- Structural and electrical behaviour studies in K doped BaSnO3 for IT-SOFC applications
De Wua,* and Chengkai Lib
aSchool of Environment and Resources, Taiyuan University of Science and Technology, 030024, Taiyuan, China
bSchool of Materials Science and Engineering, Taiyuan University of Science and Technology, 030024, Taiyuan, ChinaThis article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
In the present work, we report the synthesis, crystal structure, and charge transport properties of K doped BaSnO3(Ba0.8K0.2O3). The powder X-ray diffraction showed the formation of single phase with cubic structure. The total conductivity was measured using complex impedance analysis over a temperature range 30-750 oC and frequency 10 Hz to 10 MHz. The maximum conductivity was found to be 6.63 × 10-3 Scm-1 at 750 oC. The calculated activation energy from ac conductivity was found to be 0.40 eV. The total conductivity was found to increase with increase in temperature.
Keywords: Crystal structure, Electron microscopy, Grain boundaries, Sol-gel preparation, Thermal analysis, X-ray techniques.
It’s time to prioritize planet-friendly technologies and renewable energy sources in order to maintain a safe and clean environment for all living things, both now and in the future. One of the primary necessities for a sustainable global economy is energy. Across the globe, one of the most important and pressing challenges is clean energy. Science and technology researchers have been working hard on many different facets of finding a solution to the energy dilemma. Sophisticated techniques have been devised to swap out limited fossil fuels for environmentally sustainable substitutes. Promising energy conversion and storage technologies, including fuel cells, batteries, solar cells and hydrogen production are being developed to reduce carbon dioxide emissions. The performance of these energy systems depend crucially on the properties of their component materials, thus requiring the development of innovative materials. However these materials must be economically competitive to be commercially visible. One of the challenges faced in the development of new materials for energy application is their unique compositions, structures and morphologies that help the fast transport of ionic and electronic defects which in turn help surface electrochemical reactions.
The solid oxide fuel cell (SOFC) exhibits great efficiency, minimal environmental pollution, low susceptibility to contaminants, and strong fuel adaptability, making it one of the most promising energy-generating technologies shortly. However, the 700-1000 °C operating temperature of SOFCs raises the cost of materials, assembly, design, and maintenance. It also accelerates the rate at which the materials employed in fabrication degrade [1]. Because they provide greater material selection freedom and lessen material deterioration, intermediate and low temperature SOFCs are therefore chosen because they lower overall costs. Lowering the operating temperature, however, will cause the cell’s resistance to increase and its overall output to decrease. Thus, there is room for the creation of novel materials with reduced resistance. During the last decades oxides with different structures (ZnO, Fluorites, Apatites) have been intensively studied with respect to their ionic conduction properties. Later, in oxide materials it was considered that acceptor or donor doping is an effective method to modify the defect concentrations and to obtain the desired electrical or transport properties. Shortly, the research has moved to some ternary or higher oxides having two cations with different valences, but similar in size. And further doping was carried out in such oxides to enhance the desired property. Using perovskites. Perovskite-type oxides with general formula ABO3 have simple and flexible structures that can easily accommodate suitable dopants to create point defects and oxygen non-stoichiometry so that they can form a variety of technologically important materials for several applications. Perovskite stannates BaSnO3 have been of interest due to their dielectric, gas sensing properties etc [2, 3]. The gas sensing and proton conducting properties of BaSnO3 are associated with the structural defects that are responsible for these properties. These defects could also facilitate ion and electronic conductivity [4-10]. In the present work BaSnO3 is synthesized by wet chemical route. After thermal and structural analysis total conductivity of the sintered pellet is studied at various temperatures. In the present work K has doped in the A-site of BasnO3 to improve the electrical properties. Herein, we demonstrate that we have successfully synthesized highly crystalline and discrete doped BaSnO3 nanoparticles, which enable the electrical properties of BaSno3.
Synthesis of the perovskite
The doped BaSnO3 perovskite was prepared by wet chemical method (combustion method). Commercially available high purity Ba(C2H3O2)2, (99.9% purity from Sigma-Aldrich) and K2O3Sn·3H2O (99.9% purity from Sigma-Aldrich) were used as precursors. Stoichiomertic amount of the precursors were dissolved in deionized water along with citric acid and poly ethylene glycol. Then the solution was heated and stirred continuously till it turned into a brown porous dried gel. Further the gel was burned at the atmosphere to remove the polymers in it. After obtaining the appropriate calcination temperature through thermal analysis, the dried gel was calcined at 1000 °C for 4 h to obtain single phase perovskite. The calcined powder is pressed into pellet of 10 mm diameter and 1.6 mm thickness using a hydraulic press by applying a pressure of 1800 kg/cm2. Later the pellet was sintered at 1200 °C for 10 h and used for further characterizations and total conductivity measurements.
Characterization
The dried gel was subjected to thermogravimetry/differential scanning calorimetry (TG/DSC) performed using NETZSCH STA 449F3 in static air atmosphere in the temperature range from room temperature to 1000 °C at a heating rate of 10°C min-1 to determine the temperature range of the perovskite phase formation. Phase purity check up and structural characterisation was carried out by X-ray diffraction (XRD) with Cu Kα radiation using PHILIPS X-ray Diffractometer. Unit cell dimensions of the crystal structure were refined by Rietveld (Le-Bail fitting) method using X-pert high score plus program. Morphology and crystal structure of the perovskites along with selected area electron diffraction (SAED) patterns were examined through transmission electron microscopy (TEM) using a JEOL JEM 2100. Electrical behavior were studied by impedance measurement using a computer controlled impedance analyzer in the frequency ranging from 50 Hz to 10 MHz. For better ohmic contact a high temperature curing silver paste was applied on both faces of the pellet.
Thermal Analyses using TG/DSC
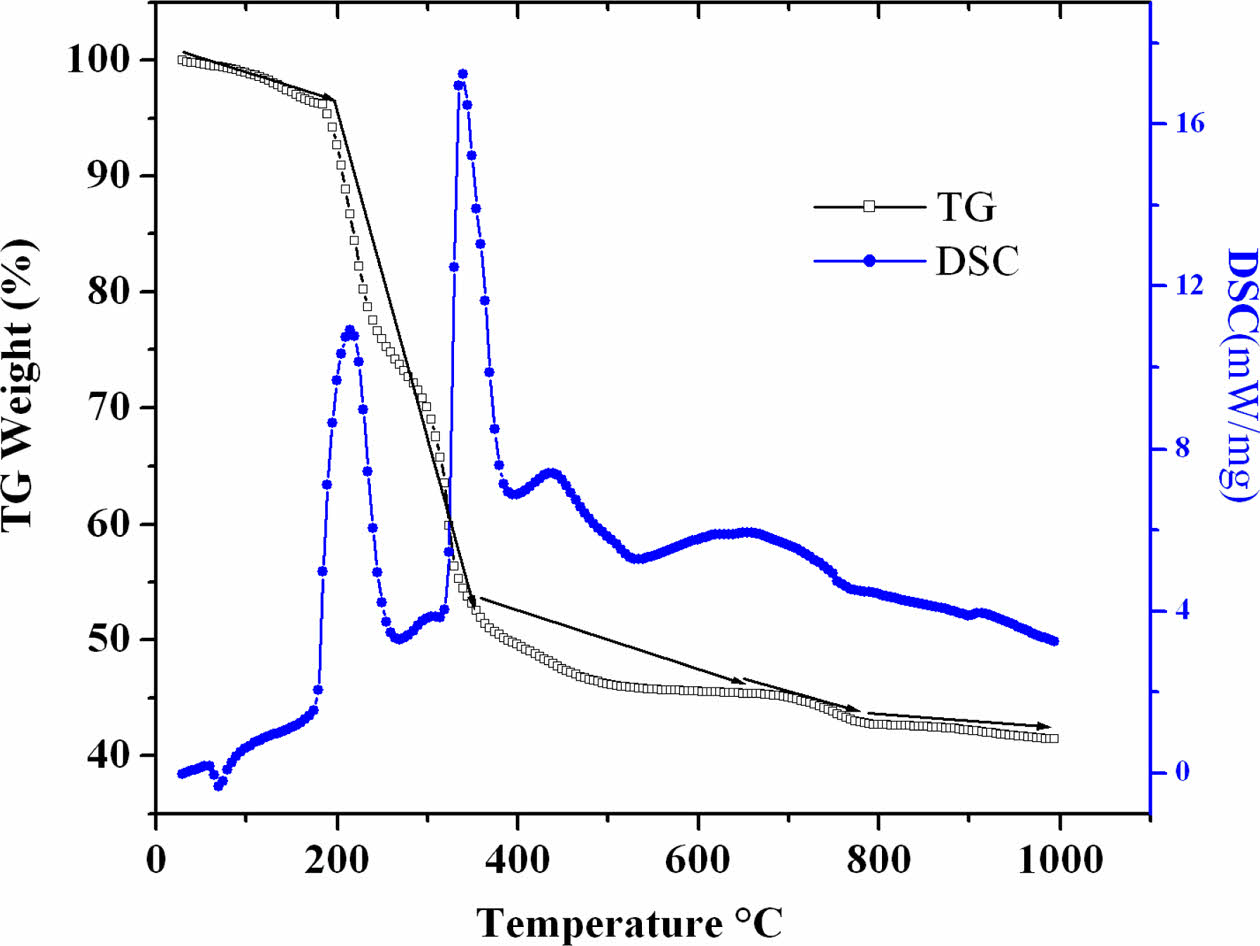
The TG/DSC analysis of the dried gel obtained during sol-gel synthesis (Fig. 1) shows the weight loss in several steps. In the TG curve the weight loss from room temperature to 200 °C is due to the removal of adsorbed and structural water from the sample with an exothermic peak in DSC curve. The second stage between 200 and 340 °C is also accompanied by an exothermic peak in the DSC curve and the weight loss is due to the decomposition of acetate and citrate complexes. The third stage in the range from 340-660 °C, represents the loss of organics and carbon dioxide from metal oxides. In this temperature range the DSC curve exhibits an exothermic peak. The fourth stage from 660-790 °C is due to the formation of oxides, concern to a small endothermic peak in DSC curve. After 790 °C there is no significant mass change in the TG curve but a small exothermic peak was observed in DSC curve. This infers that the proper crystallization of Ba0.8K0.2O3 takes place after 915 °C. Therefore the dried gel was calcined at 1000 °C for 4 h to obtain a phase pure doped BaSnO3 structure.
Powder X-ray diffraction (XRD) and crystal structure
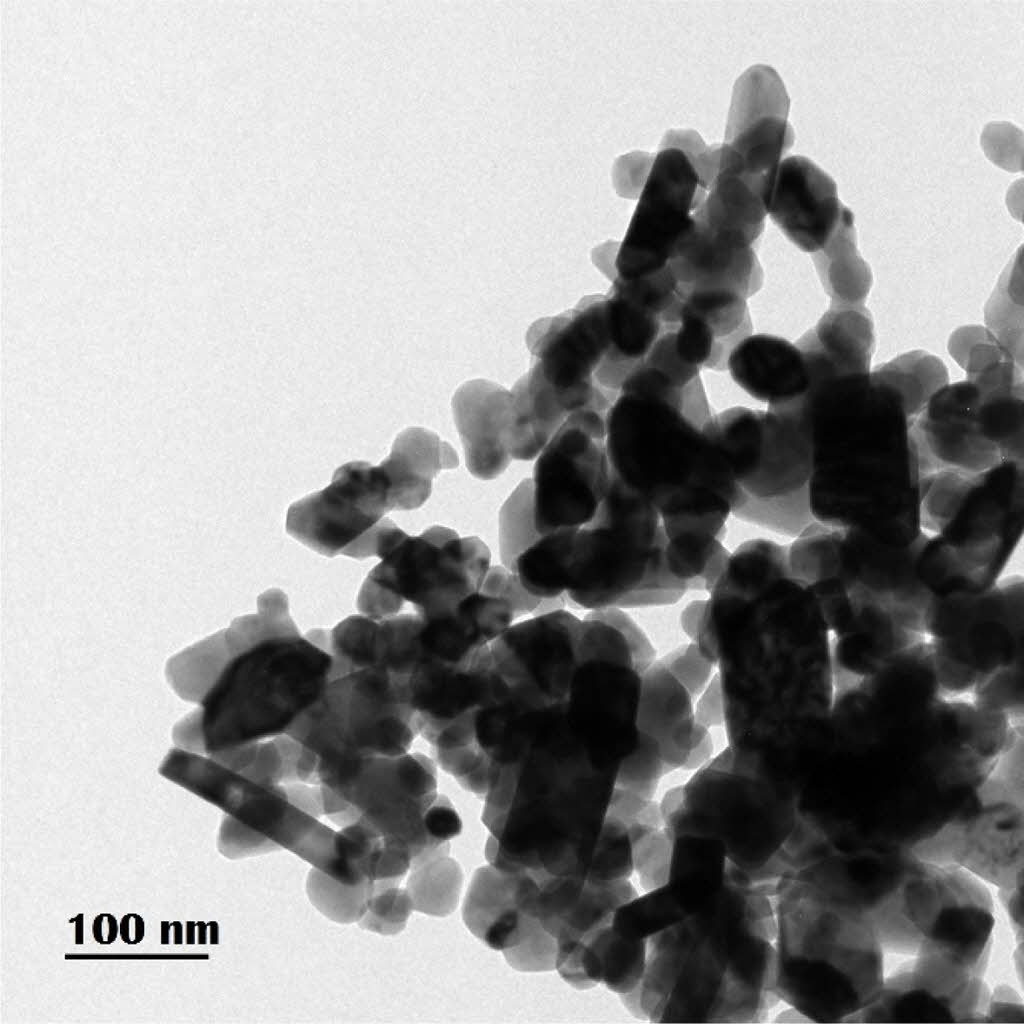
Powder XRD pattern of sintered Ba0.8K0.2O3 pellet is shown in Fig. 2. The sharp distinct peaks show the high crystalline nature of the perovskite without any additional phases. All the peaks are indexed according to a cubic crystal structure. This pattern is similar to that of earlier reported XRD patterns of cubic perovskite BaSnO3 [7] and also in good agreement with the structure given in JCPDS database (JCPDF No. 150-780).
TEM observations
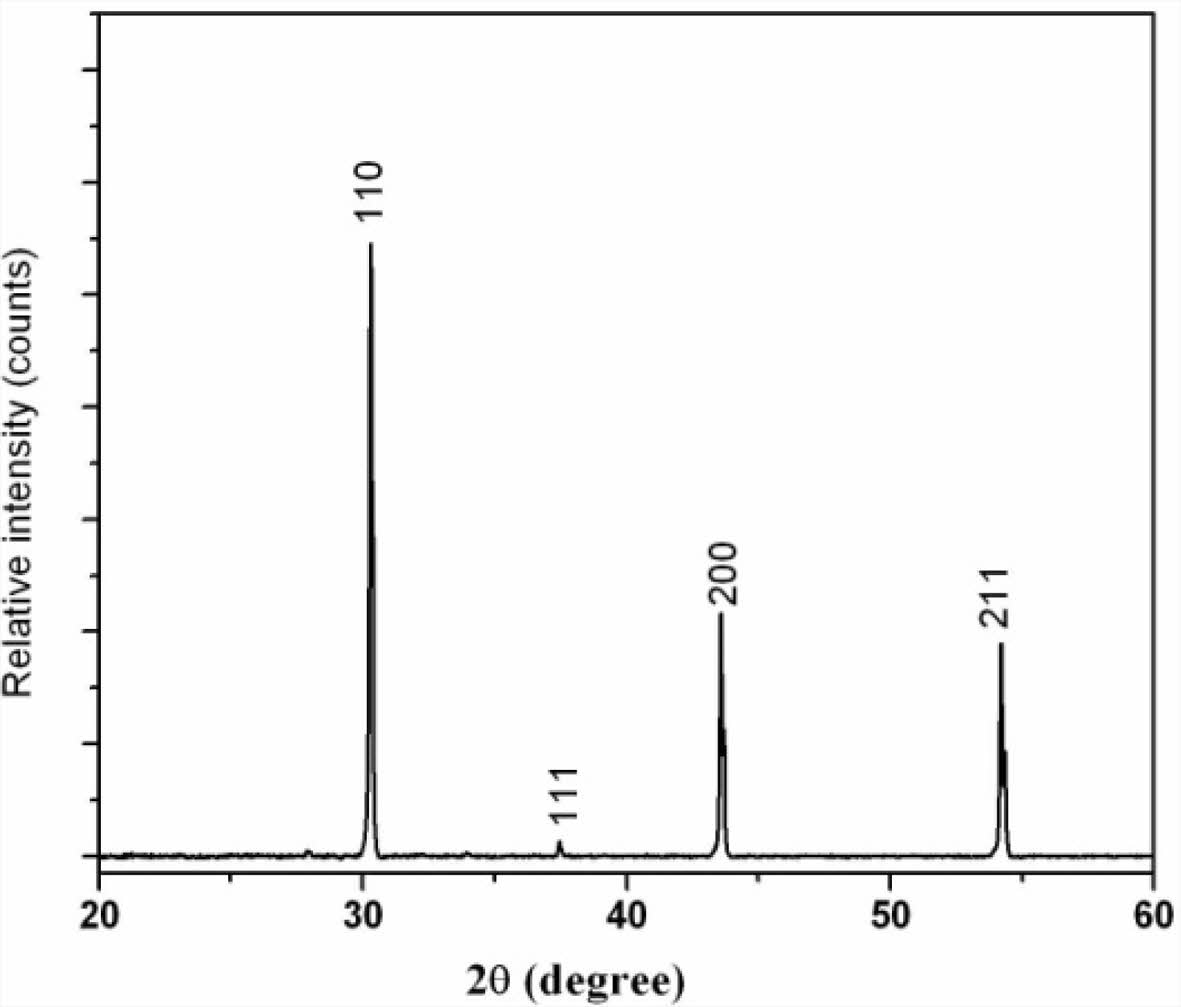
HRTEM image shown in Fig. 3a is taken from an edge of a powder particle obtained by crushing the sintered Ba0.8K0.2O3 pellet. The spacing between the lattice fringes shown in this image can be interpreted based on the crystal structure obtained through XRD.
Total conductivity studies
Impedance analysis and conductivity studies
Impedance analysis was carried out to study the total charge transport in the material. This method involves application of low amplitude ac frequency signal over the sample and taking apart the real and imaginary parts of impedance [8]. The impedance measurements were carried out in the frequency range of 10 Hz to 10 MHz in the temperature range of 30-750 °C.
The total conductivity is calculated using the following equation

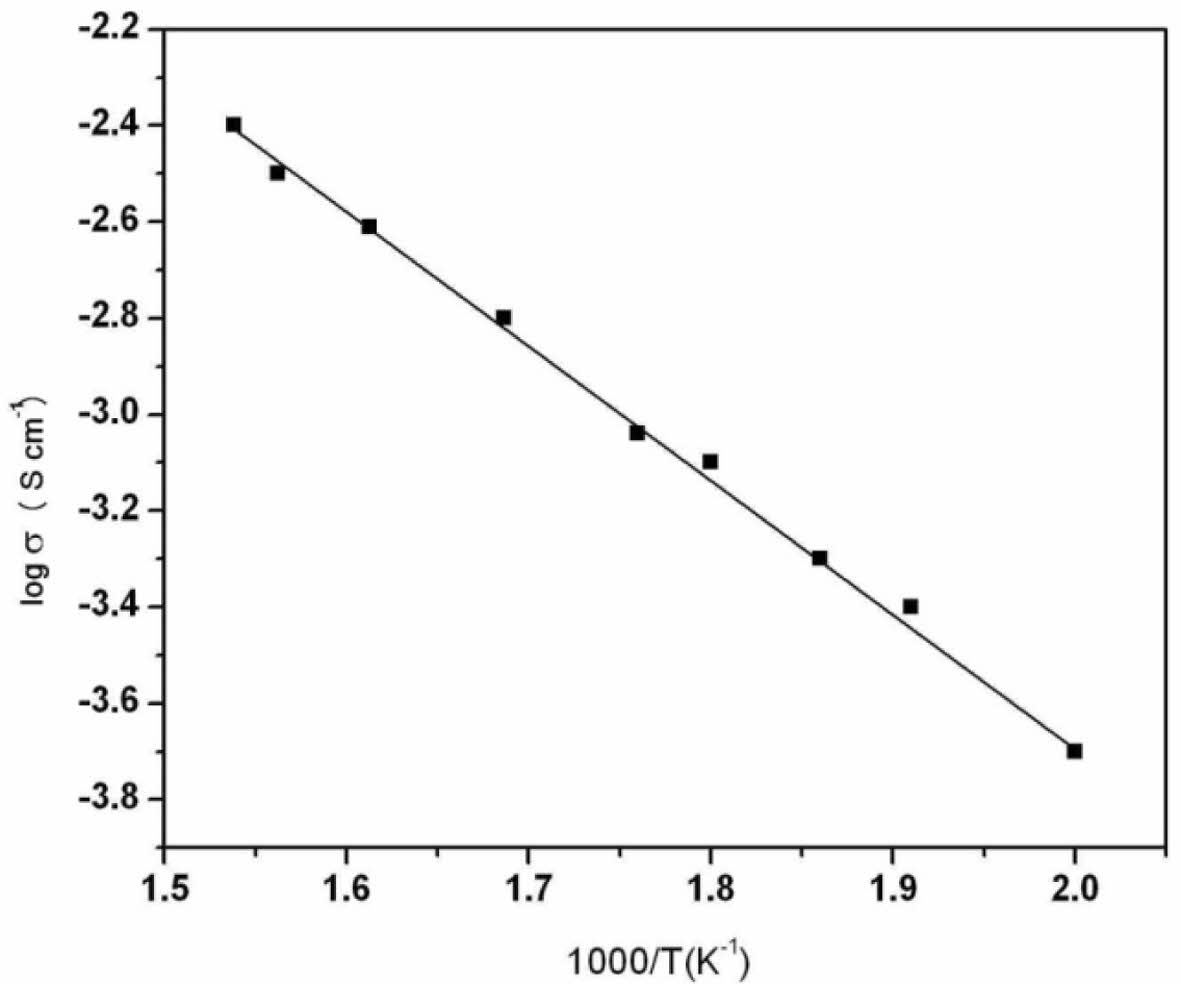
were Rb is the bulk resistance of the sample, l the thickness of the sample and A- the area of the sample. Fig. 5 shows the total conductivity (log σ) as a function of inverse of absolute temperature (1000/T). The total conductivity data of the compound reveals that it is semiconducting in nature. The nature of variation obeys Arrhenius equation

where Ea is the activation energy, T is the absolute temperature, σ0 is the pre-exponential factor and KB is the Boltzmann constant. The maximum conductivity was found to be 3.63 × 10-3 Scm-1 at 650 oC. The Arrhenius relationship shows that conductivity is a thermally activated process [10] and the value of activation energy is calculated to be 0.50 eV. The conductance could be due to the hopping of ions between allowed sites. As explained in previous studies on the same perovskite [2], the major contribution of the conductivity is due to the inherent defects in the form of oxygen vacancies in the crystal. The number of vacancies possibly increases with increase in temperature thereby increasing the number of sites available for conduction. This results in an increase in total conductivity with increase in temperature.
|
Fig. 1 TG/DSC of BaSnO3 dried gel prepared by sol-gel method. |
|
Fig. 2 Powder X-ray diffraction of BaSnO3 sintered at 1000 °C for 4 h. |
|
Fig. 3 (a) TEM image of a doped BaSnO3. |
|
Fig. 4 Variation of log conductivity with respect to reciprocal temperature for BaKSnO3. |
Phase pure BaSnO3 was prepared by chemical method. XRD pattern confirms the formation of cubic perovskite structure. The impedance analysis shows that the conductivity increases with increase in temperature.
- 1. Y. Wang, A. Chesnaud, E. Bevillon, and G. Dezanneau, Solid State Ion. 214 (2012) 45-55.
-

- 2. J. Cerda, J. Arbiol, G. Dezanneau, R. Dı´az, and J. R. Morante, Sens. Actuators B Chem. 84 (2002) 21-25.
-

- 3. S. Upadhyay, O. Prakash and D. Kumar, J. Mater. Sci. 16 (1997) 1330-1332.
-

- 4. T. Maekawa, K.Kurosaki, S. Yamanaka, J. Alloys Compd. 416[1-2] (2006) 214-217.
-

- 5. J. Cerda, J. Arbiol, R. Diaz, G. Dezanneau, and J. R. Morante; Mater. Lett. 56 (2002) 131-136.
-

- 6. L. Li, and J. C. Nino, Int. J. Hydrogen Energy 38[3] (2013) 1598-1606.
-

- 7. W. Lu, and H. Schmidt, Ceram. Int. 34 (2008) 645-649.
-

- 8. S. Tao, F. Gao, X. Liu, and O.T. Sorensen, Sens. Actuators B Chem. 71(2000) 223-227.
-

- 9. H. Ohta, M. Orita, M. Hirano, H. Tanji, H. Kawazoe, and H. Hosono, Appl. Phys. Lett.76 (2000) 2740.
-

- 10. M. Bradha,T. Vijayaraghavan, and A. Ashok, Mater. Lett. 125 (2014) 187-190.
-

- 11. H. Tetsuka, Y. J. Shen, K. Tezuka, H. Imoto, and K. Wasa,J. Vac. Sci. Technol. A 24 (2006) L4-L6.
-

 This Article
This Article
-
2024; 25(6): 1052-1055
Published on Dec 31, 2024
- 10.36410/jcpr.2024.25.6.1052
- Received on Oct 2, 2024
- Revised on Dec 14, 2024
- Accepted on Dec 14, 2024
 Services
Services
Shared
 Correspondence to
Correspondence to
- De Wu
-
School of Environment and Resources, Taiyuan University of Science and Technology, 030024, Taiyuan, China
Tel : +086 13603510228 - E-mail: 385838111@qq.com






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.