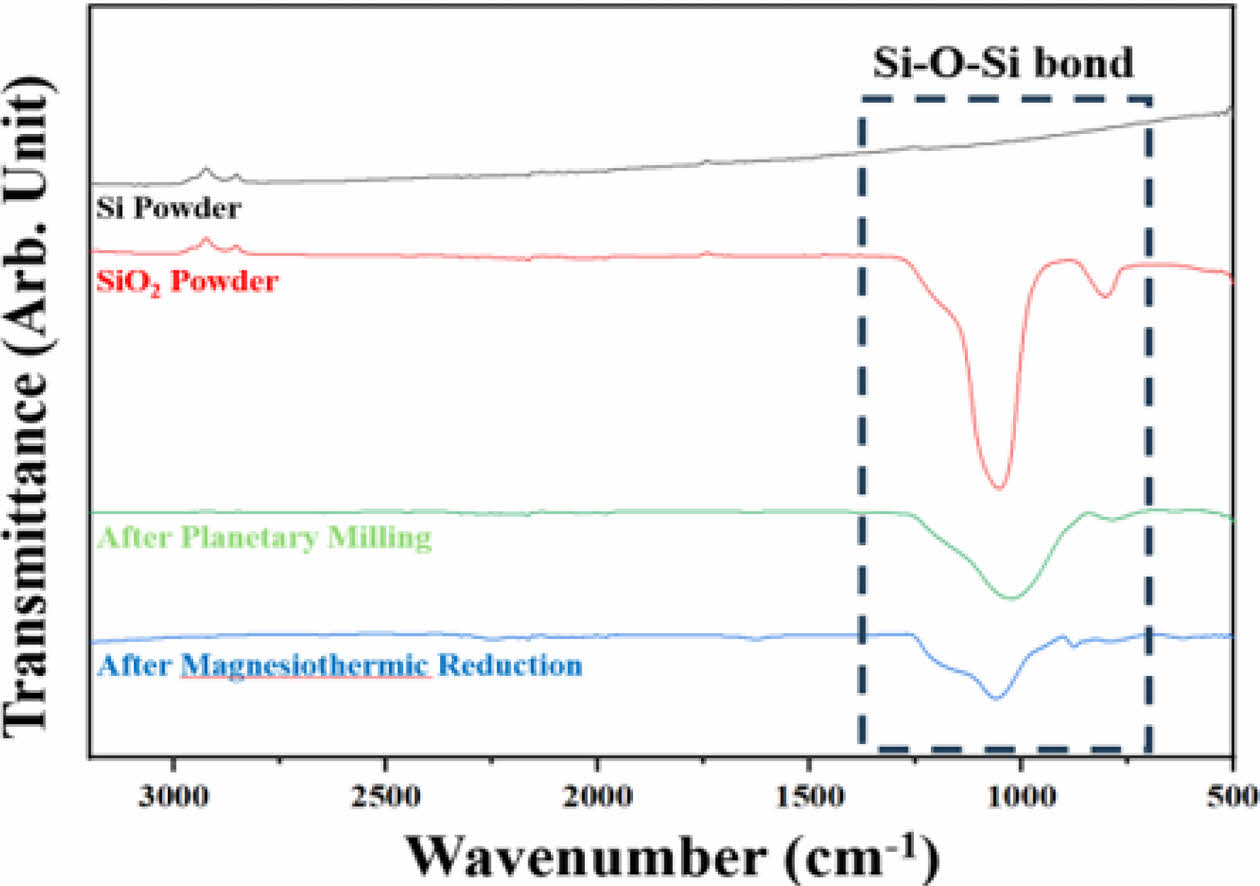
A FT-IR analysis was conducted on the Si and SiO2 powders used as starting materials, powders after planetary milling, and the final powder after magnesiothermic reduction and acid treatment. The changes in the Si–O–Si bonding of each sample are displayed in Fig. 7. The variations in the intensity of the Si–O–Si bonding are notable around the wavenumber of 1,000 cm−1 [23, 24]. The FT-IR spectra of Si powder (the starting material) do not display any Si–O–Si bonding peaks. In contrast, the synthesized SiO2 powder, which consists of a SiO4 tetrahedron structure, exhibits the most prominent peaks. Subsequently, the intensity of the peaks corresponding to Si–O–Si bonding decreases after planetary ball milling and magnesiothermic reduction. Specifically, the peaks of Si–O–Si bonding in the spectra of the final powders, which underwent magnesiothermic reduction and acid treatment, suggest the formation of an oxide film on the surface of the Si powder due to oxidation when exposed to air during the sample preparation process involving the residual amorphous SiOx (x > 0) powder.
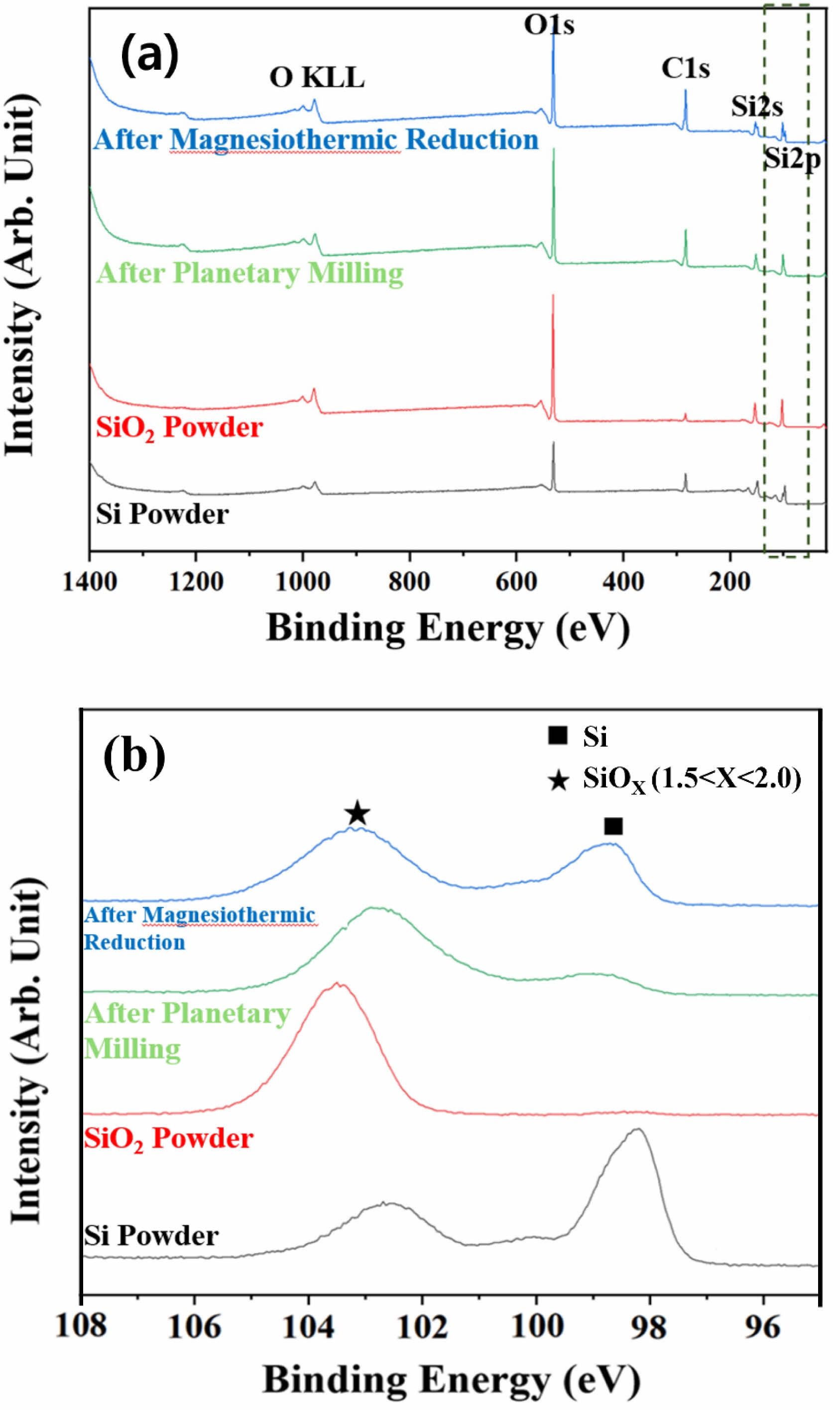
An XPS analysis was performed based on the FT-IR spectroscopy results to further evaluate the Si–O–Si bonding state at approximately 1,000 cm−1, and the results are shown in Fig. 8. As the Si2p peak is detected for all sample powders, the x value of SiOx can be analyzed by examining the narrow range of this peak [25, 26]. In the study conducted by Miyachi et al. [27] a XPS analysis revealed that SiOx can be characterized as Si0+(Si) when x = 0 or Si4+(SiO2) when x = 2. The x value continuously varies within the binding energy range of 98-104 eV. With decreasing binding energy, the SiOx state with a low oxygen content becomes more apparent. In the narrow range graph corresponding to Si2p (Fig. 8(b)), peaks of the Si powder are observed with binding energy of approximately 98.5 and 102.5 eV. Each peak corresponds to Si0+ and Si3+, representing Si and SiOx (1.5
|
Fig. 1 FE-SEM micrographs of (a) spherical SiO2 powder prepared via Stöber process, (b) commercial Si powder, and (c) commercial Mg powder. |
|
Fig. 2 Particle size distribution of (a) commercial Si powder and (b) SiO2 and Si powder mixtures after planetary milling. |
|
Fig. 3 (a) XRD patterns of mixed Si and SiO2 powders before and after planetary milling and (b) corresponding magnified pattern after planetary milling. |
|
Fig. 4 XRD patterns of magnesiothermic reduction of SiOx powder according to reducing agent without NaCl addition. |

|
Fig. 5 FE-SEM micrographs of (a) magnesiothermic reduction of SiOx powder using Mg as the reducing agent without the addition of NaCl and (b) magnified image of SiOx powder in (a). |
|
Fig. 6 (a) XRD patterns of magnesiothermic reduction of SiOx powder with addition of NaCl, water washing, and acid treatment. (b) FE-SEM micrograph of Si powder from (a). |
|
Fig. 7 FT-IR spectra of starting Si and amorphous SiO2 powders, planetary-ball-milled powder, and final reduced powder obtained by applying the magnesiothermic reduction process after acid treatment and rinsing with DI water. |
|
Fig. 8 (a) XPS result of the starting Si and amorphous SiO2 powder, planetary-ball-milled powder, and final reduced powder obtained by applying the magnesiothermic reduction process and acid treatment. (b) Narrow range spectra of the Si2p peak. |
Spherical and amorphous SiO2 powder having a particle diameter of 100 nm was synthesized by the Stöber process and then reduced to produce nano scale SiOx powder containing Si, which is suitable for use as anode materials in lithium ion batteries. Commercial Si powder was added to the synthesized SiO2 powder, and SiOx powder was prepared via planetary milling. Subsequently, Mg powder was added, and the mixture underwent heat treatment in a vacuum. The thermally reduced powder was then subjected to acid treatment and DI water washing to remove the residual Mg by-products. The XRD analysis of the finally fabricated SiOx powder revealed a mixture of metallic Si and amorphous SiOx. FT-IR spectroscopy and XPS analyses of the fabricated SiOx powder were conducted to determine the x value, which varied in the range of 0~2, thereby confirming the presence of Si as Si0+ and amorphous type SiOx (0
This work was supported by Research Funds of Sabbatical Year of Mokpo National University in 2024.
- 1. M. Gu, Y. He, J. Zheng, and C. Wang, Nano Energy 17 (2015) 366-383.
-

- 2. H. Zhang, Y. Yang, D. Ren, L. Wang, and X. He, Energy Storage Mater. 36 (2021) 147-170.
-

- 3. J. Guo, X. Chen, and C. Wang, J. Mater. Chem. 20 (2010) 5035-5040.
-

- 4. B. Jarulertwathana and T. SaraKonsri, J. Ceram. Process. Res. 15[6] (2014) 389-392.
-

- 5. J. Lee, Y Ahn, and S. Lee, J. Ceram. Process. Res. 24[6] (2023) 1010-1024.
-

- 6. K. Kong, G. Xu, Y. Lan, C. Jin, Z. Yue, X. Li, F. Sun, H. Huang, J. Yuan, and L. Zhou, Appl. Surf. Sci. 515 (2020) 146026.
-

- 7. W. Chen, Z. Fan, A. Dhanabalan, C. Chen, and C. Wang, J. Electrochem. Soc. 158[9] (2011) A1055-A1059.
-

- 8. W.C. Cho, H.J. Kim, H.I. Lee, M.W. Seo, H.W. Ra, S.J. Yoon, T.Y. Mun, Y.K. Kim, J.H. Kim, B.H. Kim, J.W. Kook, C.Y. Yoo, J.G. Lee, and J.W. Choi, Nano Lett. 16 (2016) 7261-7269.
-

- 9. J.C. Kwon, B.H. Oh, and S.J. Lee, Korean J. Mater. Res. 33[12] (2023) 530-536.
-

- 10. Y.T. Park and K.T. Lee, J. Ceram. Process. Res. 17[1] (2016) 1-4.
-

- 11. B.E. Nilssen and R.A. Kleiv, SN. 12[10] (2020) 2413-2423.
-

- 12. T.T.T. Hien, T. Shirai, and M. Fuji, J. Ceram. Soc. Jpn. 120[10] (2012) 429-435.
-

- 13. I.S. Curtis, R.J. Wills, and M. Dasog, Nanoscale 13 (2021) 2685-2692.
-

- 14. A. Rasouli, M. Tsoutsouva, J. Safarian, and G. Tranell, Materials 15[19] (2022) 1-14.
-

- 15. Y. Iwadate, K. Ohgane, and T. Ohkubo, Electrochemistry (Tokyo, Jpn.) 86[4] (2018) 198-201.
-

- 16. P. Lv, H. Zhao, C. Gao, T. Zhang, and X. Liu, Electrochim. Acta, 152 (2015) 345-351.
-

- 17. J. Yang, T. Mori, and M. Kuwabara, ISIJ Int. 47[10] (2007) 1394-1400
-

- 18. J. Entwistle, A. Rennie, and S. Patwardhan, J. Mater. Chem. A. 6[38] (2018) 18344-18356.
-

- 19. C. Li, C. Liu, W. Wang, Z. Mutlu, J. Bell, K. Ahmed, R. Ye, M. Ozkan, and C.S. Ozkan, Sci. Rep. 7[917] (2017) 1-11.
-

- 20. Z. Favors, W. Wang, H.H. Bay, Z. Mutlu, K. Ahmed, C. Liu, M. Ozkan, and C.S. Ozkan, Sci. Rep. 4 (2014) 1-7.
-

- 21. J. Yang, Y. Takeda, N. Imanishi, C. Capiglia, J.Y. Xie, and O. Yamamoto, Solid State Ionics. 152-153 (2002) 125-129.
-

- 22. J.K. Lee and J.R. Yoon, J. Ceram. Process. Res. 21[5] (2020) 533-538.
-

- 23. G. Kandilioti, A. Siokou, V. Papaefthimiou, S. Kennou, and V. G. Gregoriou, Appl. Spectrosc. 57[6] (2003) 628-635.
-

- 24. Y. Park, M. Boyer, G.S. Hwang, and J. Lee, J. Electroanal. Chem. 833 (2019) 552-559.
-

- 25. W. Wu, M. Wang, R. Wang, D. Xu, H. Zeng, C. Wang, Y. Cao, and Y. Deng, J. Power Sources. 407 (2018) 112-122.
-

- 26. Y. Park, Y. Myung, and J. Lee, ACS Appl. Energy Mater. 3 (2020) 8803-8811.
-

- 27. M. Miyachi, H. Yamamoto, H. Kawai, T. Ohta, and M. Shirakata, J. Electrochem. Soc. 152[10] (2005) A2089-A2091.
-

 This Article
This Article
-
2024; 25(6): 1115-1121
Published on Dec 31, 2024
- 10.36410/jcpr.2024.25.6.1115
- Received on Aug 9, 2024
- Revised on Nov 7, 2024
- Accepted on Dec 18, 2024
 Services
Services
- Abstract
introduction
experimental procedure
results and discussion
conclusions
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Sang-Jin Lee
-
aDepartment of Advanced Materials Science and Engineering, Mokpo National University, Muan 58554, Republic of Korea
bResearch Institute of Ceramic Industry and Technology, Mokpo National University, Muan 58554, Republic of Korea
Tel : +82-61-450-2493 Fax: +82-61-450-2498 - E-mail: lee@mnu.ac.kr






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.