- Effect of different Ba-sources on the sonochemically activated solid-state synthesis of BaTiO3 powders
Hae Won Leea, Min Ho Janga, Ga Young Leea, Gil-Geun Leeb, Woo Hyun Namc and Young Soo Lima,b,*
aDepartment of Smart Green Technology Engineering, Pukyong National University, Busan 48513, Republic of Korea
bDepartment of Materials System Engineering, Pukyong National University, Busan 48513, Republic of Korea
cElectronic Convergence Materials Center, Korea Institute of Ceramic Engineering and Technology, Jinju 52851, Republic of KoreaThis article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
We report the impact of Ba sources in the sonochemically activated solid-state synthesis of BaTiO3 powders. A water-soluble Ba(CH3COO)2 and TiO2 powders were sonochemically mixed in aqueous medium with controlled pH values and subsequently calcined following a drying process. For a comparative study, solid-state BaCO3 powders were also used to synthesize BaTiO3 powders under the same ultrasonication, drying, and calcination conditions. Phase conversion ratio to BaTiO3 and structural properties of the resulting powders for each case were characterized and compared in detail. We expect that this study will be helpful in understanding the process for synthesizing and doping BaTiO3 powders using water-soluble raw materials.
Keywords: BaTiO3, Powder, Sonochemical, Solid-state reaction.
Solid-state synthesis of BaTiO3 has been carried out conventionally by using BaCO3 and TiO2 raw powders, as described in (1) [1-4].
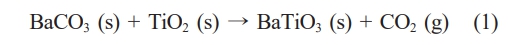
In this process, the reaction takes place by coupled diffusion of Ba2+ and O2- ions into the TiO2 lattice from the contact point between the raw materials [3-5]. Therefore, the intimate and uniform mixing of BaCO3 and TiO2 to increase the initial contact surface area have been considered critical factors for the process. Accordingly, efforts have been made to promote the solid-state reaction of BaTiO3 by utilizing nanometer-sized ultrafine raw materials or by reducing the particle size through high-energy milling processes [6-17].
Recently, it has been reported that the mixing process of raw materials can be effectively performed by using ultrasonication process within only a few minutes and that the solid-state reaction in (1) can be accelerated by the sonochemical activation effect [18-21]. We reported that when the raw materials were dispersed in aqueous medium, the sonochemical mixing process was strongly affected by pH of the aqueous medium due to the pH-dependent partial solubility of BaCO3 in water [18].
Therefore, it is also intriguing to study the solid-state reaction using a water-soluble source instead of the solid-state source of BaCO3 with a partial solubility since the solubility of Ba source was proved to be a critical factor for the sonochemically activated solid-state reaction in our previous study [18].
In this study, the effect of Ba-sources for sonochemically activated solid-state synthesis of BaTiO3 powders is presented. We ultrasonicated the mixtures of TiO2 powder with different Ba sources of water-soluble Ba(CH3COO)2 and solid-state BaCO3 in aqueous medium with adjusted pH values and compared the properties of resulting powders. The results revealed that the phase conversion ratio through the solid-state reaction was critically dependent on the type of Ba source. Detailed microstructural and crystal structural characterizations were conducted on each resulting BaTiO3 powder and compared.
Sample preparation
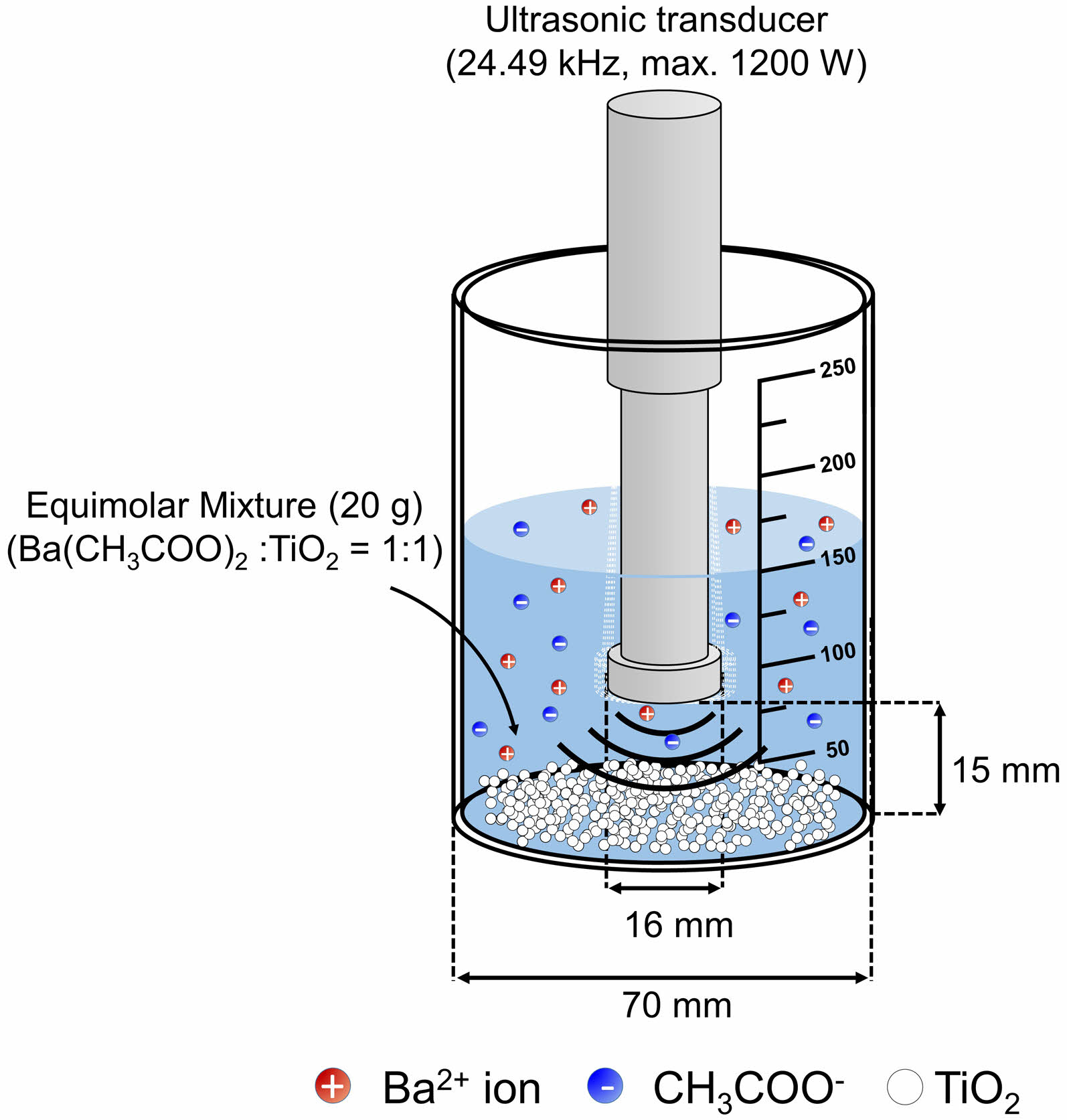
Barium acetate (Ba(CH3COO)2, ≥99%, Alfa Aesar, USA) and titanium dioxide (TiO2, ≥99%, Sigma-Aldrich, USA) were used as raw materials, with the pH of distilled water controlled using potassium hydroxide (KOH) and hydrochloric acid (HCl) solutions. 150 ml of distilled water with controlled pH values (3, 5, 7, 9 and 11) was placed in a 250 ml beaker, and Ba(CH3COO)2 and TiO2 were introduced stoichiometrically into the solvent as shown in Fig. 1. Sonochemical mixing process was carried out for 5 min at an ultrasonic power of 900 W using an ultrasonic homogenizer (Boshi Electronic Instrument, Hangzhou, China). The ultrasonicated mixtures were oven-dried at 120 °C for 12 hours, and the resulting dried powder was ground using a mortar and pestle. The powder was calcined in air at 900 °C (heating rate=5 °C/min) for 3 h for the solid-state synthesis of BaTiO3. The synthesized powder with the water-soluble Ba-source was referred to as W-BT. In addition, for a comparative study with the conventional solid-state synthesis of BaTiO3, the same experimental procedure was carried out using barium carbonate (BaCO3, 99%; Sigma-Aldrich, USA) and TiO2 as raw materials. The synthesized powder was referred to as solid-state source BaTiO3 (S-BT).
Characterizations
Crystal structures of calcined powders were investigated using an X-ray diffractometer (XRD, Ultima IV, Rigaku Corp, Tokyo, Japan) and Rietveld refinements were performed using Profex software (ver. 3.14.3). microstructural characterization was carried out using a field emission scanning electron microscope (FESEM, Mira 3, Tescan, Brno, Czech) and a transmission electron microscope (TEM, Titan Themis Z, FEI Company, USA). In addition, particle size analysis of the powder was conducted using a particle size analyzer (PSA, LA-950, Horiba Ltd., Japan).
|
Fig. 1 A schematic of sonochemical mixing process. |
Effect of pH on the particle size of ultrasonicated raw materials
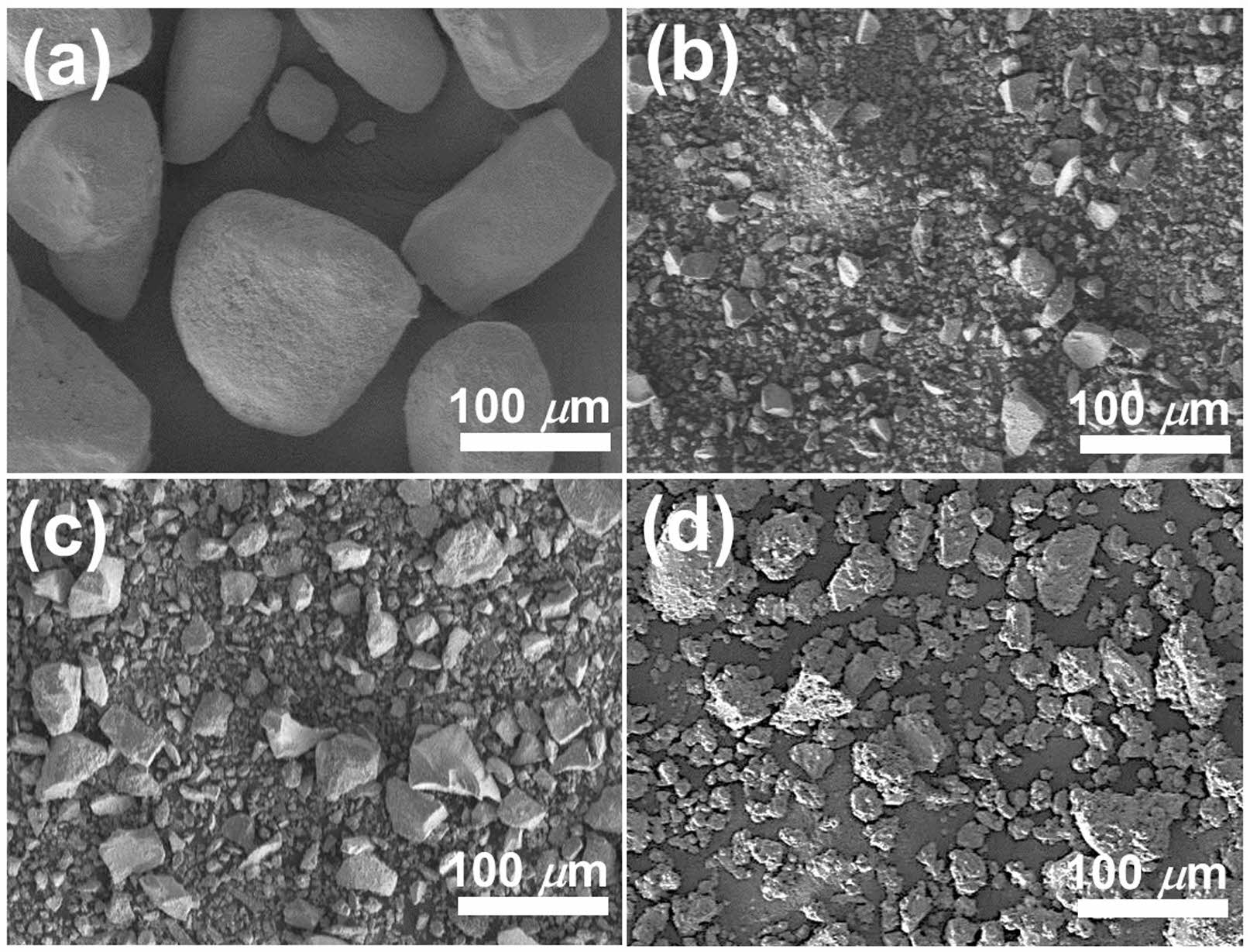
Fig. 2(a) shows a SEM micrograph of water-soluble Ba(CH3COO)2 raw powder before ultrasonication, and (b)-(d) represent their microstructures after ultrasonication in aqueous medium at various pH levels and the subsequent drying process. All dried powders after ultrasonication exhibited a smaller particle size than untreated powder and showed a tendency that the particle size increased with the increase in pH of solvent. It has been reported that the solubility of the barium compounds decreases with an increase in the pH [22]. Therefore, Ba(CH3COO)2 dissolved in high pH aqueous medium recrystallizes rapidly during drying, and the particles formed through this process will grow in size. This can be considered the factor influencing the pH-dependent particle size after dissolution with ultrasonication and subsequent recrystallization.
Fig. 3(a) shows a SEM micrograph of solid-state BaCO3 raw powder before ultrasonication, and (b)-(d) represent their microstructures after ultrasonication in aqueous medium at various pH levels and the subsequent drying process. It has been reported that BaCO3 is partially soluble in water and its partial solubility is dependent on the pH [18, 23, 27]. However, although the degree of the fragmentation in BaCO3 powder decreased with increasing pH [18], the changes in size with respect to pH were not significant and all resulting powders exhibited a smaller particle size and a narrower size distribution compared to the Ba(CH3COO)2 powders in Fig. 2.
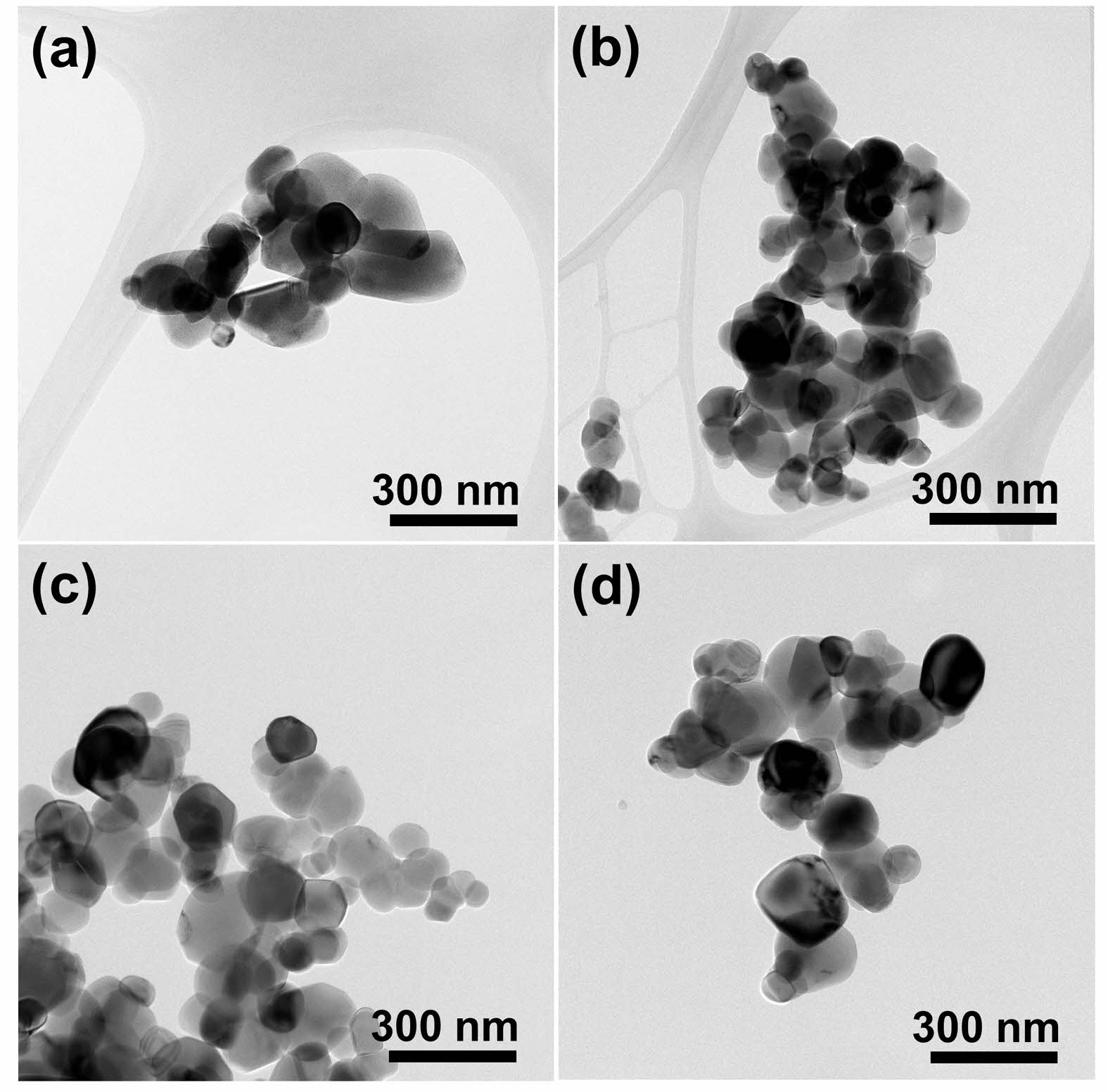
Fig. 4(a) shows a TEM micrograph of TiO2 raw powder before ultrasonication, and (b)-(d) represent their microstructures after ultrasonication in aqueous medium at various pH levels and the subsequent drying process. Regardless of the pH, no significant change was observed in the particle size of TiO2 before and after the sonochemical process and the particle size was ~80 nm for all samples. It has been reported that TiO2 is chemically stable and does not dissolve in water [24], and the influence of pH on the TiO2 particle size was negligible also in this experiment.
Effect of pH on the solid-state reaction for BaTiO3
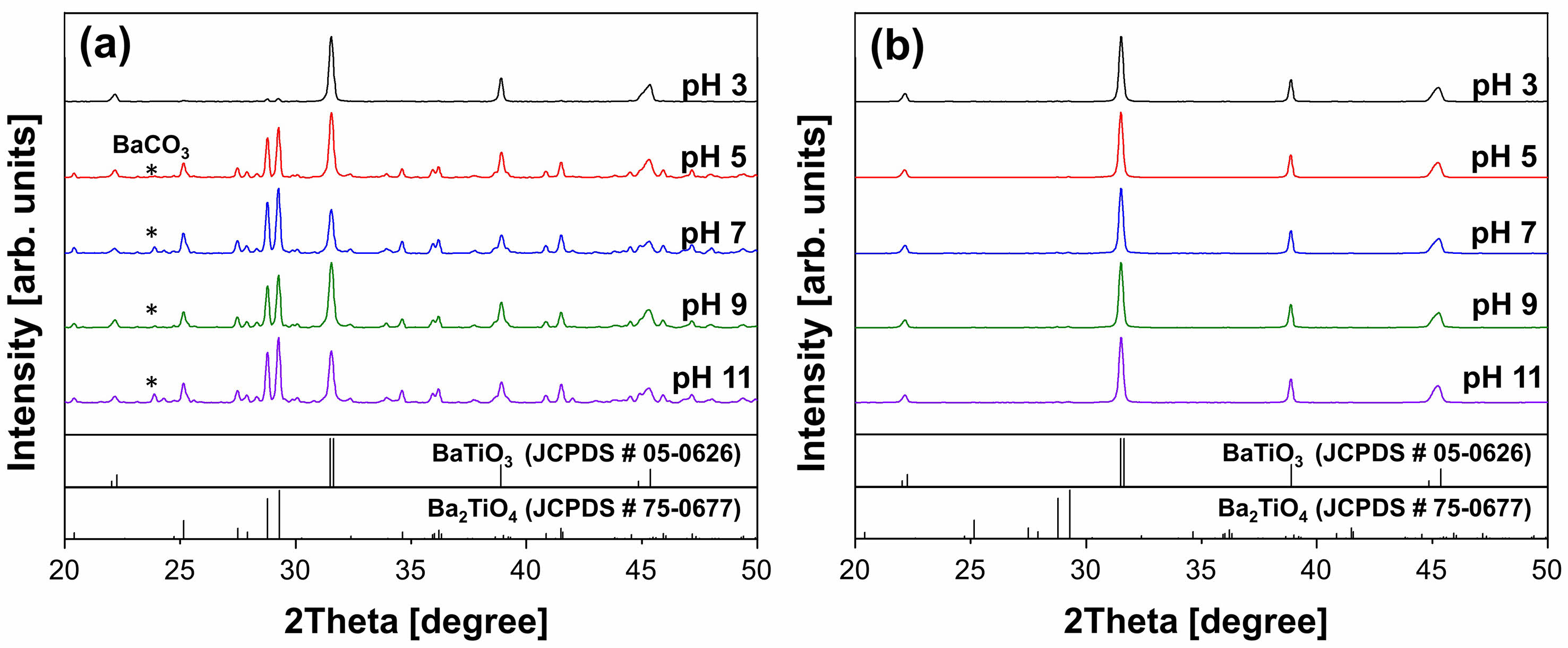
Fig. 5(a) shows XRD patterns of W-BT powders synthesized by the calcination of sonochemical mixtures of water-soluble Ba(CH3COO)2 and TiO2 at 900 oC for 3 h. For pH = 3, the powder was composed of major BaTiO3 phase and a secondary Ba2TiO4 phase. BaCO3 phase started to appear from pH = 5, and multiphase of BaTiO3, Ba2TiO4 and BaCO3 were observed in W-BT powders up to pH = 11.
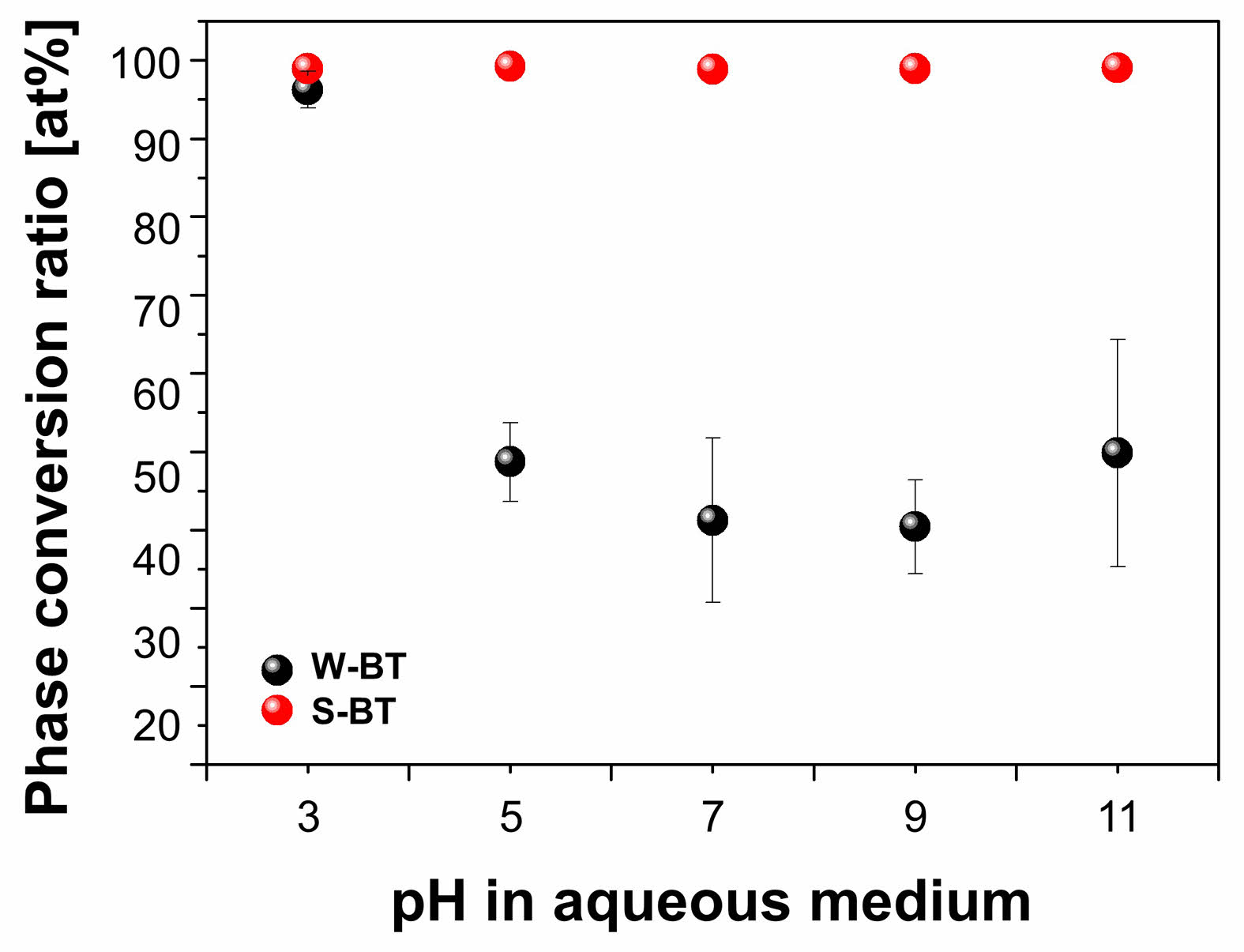
Rietveld refinements on the XRD patterns were performed to investigate the phase conversion ratio to BaTiO3 in the powders depending on the starting materials and pH. As shown in Fig. 6, a high conversion ratio of ~95% was obtained in the solid-state synthesized W-BT only for pH = 3. For higher pH values (pH = 5-11), a relatively low BaTiO3 phase conversion ratio of 40-50% was obtained in W-BT powders. On the other hand, S-BT, which uses conventional solid-state source of BaCO3 and TiO2, exhibited almost single perovskite phase regardless of pH as shown in Fig. 5(b) and Fig. 6.
Because the solid-state reaction takes place through the coupled diffusion of Ba2+ and O2- ions into the TiO2 lattice at the contact point between the starting materials [3-5], phase conversion ratio is determined by the properties of starting powders such as particle size, distribution, and surface area. Therefore, there have been reports on the enhancement of the solid-state reaction for BaTiO3 by using nanosized raw materials to maximize the contacting area [6-17]. In Figs. 2 and 3, we observed the particle size of ultrasonicated Ba sources under different pH values. Regardless of pH values, the particle size of BaCO3 source was much smaller than that of Ba(CH3COO)2 source and that is the reason for that the conversion ratio in S-BT was much higher than that in W-BT. Among the W-BT powders, the conversion ratio was much higher at pH = 3 than at pH ≥ 5 and this can be attributed to the fact that the particle size of Ba(CH3COO)2 was much smaller than the others, as shown in Fig. 2.
On the other hand, the conversion ratio was higher than 98% for all S-BT powders regardless of the pH in the aqueous medium. In this experiment, the calcination of the ultrasonicated mixtures for S-BT was carried out only at 900 oC for 3 h and this is relatively low temperature for the solid-state reaction for BaTiO3 as compared to the conventional calcination temperatures in literature [1, 18, 19]. Therefore, the high conversion ratio of S-BT in this experiment shows the effect of the sonochemical activation on the enhancement of the solid-state reaction and this result is quite consistent with our previous reports [18, 19].
Structural characterizations of W-BT and S-BT
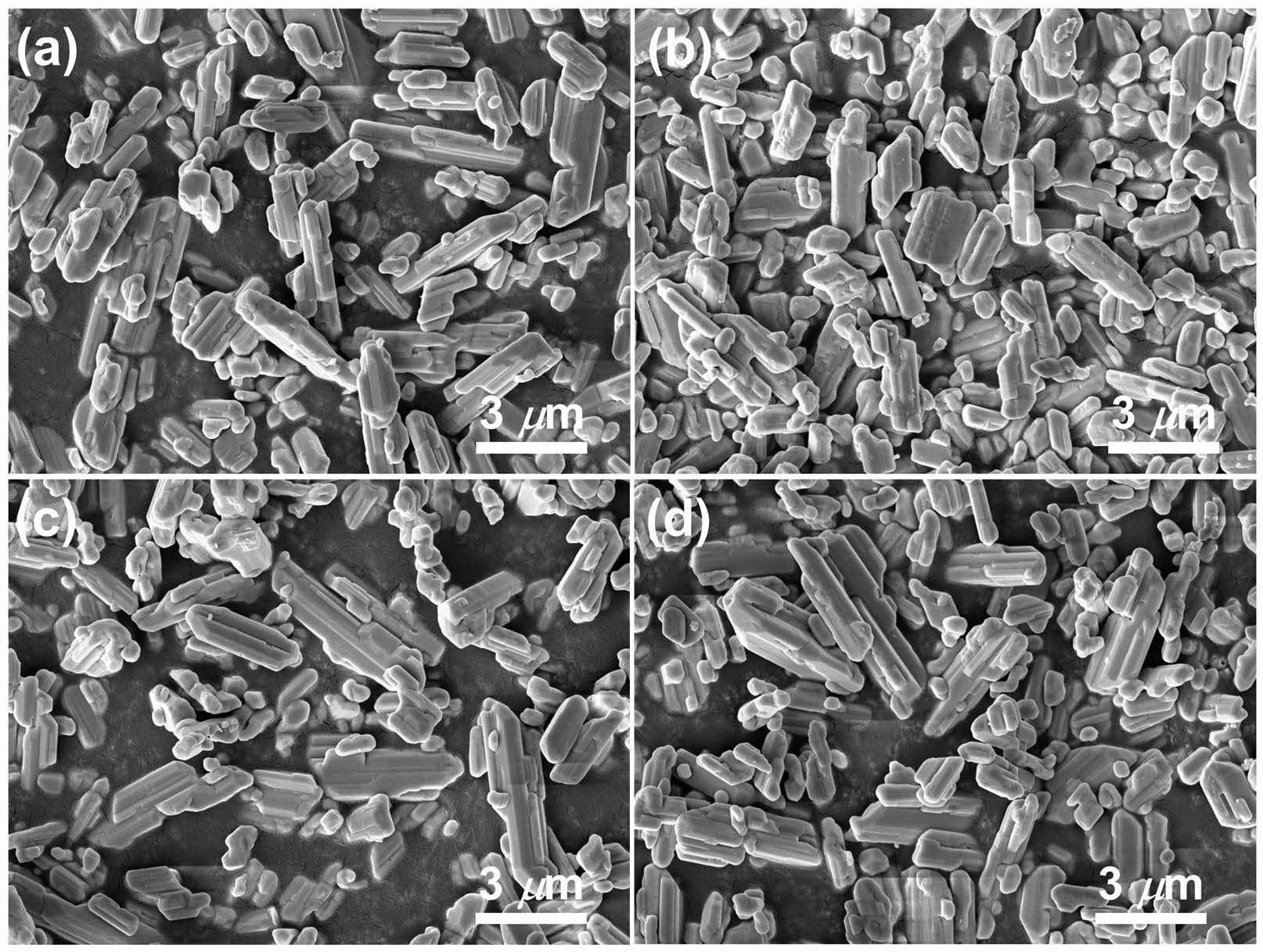
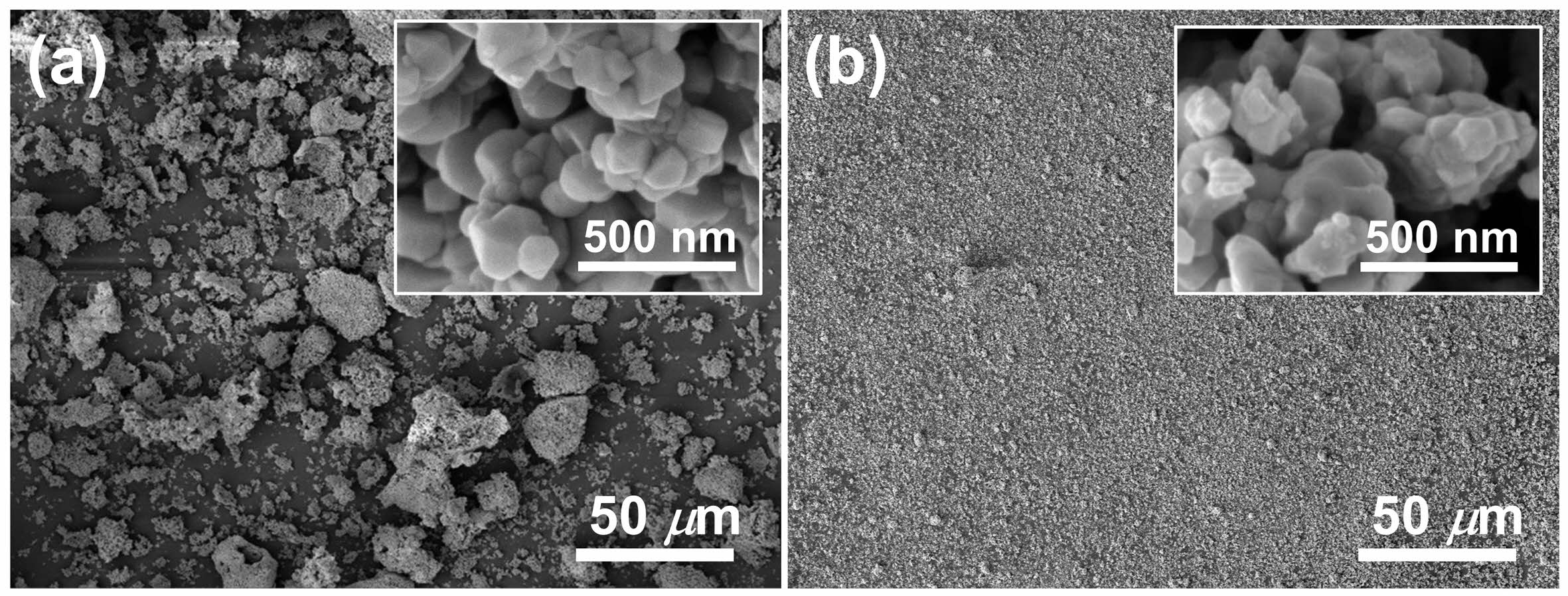
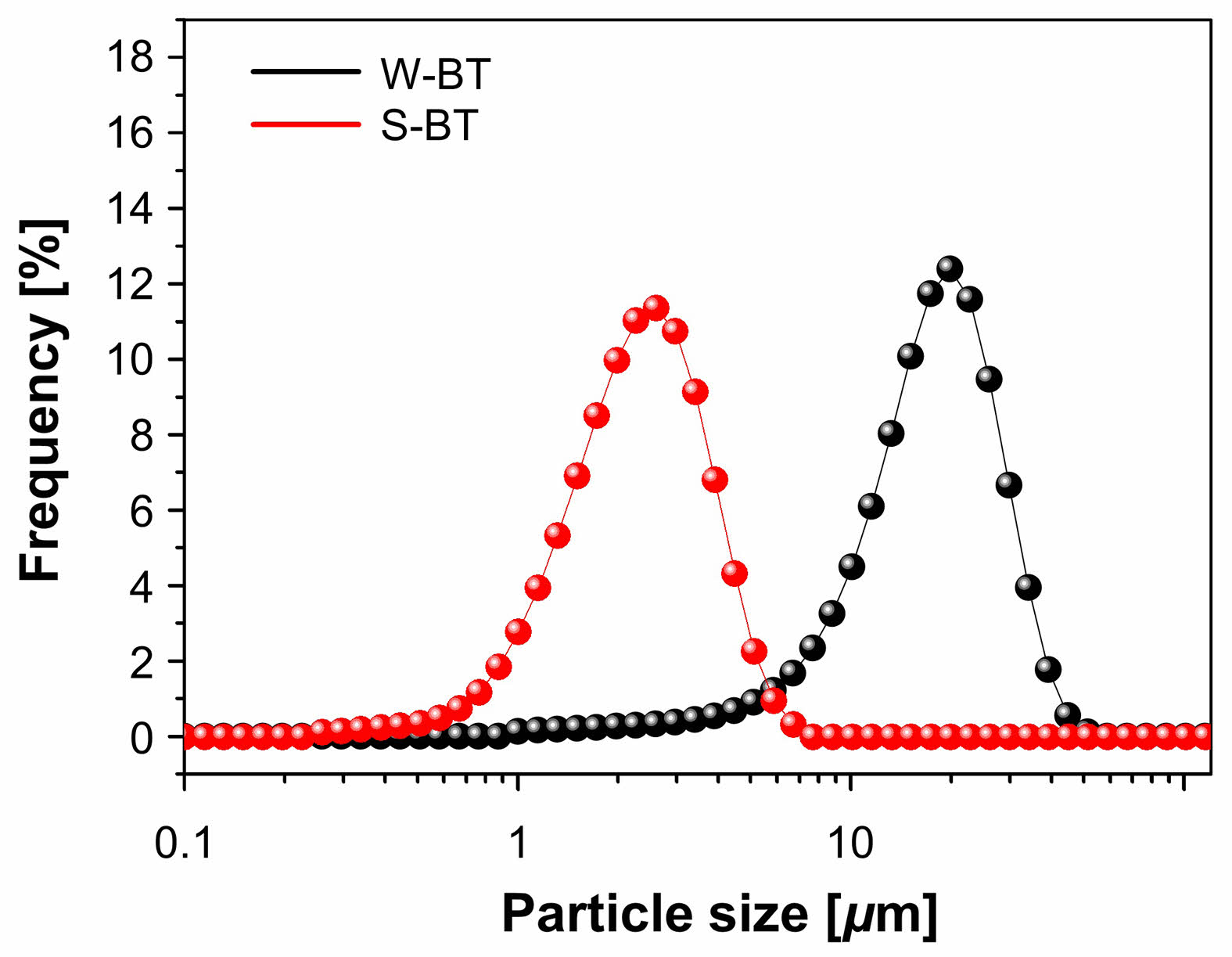
For microstructural characterizations, we selected W-BT and S-BT powders synthesized at pH = 3 as they both exhibited a sufficiently high conversion ratio for comparative analysis. Fig. 7(a) shows SEM micrographs of the W-BT powder and the enlarged micrograph is shown in the inset. Strongly agglomerated particles (> 10 µm) were easily observed in W-BT even though its primary particle size was ~160 nm as shown in the inset. On the other hand, Fig. 7(b) shows that the agglomeration in S-BT was much weaker than that in W-BT. Even in this case, the primary particle size was measured to be ~160 nm. It has been reported that the crystallite size of solid-state synthesized BaTiO3 powder is predominantly affected by the initial TiO2 particle size [25, 26]. Because the particle size of TiO2 was not changed by the sonochemical process in aqueous medium as shown in Fig. 4, this result is consistent with the reported results. With this result, it was found that different barium sources only affect the degree of agglomeration of primary particles, not the primary particle size, in this set of BaTiO3 powders. To investigate the degree of the agglomeration quantitatively, we also carried out the particle size analysis (PSA) for the powders and the results are shown in Fig. 8. The D50 of W-BT was measured to be 16.7 µm, while for S-BT, it was 2.2 µm. These quantitative results are quite consistent with the qualitative observations in Fig. 7.
The structural characterizations of W-BT and S-BT powders were conducted through Rietveld refinements using the XRD patterns shown in Fig. 5, and the summarized results are presented in Table 1. The lattice parameters, volume and tetragonality were almost similar in both powders. Therefore, it was found that Ba sources do not affect the crystallographic properties of the resulting primary BaTiO3 particles, but rather only influence the degree of agglomeration.
|
Fig. 2 SEM micrographs of (a) as-is and (b)-(d) ultrasonicated and subsequently dried Ba(CH3COO)2 powders at pH=3, 7 and 11, respectively. |
|
Fig. 3 SEM micrographs of (a) as-is and (b)-(d) ultrasonicated and subsequently dried BaCO3 powders at pH=3, 7 and 11, respectively |
|
Fig. 4 TEM micrographs of (a) as-is and (b)-(d) ultrasonicated and subsequently dried TiO2 powders at pH = 3, 7 and 11, respectively. |
|
Fig. 5 XRD patterns of solid-state synthesized BaTiO3 powder using (a) water-soluble Ba(CH3COO)2 and (b) solid-state BaCO3 for Ba source. |
|
Fig. 6 Phase conversion ratio to BaTiO3 as a function of pH in aqueous medium with different barium sources. |
|
Fig. 7 SEM micrographs of (a) W-BT and (b) S-BT powders. |
|
Fig. 8 PSA results of W-BT and S-BT powders |
|
Table 1 Refined structural parameters for W-BT and S-BT powders at room temperature |

In summary, we reported the effect of Ba-sources on the sonochemically activated solid-state synthesis of BaTiO3 powders. We ultrasonicated the mixtures of TiO2 powder with different Ba sources of water-soluble Ba(CH3COO)2 and solid-state BaCO3, and we compared the properties of resulting powders. When we used water-soluble source of Ba(CH3COO)2, the phase conversion to BaTiO3 showed a strong pH dependence. Except for pH = 3, the conversion ratio of W-BT powders was quite low (40-50%) and this was mainly attributed to the change in the particle size of Ba source during the ultrasonication and drying process depending on pH in the aqueous medium. On the other hand, when using the solid-state BaCO3 powder, the conversion ratio surpassed 98% for all S-BT powders under the same calcination condition (900 oC for 3 h in air). Considering that the BaCO3 was micron-sized, this high conversion ratio indicates that the solid-state reaction to BaTiO3 was significantly accelerated by the sonochemical mixing process compared with the conventional solid-state reaction employing a ball-mill process. Detailed microstructural and crystal-structural characterizations of W-BT and S-BT powders revealed that the properties of primary BaTiO3 particles were not significantly affected by the Ba sources. However, the degree of agglomeration of primary particles was strongly associated with the type of Ba source. We expect that this study will be helpful in understanding the process for synthesizing and doping BaTiO3 powders using water-soluble raw materials.
This work was supported by a Research Grant from Pukyong National University (2023).
- 1. G.H. Haertling, J. Am. Ceram. Soc. 82[4] (1999) 797-818.
-

- 2. L.K. Templeton and J. A. Pask, J. Am. Ceram. Soc. 42[5] (1959) 212-216.
-

- 3. A. Beauger, J.C. Mutin, and J.C. Niepce, J. Mater. Sci. 18 (1983) 3041-3046.
-

- 4. A. Beauger, J.C. Mutin, and J.C. Niepce, J. Mater. Sci. 18 (1983) 3543-3550.
-

- 5. D.-H. Yoon and B.I. Lee, J. Ceram. Proc. Res. 3[2] (2002) 41-47.
- 6. T.-T. Lee, C.-Y. Huang, C.-Y. Chang, I.-K Cheng, C.-L. Hu, C.-T. Lee, and M. Fujimoto, J. Mater. Res 27[19] (2012) 2495-2502.
-

- 7. R. Ashiri, RSC Adv. 6[21] (2016) 17138-17150.
-

- 8. J.L. Clabel H, I.T. Awan, A.H. Pinto, I.C. Nogueira, V.D.N. Bezzon, E.R. Leite, D.T. Balogh, V.R. Mastelaro, S.O. Ferreira, and E. Marega Jr, Ceram. Int. 46[3] (2020) 2987-3001.
-

- 9. V.P. Pavlović, D. Popović, J. Krstić, J. Dojčilović, B. Babić, and V.B. Pavlović, J. Alloys Compd. 486[1-2] (2009) 633-639.
-

- 10. D.F.K. Hennings, B.S. Schreinemacher, and H. Schreinemacher, J. Am. Ceram. Soc. 84[12] (2001) 2777-2782.
-

- 11. M.T. Buscaglia, M. Bassoli, V. Buscaglia, and R. Alessio, J. Am. Ceram. Soc. 88[9] (2005) 2374-2379.
-

- 12. M.T. Buscaglia, M.T. Bassoli, V. Buscaglia, and R. Vormberg, J. Am. Ceram. Soc 91[9] (2008) 2862-2869.
-

- 13. S.-S. Ryu and D.-H. Yoon, J. Mater. Sci. 42 (2007) 7093-7099.
-

- 14. L.B. Kong, J. Ma, H. Huang, R.F. Zhang, and W.X. Que, J. Alloys. Compd. 337[1-2] (2002) 226-230.
-

- 15. S.-S. Ryu, S.-K. Lee, and D.-H. Yoon, J. Electroceram. 18 (2007) 243-250.
-

- 16. R. Yanagawa, M. Senna, C. Ando, H. Chazono, and H. Kishi, J. Am. Ceram. Soc. 90[3] (2007) 809-814
-

- 17. D.-H. Yoon, J. Ceram. Proc. Res. 7[4] (2006) 343-354.
- 18. H.W. Lee, N.W. Kim, W.H. Nam, and Y.S. Lim, Ultrason. Sonochem. 82 (2022) 105874.
-

- 19. S.H. Jin, H.W. Lee, N.W. Kim, B.-W. Lee, G.-G. Lee, Y.-W. Hong, W.H. Nam, and Y.S. Lim, J. Eur. Ceram. Soc. 41[9] (2021) 4826-4834.
-

- 20. S. Utara and S. Hunpratub, Ultrason. Sonochem. 41 (2018) 441-448.
-

- 21. H.Z. Akbas, Z. Aydin, O. Yilmaz, and S. Turgut, Ultrason. Sonochem. 34 (2017) 873-880.
-

- 22. E. Nielsen and O. Ladefoged, Fundam. Appl. Toxicol. 19 (2013) 527-537.
- 23. C.-C. Li and J.-H. Jeon, J. Am. Ceram. Soc. 85[12] (2002) 2977-2983.
-

- 24. M. Kakihana, M. Kobayashi, K. Tomita, and V. Petrykin, Bull. Chem. Soc. Jpn. 83[11] (2010) 1285-1308.
-

- 25. H.-T. Kim, J.-H. Kim, W.-S. Jung, and D.-H. Yoon, J. Ceram. Proc. Res. 10[6] (2009) 753-757.
-

- 26. E. Song, D.H. Kim, E.J. Jeong, M. Choi, Y. Kim, H.J. Jung, and M.Y. Choi, Environ. Res. 202 (2021) 111668.
-

- 27. B.I. Lee, M. Wang, D. Yoon, and M. Hu, J. Ceram. Proc. Res. 4[1] (2003) 17-24.
 This Article
This Article
-
2024; 25(5): 737-741
Published on Oct 31, 2024
- 10.36410/jcpr.2024.25.5.737
- Received on Mar 12, 2024
- Revised on Jun 6, 2024
- Accepted on Jun 11, 2024
 Services
Services
- Abstract
introduction
experimental
results and discussion
conclusions
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Young Soo Lim
-
aDepartment of Smart Green Technology Engineering, Pukyong National University, Busan 48513, Republic of Korea
bDepartment of Materials System Engineering, Pukyong National University, Busan 48513, Republic of Korea
Tel : +82-51-629-6384 Fax: +82-51-629-6373 - E-mail: yslim@pknu.ac.kr






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.