- Design and performance study of high-reliable alumina ceramic composites for sports fitness equipment
Dandan Zhao*
Xinxiang Vocational and Technical College, Xinxiang, Henan 453006, China
This article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Alumina ceramics have been identified as a potential material for fitness equipment due to their unique properties like hardness, high wear resistance, and chemical stability. The brittle nature of alumina ceramics limits their use in areas where toughness and impact resistance are essential. To meet the high toughness and crack resistance requirements of alumina, researchers have searched for possible composite structures mimicking natural material. In this study, alumina ceramic composites were prepared using advanced materials like hexagonal boron nitride (hBN) and graphene oxide (GO) platelets, which can support stress redistribution, energy dissipation, and crack deflection at the interfaces. The spark plasma sintering temperature for the alumina-BN or GO composite preparation was optimized as 1400 °C. The mechanical properties of the composites were improved compared to the control alumina matrix. The phase of BN and GO in the alumina matrix remained stable during the SPS process, while at higher concentrations, GO transformed into a more ordered graphitic structure and contributed to better mechanical properties. The microstructure analysis confirmed the uniform distribution of BN and GO platelets in the alumina matrix. The novel alumina composites prepared using BN or GO in the present study are ideal for the manufacturing of ceramic material-based fitness equipment.
Keywords: Alumina ceramic, Composite, Boron nitride, Graphene oxide, Sports Fitness equipment.
The fitness equipment industry demands advanced durable materials that tolerate repetitive stress and varying loads. The metals and polymers used in current fitness equipment provide ideal strength, toughness, and ductility. These conventional materials lack the thermal stability, wear resistance, and durability offered by ceramics. The use of alumina ceramics in fitness equipment material is an emerging area due to their hardness, high wear resistance, and chemical stability [1]. The toughness and impact resistance are critical criteria for the manufacturing of fitness equipment and the inherent brittleness of alumina limits its applications in this sector. The plastic deformation of ceramic materials before fracture is restricted by the atomic structure and causes fracture under high-stress conditions [2]. Recent research focuses on innovative structural designs and modifications of alumina ceramics to enhance toughness and damage resistance, addressing its inherent limitations.
The composites of alumina ceramics with improved toughness and damage resistance are emerging as promising materials for fitness equipment. Biomimetic structural designs, inspired by natural materials such as bone, nacre, wood, etc., are suggested for making alumina ceramic composites with exceptional mechanical properties [3]. The introduction of a layered structure, fibers, and graded interfaces in the ceramic matrix overcomes the inherent brittleness of alumina ceramics. Such material design can significantly improve the toughness and damage resistance of alumina ceramics and make them more suitable for fitness equipment. In composites, fibers carry and distribute the loads, interfaces in layered structures can absorb and redirect stress, and graded interfaces distribute stress more evenly [4]. The biomimetic designs involve combinations of stress redistribution, energy dissipation, and crack deflection at the interfaces. These mechanisms provide toughness and damage resistance to alumina ceramic materials. In addition to structural designs, the integration of materials that can respond to stress and self-heal cracks is becoming popular in alumina ceramic composite research [5].
The addition of Hexagonal boron nitride (hBN) to the alumina matrix is a promising approach to improving the material’s toughness, damage resistance, and self-healing potential. Hexagonal boron nitride, when incorporated into the alumina matrix, acts as a lubricating agent due to its layered structure. The layered structures can slide over each other and reduce the friction at the crack surface, arresting the propagation of cracks [6]. During high-temperature oxidation, BN forms boron oxide that seals cracks and acts as a self-healing component in composites [7]. The synergetic effects of these mechanisms due to the presence of BN in the ceramic matrix are vital for the development of high-toughness, crack-resistant durable materials. The exceptional mechanical properties of two-dimensional materials are widely utilized in various composites [8]. The incorporation of GO in the alumina matrix improved the composite’s mechanical strength, toughness, and fracture resistance. GO sheets dispersed in the matrix create a network within ceramic materials. They absorb and redistribute stress created within the material, preventing crack formation and propagation [9].
The optimization of spark plasma sintering (SPS) process conditions is a critical step in determining the final properties of alumina composite materials. The optimum sintering temperature is crucial for obtaining an alumina composite material with ideal mechanical properties [10]. The development of a high-performance alumina composite material can replace conventionally used materials in the fitness equipment industry with high-strength and durable products. Innovative alumina material designs, composites, and processing conditions are essential for obtaining a balance between hardness, toughness, and other desirable properties of materials. The current study fabricated alumina-matrix composites with different concentrations of BN or GO. The sintering temperature was optimized for manufacturing composites through the SPS process. The phase changes of BN and GO in the alumina matrix during the sintering process were evaluated. Mechanical properties and microstructural features of the composites were compared with the control matrix material.
Materials
Nextel™ 610 (Al₂O₃ fibers, 10-12 µm, Shanghai Dashai New Materials Co., Ltd) serves as the primary reinforcement in the composite. Alumina (Al₂O₃) Nanopowder (80-120 nm, Suzhou Lida High Tech Special Materials Co., Ltd) acts as the matrix material, filling the spaces between the fibers. Alumina Sol (AlOOH, 3 nm, Dalian Snow Chemical New Materials Technology Co., Ltd) aids in bonding the fibers and matrix together. Hexagonal Boron Nitride (h-BN, 7 µm, Shanghai Shinori New Materials Co., Ltd) is added as a solid lubricant for toughness and crack-healing properties. The Graphene Oxide (GO) solution (Shanghai Shinori New Materials Co., Ltd) combined with Polyvinyl Alcohol (PVA) and Polyethyleneimine (PEI), further contributes to toughness and the crack-healing mechanism.
Composite Preparation
For BN-based alumina matrix composites, the alumina base matrix was prepared first, consisting of Alumina nanopowder, alumina sol, and alumina fiber (Nextel™ 610) in a ratio of 70:20:10. Subsequently, two types of composite mixtures were made by incorporating 5% and 10% BN powder into the alumina matrix. The components were carefully blended in a pre-grinding machine (MTI Corporation) to ensure that the fibers were uniformly distributed throughout the matrix without being damaged. Dried the alumina matrix-BN mixture in a drying oven at a moderate temperature, such as 80 °C, to remove any excess moisture. For GO-based alumina matrix composites, dispersed the required amount of GO in isopropyl alcohol to create a homogeneous solution. Used an ultrasonic bath to achieve proper dispersion. Gradually added 1% polyvinyl alcohol (PVA) to the GO solution while stirring continuously until fully dissolved, then incorporated 1% polyethyleneimine (PEI) into the GO-PVA solution. Gradually add the prepared solution to the dried alumina base matrix, which contains alumina nanopowder, alumina sol, and alumina fiber (Nextel™ 610) in a ratio of 70:20:10. Two types of composite mixtures were then prepared with 5% and 10% GO by weight in the alumina base matrix. Stirred continuously to ensure uniform dispersion of the alumina particles in the solution and to achieve even distribution of the fibers throughout the slurry, preventing clumping. The slurries were then maintained at 110 °C for 24 hours, after which the dried powders were used for further processing. The alumina powder–alumina sol–Nextel™ 610 fibers mixture (70:20:10) was used as a control to compare the performance of the BN or GO in the final composite materials.
Finally, the mixed powder was placed in a graphite die with an inner diameter of 20 mm and a thickness of 3 mm, and the mixture was sintered using an SPS apparatus (Shanghai Sile Instrument Co., Ltd). The sintering process proceeded as follows: the temperature was raised to 1400 °C at a rate of 50°C/min, then increased to 1600 °C at a rate of 10 °C/min. During the SPS process, the pressure was gradually increased to a final holding pressure of 50 MPa, with a holding time of 15 minutes. Once the process was completed, the sample was cooled to room temperature under a pressure of 50 MPa for 30 minutes. After completing the above steps, a disc-shaped sample with a diameter of 20 mm and a thickness of approximately 2 mm was produced. The linear shrinkage of the specimens during the SPS process was monitored continuously.
Characterization of composites
The sintered samples were cut into bars, polished, and prepared for further characterization studies. The bulk densities of the samples were measured and then converted to relative densities based on their theoretical densities. The bulk density of the sintered samples containing 5 wt% and 10 wt% BN or GO, as well as the control sample, was measured using Archimedes' method in distilled water. Theoretical densities were calculated using the rule of mixtures, assuming densities of 3.98 g/cm³ for Al₂O₃, 2.25 g/cm³ for hBN, and 2.2 g/cm³ for GO. The phase analysis of the powders and sintered alumina-BN composites was conducted using an X-ray diffractometer (XRD, D8 ADVANCE, Bruker, Germany) equipped with a Cu Kα X-ray source. A Raman spectrometer (DXR-3xi, Thermo Fisher, USA) with an excitation laser wavelength of 532 nm, covering the range from 100 to 3800 cm⁻¹, was used to perform phase analysis on the alumina-GO composites. Young's modulus was measured on polished test bars (2 mm width × 4 mm thickness × 20 mm length) using the resonant frequency method with a Buzz-o-sonic Lab Kit 5.9 (BuzzMac International LLC), with a minimum of five readings taken for each sample. The fracture toughness of the samples (2 × 4 × 25 mm with a 2 mm notch) was measured using the single-edge V-notched beam (SEVNB) method, with a span length of 20 mm and a crosshead speed of 0.05 mm/s. Five samples were tested for each material. Flexure strength was determined using the three-point bending method on bar specimens measuring 2 mm × 4 mm × 25 mm. The tests were performed with a span length of 20 mm and a crosshead speed of 0.05 mm/min, with five bars tested for each material. The hardness of the sintered compacts was measured using a Vickers hardness tester (MVS-1000IMT2) with a 1 kg applied load and a 10-second dwell time. Each sample was tested with at least five indentations to calculate an average value. The microstructure of the composites was examined using a field emission scanning electron microscope (FESEM) (SEM, Apreo S Hivac, FEI, USA) equipped with an energy-dispersive X-ray spectrometer (EDS).
The use of alumina ceramics in fitness equipment materials is an emerging area due to their hardness, high wear resistance, and chemical stability [1]. The toughness and impact resistance are critical criteria for the manufacturing of fitness equipment, and the inherent brittleness of alumina limits its applications in this sector. Incorporating toughening and self-healing components into alumina ceramics provides damage resistance to materials and enhances properties suitable for application in fitness equipment [3]. This study proposes a novel composite preparation of alumina ceramic with boron nitride and graphene oxide, using spark plasma sintering at an optimized temperature. The phase changes of the additives (BN or GO) and the composites’ mechanical properties and microstructure were evaluated.
Determination of optimal sintering temperature
Spark plasma sintering (SPS) is an effective method for preparing alumina ceramic composite materials. Its short densification time and high localized temperature help limit undesired grain growth during the process. The spark plasma sintered composites typically result in near-fully dense bodies, which is expected due to the distinct advantages of the SPS process, including the vacuum environment and applied pressure [10]. The optimal sintering temperature must be high enough to achieve full densification but low enough to avoid unacceptable phase formation. The sintering temperature of alumina typically ranges from about 1200 °C to 1700 °C and strongly depends on factors such as the starting powder’s grain size, purity, and other sintering conditions [11]. When fabricating composites using Nextel™ 610 fibers, alumina powder, alumina sol, and boron nitride (BN) or graphene oxide (GO) through SPS, the density of the resulting materials is significantly influenced by the sintering temperature and the amount of reinforcing agents incorporated. This study evaluated the optimal sintering temperature for the alumina matrix composite with BN or GO. The SPS process was carried out over a wide range of temperatures to establish the optimal sintering conditions for all the composites investigated. The sintering temperature for the current composites was optimized by assessing the shrinkage and relative density of the composites obtained during the SPS process.
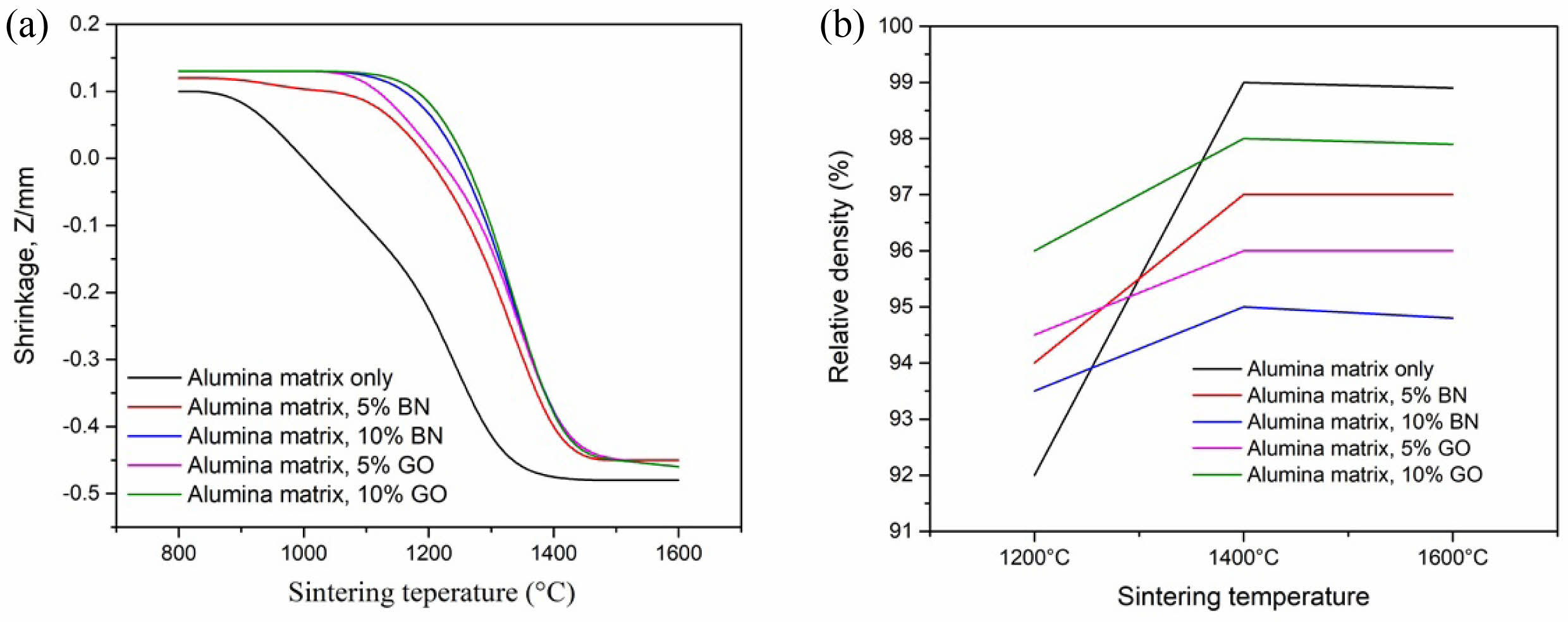
The shrinkage behavior of the composites varies with temperature across different compositions. Fig. 1a shows the shrinkage of various composites from 800 °C to 1600 °C. The shrinkage of the alumina matrix begins at 1100 °C and concludes at 1350 °C. In composites containing BN and GO, the starting temperature for shrinkage is higher than that of the pure alumina matrix and increases with the percentage of these additives. The alumina matrix with 5% BN and GO starts shrinking at 1200 °C and stabilizes at 1400 °C. For composites with 10% BN and GO, shrinkage begins at 1250 °C and then stabilizes at 1400 °C. No further significant shrinkage was observed in the composites between 1400 °C and 1600 °C. Based on our experiment, the ideal sintering temperature for the alumina matrix and composites is around 1400 °C, with the shrinkage starting temperature increasing as the BN and GO content increases.
The composite materials were further characterized for their relative density to assess the sintering performance. The relative density of the composites at different sintering temperatures of 1200 °C, 1400 °C, and 1600 °C was evaluated (Fig. 1b). The overall density of composites decreases at 5 and 10% BN loading due to the lower density of BN than alumina. The theoretical density of the alumina matrix alone is approximately 3.9 to 3.95 g/cm³. The composite with 5% BN decreased the theoretical density to 3.85 g/cm³. The density again reduced to 3.75 g/cm³ at 10% BN content. Archimedes' method measured the actual density of composites prepared at sintering temperatures of 1200 °C, 1400 °C, and 1600 °C, and the relative density was calculated. The relative density of the alumina control matrix reached up to 99% at 1400 °C. The 5% and 10% BN alumina composites achieved a maximum density of 97% and 95%, respectively, at 1400 °C. The addition of GO (2.2 g/cm³ density) into the alumina matrix reduced the density of the composites based on the volume fraction. Alumina-GO composites showed better densification at a sintering temperature of 1400 °C. The relative density of 5% and 10% GO-alumina composites reached up to 98% and 96%, respectively.
A higher sintering temperature (1400 °C) leads to better densification in composites due to enhanced diffusion of components in the alumina matrix and better bond formation. The density reduction was more prominent in alumina composites with the BN than GO, and the overall density reduction was 95-98% at different concentrations. The density reduction was more distinct at a higher volume fraction of the BN or GO in the alumina matrix.
Evaluation of phase change in composites due to the SPS process
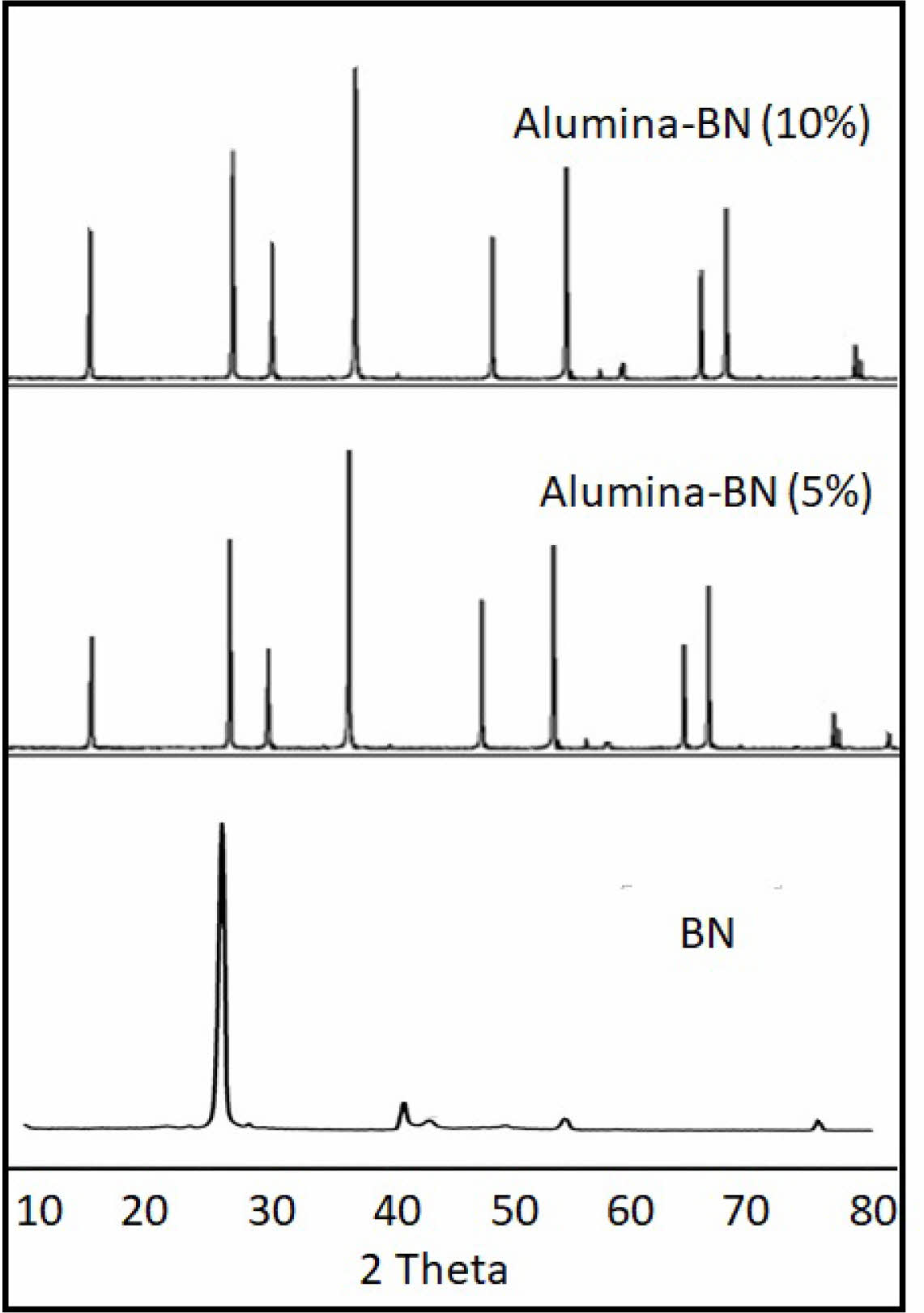
Alumina (Al₂O₃) materials are known for their high hardness and thermal stability, making them a popular matrix material in composites [12]. Hexagonal boron nitride (h-BN) is a widely used 2D material in composites due to its characteristics like thermal stability, chemical resistance, and lubricating properties. In normal atmospheric conditions, the h-BN is stable up to 1400 °C and undergoes phase transformations under specific conditions. Spark Plasma Sintering (SPS) is becoming more popular compared to conventional sintering due to its relatively low temperatures and shorter processing time. The high heating rates and applied pressure during the sintering process may influence the phase stability of the components [13]. At 1400 °C, chemical reactions that occur between h-BN and alumina are limited. Impurities in the powder materials or specific oxygen conditions promote reactions and change the phase of h-BN. The XRD spectrum of materials before and after the SPS process was evaluated to detect any phase changes in h-BN. The XRD profiles for the 5% and 10% BN composites did not show any additional peaks corresponding to phase changes in alumina and h-BN. The peaks indicate similar h-BN or alumina phases, which were observed before sintering (Fig. 2). The lack of additional XRD peaks confirms the stability of h-BN in the alumina matrix during the SPS process at 1400 °C.
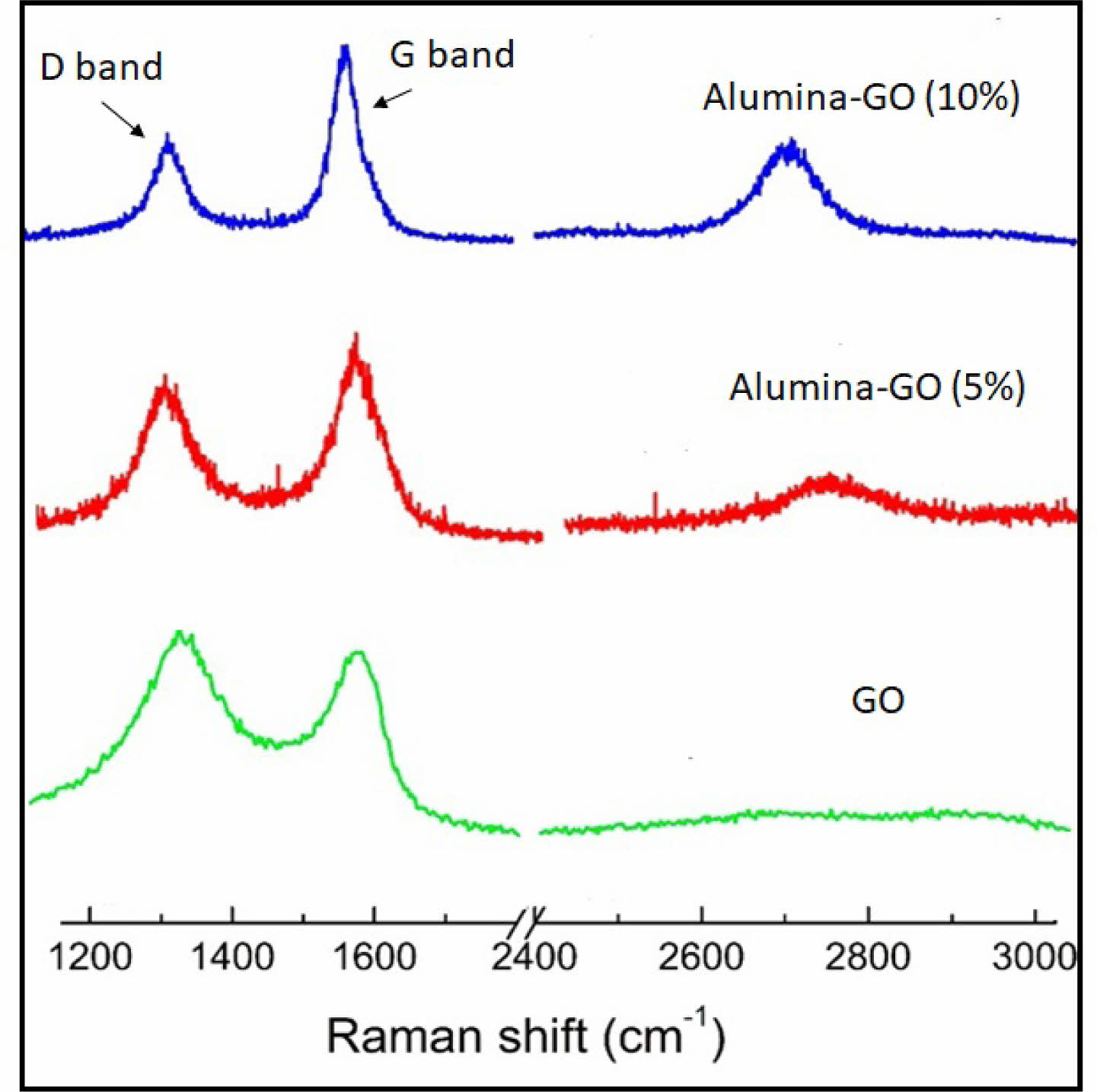
Graphene oxide (GO) is known for its lower thermal stability than h-BN and decomposition occurs at high temperatures. The oxygen functional groups in GO are the key accelerators of its decomposition [9]. At high temperatures, GO loses its oxygen groups and is reduced to graphene structures. The graphitization process continues at high temperatures and the matrix characteristics may influence these processes. The alumina matrix can act as a barrier to graphitization during the SPS process and may provide more stability to GO in the matrix. Even though the high temperature catalyzes the reduction process of GO, it may vary depending on the interaction with alumina and the other conditions. During SPS at 1400 °C, GO is expected to undergo a phase change and form more ordered graphitic structures. The degree of graphitization depends on factors like the duration of temperature, the pressure applied during SPS, the interaction with the alumina matrix, and the volume fractions used. Raman spectroscopy was used to confirm the phase change of GO in alumina composites. The degree of reduction and graphitization of GO components was analyzed by evaluating the pattern of the G-bands and the D-bands in composites. The D band is associated with defects in GO, and the higher intensity of the D band predicts the stable nature of GO. The G band is associated with sp² carbon in a more ordered graphitic structure [14]. The high intensity of the G band suggested the phase change of GO under the SPS conditions. The high intensity of the G band after the sintering process proved the phase change of GO to graphitic structures (Fig. 3). During SPS at 1400°C, the phase change was prominent in an alumina composite with 10% GO. The formation of more graphitized, ordered structures may contribute to better densification, as observed in the relative density of GO composites compared to BN composites (Fig. 1b). The differences in phase transformation potential between BN and GO at a sintering temperature of 1400 °C affect the other properties of BN- or GO-based composites.
Mechanical properties of composites
Young’s modulus
The elastic modulus (also known as Young's modulus)
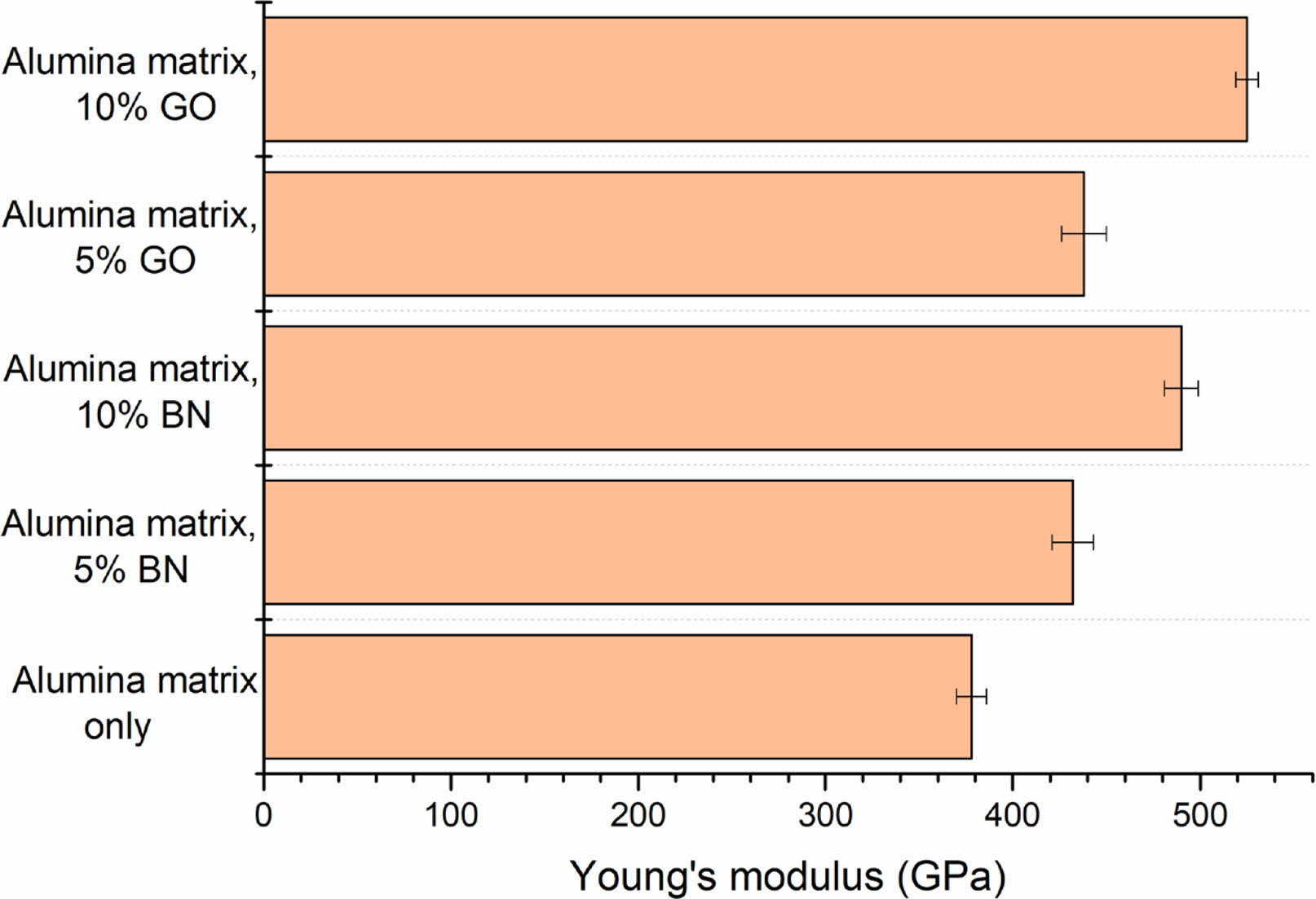
of a composite material reflects its stiffness, representing the material's ability to resist deformation under stress. For the composite made from an alumina matrix (Nextel™ 610 fibers, alumina powder, alumina sol) and boron nitride (BN) or graphene oxide (GO) sintered using Spark Plasma Sintering (SPS), the elastic modulus will be influenced by the individual properties of the constituents, their volume fractions, and the microstructure achieved through sintering. The elastic modulus of the composite increases with the addition of BN and GO due to their higher modulus compared to alumina [8]. However, this effect can be partially offset by optimizing the sintering temperature. Sintering at 1400 °C yields a higher elastic modulus due to better densification and reduced porosity.
The Young’s modulus of the control alumina matrix was 378 ± 8 GPa. The composites with 5% BN resulted in a lesser increase of the elastic module to the alumina matrix. The 10% BN content resulted in a higher Young’s modulus due to the significant distribution of BN in the alumina matrix. The alumina matrix with 5% and 10% BN shows Young’s moduli of 432 ± 11 GPa and 490 ± 9 GPa, respectively. A very high intrinsic elastic modulus of GO significantly contributes to Young’s modulus of composites. The 5% GO provided Young’s modulus of 438 ± 12 GPa to composites. Even in low concentrations, GO is well dispersed in the alumina matrix and an effective load transfer occurred from the alumina matrix to the GO sheets, and the elastic modulus of composites increased. Even though dispersion becomes more challenging, the higher concentrations of GO (10%) can further enhance Young’s modulus of composites. With 10% GO at 1400 °C, highly ordered graphene platelets may form, increasing the modulus of the composite material to up to 525 ± 6 GPa (Fig. 4).
Adding BN and GO generally increases the elastic modulus compared to the base composite of Nextel™ 610 fibers and alumina. The increase is more significant with 10% GO, assuming the results of phase changes and improved densification. Despite a decrease in the partial density of the composite with 10% BN, the high modulus of BN significantly raises the Young’s modulus of the composite. Both BN and GO particles can act as secondary phases, enhancing the overall stiffness of the composite material.
Fracture toughness
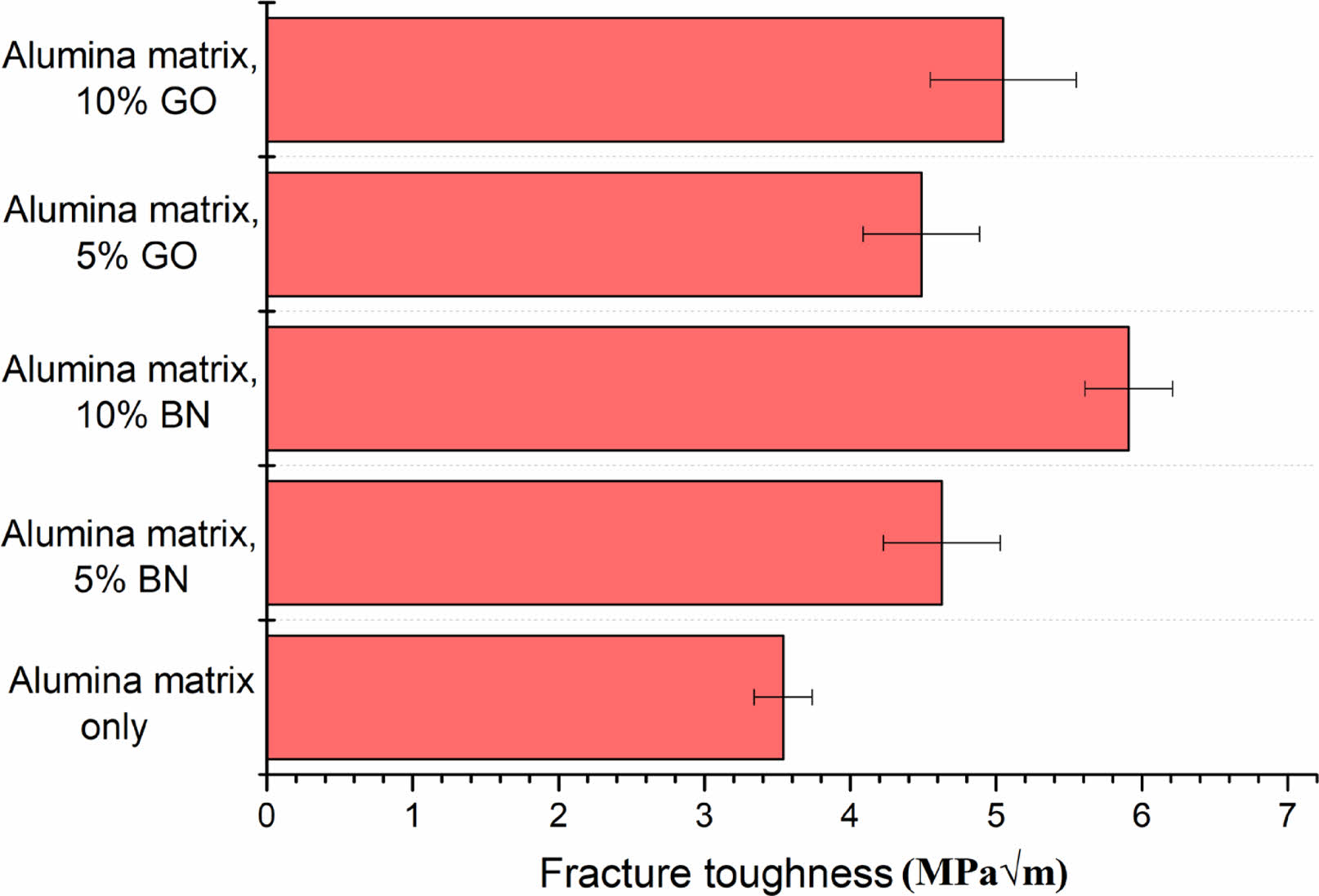
Fracture toughness is an important property demonstrating a material's resistance to crack propagation. The microstructure, the presence of reinforcement phases, and the degree of densification achieved during sintering affected the fracture toughness of composite materials [15]. The BN and GO incorporation in the alumina matrix significantly enhanced the fracture toughness of the alumina composites. The control alumina matrix showed a toughness of 3.54 ± 0.8 MPa√m. A 5% BN content in composite resulted in a slight increase in fracture toughness to 4.63 ± 0.4 MPa√m. Even though BN is well dispersed at 5%, its limited crack deflection potential was not enough to cause significant variations in toughness. The maximum fracture toughness was observed at 10% BN content and the value was 5.91 ± 0.7 MPa√m, 60% higher than the control alumina matrix. During crack propagation, toughening mechanisms like crack deflection and energy absorption functioned well and improved the performance of composites. The higher concentrations of BN might also introduce more interfaces and bond well to the alumina matrix during sintering, which could impact the overall toughness positively (Fig. 5).
Graphene oxide (GO) improves fracture toughness by creating bridges across cracks, deflecting cracks, and arresting crack growth. At 5% GO, the reinforcement provided by graphene oxide results in a moderate fracture toughness of 4.49 ± 0.4 MPa√m. Increasing the GO content to 10% is expected to further enhance fracture toughness due to increased crack bridging and deflection. However, if GO becomes highly ordered due to phase changes, it could lead to the formation of highly stiffened areas that might serve as crack initiation sites, potentially negatively impacting toughness. The alumina composite with 10% GO showed a fracture toughness of 5.05 ± 0.5 MPa√m. The highest fracture toughness was 5.91 ± 0.7 MPa√m for the alumina–BN composite containing 10 vol% BN sintered at 1400 °C (Fig. 5).
Flexure strength
Flexural strength (or bending strength), is a key mechanical property for composite materials, particularly those proposed for structural applications. The various factors affecting the bending strength are the material composition, sintering conditions, and the presence of reinforcing materials such as fibers and particulates [16]. The alumina matrix had a strength of 420 ± 5 MPa and all tested samples showed better strength than the alumina matrix. The flexural strength of composites with 5% BN was 520 ± 7 MPa. The lubricant action of BN, due to its layered structure, reduces friction during crack propagation and potentially increases the bending strength. The BN absorbs more energy before the failure of composite materials. The flexural strength reached a maximum of 580 ± 11 MPa at 10% BN loading. At higher wt%, BN distributed well in the alumina matrix and formed more interfaces within the composite. The well-distributed BN leads to increased bending strength due to enhanced load transfer between the BN interfaces and alumina matrix (Fig. 6).
The flexural strength of the alumina matrix composite with 5% GO was 522 ± 6 MPa, and with 10% GO, it was 590 ± 12 MPa. The 5% GO concentration is likely optimal for balancing improved flexural strength while maintaining good dispersion within the matrix. A higher GO content (10%) is expected to further enhance bending strength due to improved toughening mechanisms. However, a phase change and ordered graphitic structures at higher sintering temperatures (1400 °C) led to a flexural strength higher than BN composites. The highest bending strength, 590 ± 12 MPa, was observed for the alumina ceramic composite with 10% GO (Fig. 6).
Hardness
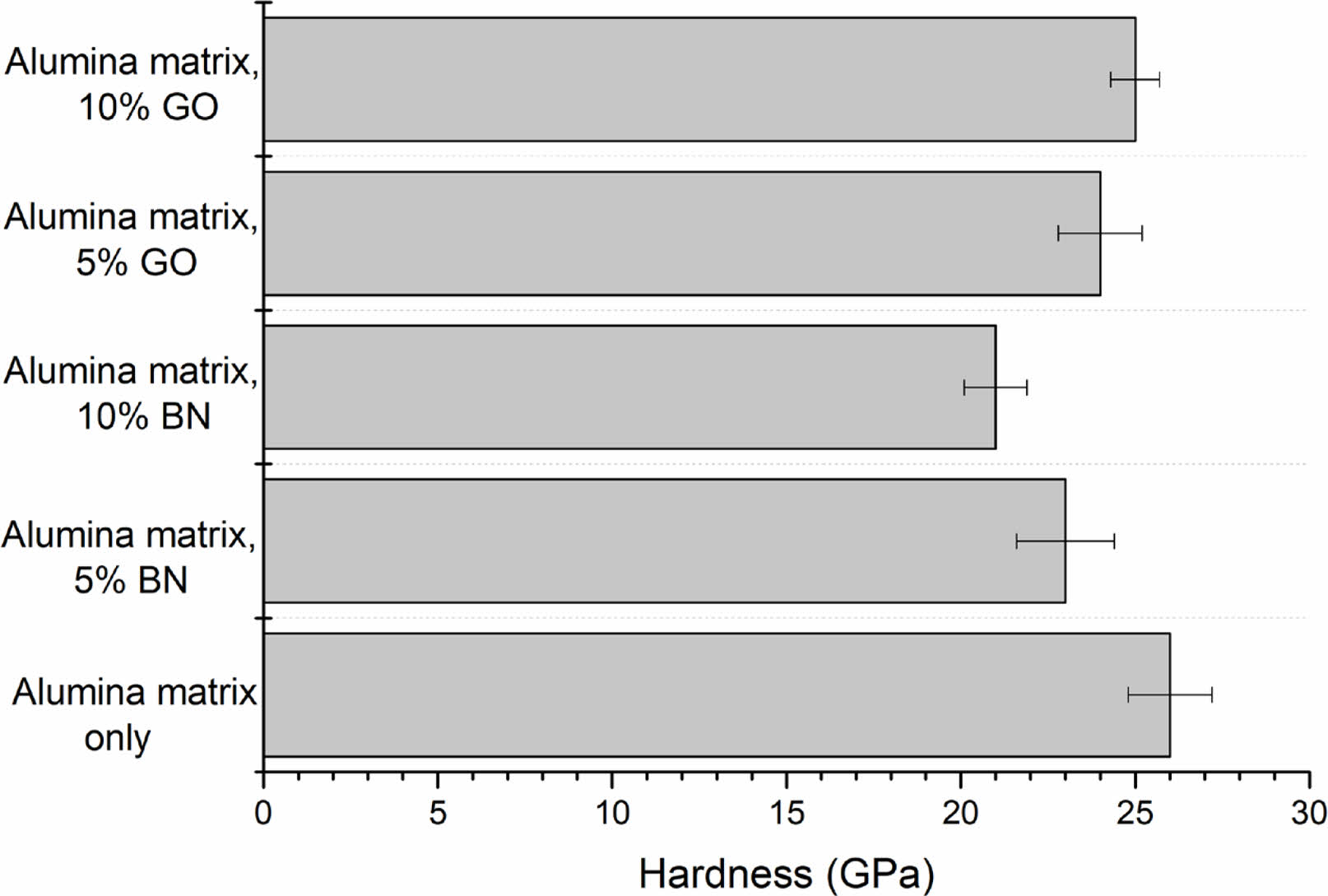
Boron nitride (BN) is well-known for its lubricating properties and has comparatively low hardness related to alumina [17]. The alumina matrix sintered at 1400 °C showed a high hardness of 26 ± 1.2 GPa. The toughness of the composite with 5% BN was improved without reducing the hardness, which was 23 ± 1.4 GPa. The alumina phase dominated the composite matrix. The hardness of the composite was significantly changed at 10% BN content. The weak interfaces formed due to more BN content and its softer nature reduced the overall hardness of the composite to 21 ± 0.9 GPa.
Graphene oxide (GO) strengthens the alumina matrix by controlling load transfer between alumina particles and enhancing the hardness of the composite. The present study showed a minor decrease in hardness (24 ± 1.2 GPa) with 5% GO. However, more GO makes phase changes during the sintering process, which may affect grain boundary movement and enhance hardness. The hardness of the composite was reduced to 25 ± 0.7 GPa at 10% GO (Fig. 7).
Microstructure analysis of composites
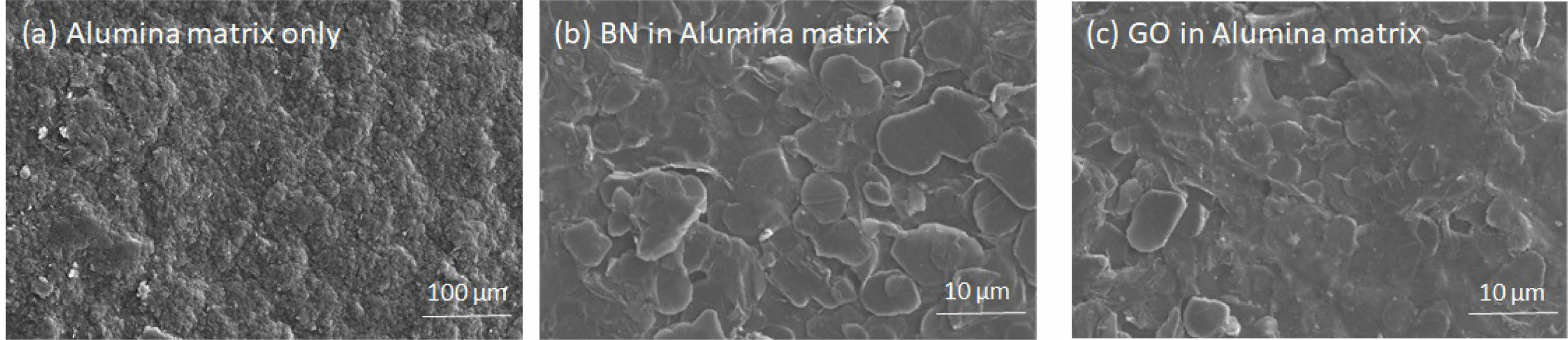
Field emission scanning electron microscopy (FESEM) was used to analyze the microstructures on the surface of alumina-BN or GO composites. Large grains and abnormal grain growth was observed on the surface of control alumina matrix (Fig. 8a). The addition of BN or GO in the alumina matrix refined the grain structure pattern, and small gains were prominent in composites. The grain size decreased with the increase of the mass fraction of BN or GO. The smaller and more uniform grains were observed in composites containing 10% BN or GO than 5%. The changes in the grain growth pattern support the toughening of the composite material through BN or GO addition [18]. The BN or GO platelets are embedded within the Al₂O₃ matrix or distributed at grain boundaries (Fig. 8b & c). Interfaces in layered structures can absorb and redirect stress [19]. The BN or GO platelets act as stress management centers and block crack propagation in composites. The interlayered structure of BN or GO decreases relative density in composites compared to the pure Al₂O₃ matrix. This indicates that a moderate amount of BN or GO effectively toughens the alumina ceramic matrix. Energy-dispersive X-ray spectroscopy (EDX) studies further confirm the homogeneous dispersion of BN/GO platelets within the alumina matrix (Fig. 9).
|
Fig. 1 Effect of sintering temperature on (a) shrinkage of alumina-BN or GO composites at 1000 to 1600 °C, (b) relative density of alumina-BN or GO at 1200, 1400 and 1600 °C. |
|
Fig. 2 XRD spectrum of BN and alumina-BN composites after sintering at 1400 °C. |
|
Fig. 3 Raman spectrum of GO and alumina-GO composites after sintering at 1400 °C. |
|
Fig. 4 Young’s modulus of Alumina - BN or GO composites with different wt%. |
|
Fig. 5 Fracture toughness of Alumina - BN or GO composites with different wt%. |

|
Fig. 6 Flexure strength of Alumina - BN or GO composites with different wt%. |
|
Fig. 7 Hardness of Alumina - BN or GO composites with different wt%. |
|
Fig. 8 SEM images of (a) alumina matrix and alumina composite with (b) BN, (c) GO. |

|
Fig. 9 SEM EDX images of (a) Al in alumina matrix, (b) nitrogen in BN-alumina composite and (c) carbon in alumina-GO composite. |
The composites of alumina ceramics with improved toughness and damage resistance are emerging as promising materials for fitness equipment. Biomimetic structural designs are suggested for making alumina composites with enhanced toughness and damage resistance. The current study separately used boron nitride and graphene oxide sheets in an alumina matrix to reduce inherent brittleness and improve crack resistance. Under optimized SPS conditions, the composite showed better mechanical properties. Higher concentrations of GO showed phase changes in the alumina matrix. The composite materials prepared in the current study are suitable for ceramic-based fitness equipment.
- 1. T.A. Otitoju, P.U. Okoye, G. Chen, Y. Li, M.O. Okoye, and S. Li, J. Ind. Eng. Chem. 85 (2020) 34-65.
-

- 2. F.O. Braga, P.H.L.M. Lopes, M.S. Oliveira, S.N. Monteiro, and E.P. Lima Jr, Compos. B. Eng. 166 (2019) 48-55.
-

- 3. D.E. Tria, A. Kouadria, J. Małachowski, Y. Bouteghrine, and A. Manaa, Proc. Inst. Mech. Eng. L. 236[12] (2022) 2516-2538.
-

- 4. X. Zeng, Q. Jing, J. Sun, and J. Zhang, Materials. 16[6] (2023) 2296.
-

- 5. Z. Long and J. Ouyang, Comput. Intell. Neurosci. 2022(1), 7490051.
- 6. J. Wang, D. Lu, W. Xuan, Y. Ji, R. Chen, S. Li, W. Li, W. Chen, S. Tang, G. Zheng, and F. Long, J. Adv. Ceram. 13[4] (2024) 496-506.
-

- 7. S. Dhanasekar, A.T. Ganesan, T.L. Rani, V.K. Vinjamuri, M.N. Rao, E. Shankar, D.P.S Kumar, and W.M. Golie, Adv. Mater. Sci. Eng. 2022[1] 6160591.
-

- 8. R. Yin, H. Wu, K. Sun, X. Li, C. Yan, W. Zhao, Z. Guo, and L. Qian, J. Phys. Chem. C. 122[3] (2018) 1791-1799.
-

- 9. B.L. Dasari, M. Morshed, J.M. Nouri, D. Brabazon, and S. Naher, Compos. B Eng. 145 (2018) 136-144.
-

- 10. S. Manafi, M. Ebrahimi, F.S. Bidabadi, and I. Mobasherpour, Ceram. Int. 45[13] (2019) 15928-15933.
-

- 11. J. Yu, Q. Zhao, S. Huang, Y. Zhao, Y. Zhou, J. Lu, L. Dong, and Y. Zhang, J. Alloys Compd. 879 (2021) 160346.
-

- 12. M.Y. Akram, V. Casalegno, M. Ferraris, G. Puchas, W. Krenkel, and S. Roszeitis, J. Eur. Ceram. Soc. 39[7] (2019) 2510-2517.
-

- 13. P. Klimczyk, P. Wyżga, J. Cyboroń, J. Laszkiewicz-Łukasik, M. Podsiadło, S. Cygan, and L. Jaworska, Diam. Relat. Mater. 104 (2020) 107762.
-

- 14. A.S. Shamshirgar, R. Ivanov, and I. Hussainova, Proc. Estonian Acad. Sci. 68[2] (2019) 140-144.
-

- 15. J.Y. Huang, J.C. Yuan, T.T. Zhu, T. Zhong, Y.F. Xu, and S.N. Luo, Ceram. Int. 48[24] (2022) 36371-36382.
-

- 16. J.J. Swab, W. Chen, J. Hogan, H. Liao, C. Lo, S. Mates, C. Meredith, J.J. Pittari III.
-

- 17. R. Rhorer and G.D. Quinn, J. Dyn. Behav. Mater. 7 (2021) 34-47.
-

- 18. J. Wu, H. Chen, X. Luo, B. Hu, S. Fu, D. Wan, Y. Bao, Q. Feng, S. Grasso, and C. Hu, Ceram. Int. 48[2] (2022) 2776-2781.
-

- 19. X. Lu, T. Dolmetsch, C. Zhang, Y. Chen, B. Boesl, and A. Agarwal, Ceram. Int. 47[10] (2021) 13970-13979.
-

- 20. W. Wang, G. Sun, Y. Chen, X. Sun, and J. Bi, Ceram. Int. 44[17] (2018) 21993-21997.
-

 This Article
This Article
-
2024; 25(5): 910-917
Published on Oct 31, 2024
- 10.36410/jcpr.2024.25.5.910
- Received on Aug 2, 2024
- Revised on Sep 23, 2024
- Accepted on Sep 27, 2024
 Services
Services
Shared
 Correspondence to
Correspondence to
- Dandan Zhao
-
Xinxiang Vocational and Technical College, Xinxiang, Henan 453006, China
Tel : +86-13283729630 Fax: +86-0373-3720570 - E-mail: 6479712@qq.com






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.