- Design and characterization of polymeric scaffolds reinforced with ZrO2-doped BG-85S bioabsorbable ceramics for training bone restoration
Mingda Hea and Guangjiu Chenb,*
aSchool of Physical Education, Chengdu Sport University, Chengdu, 610041, China
bLuzhou Vocational and Technical College, Luzhou, 646000, ChinaThis article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
This study investigates the effects of varying ZrO₂ content on the mechanical properties of BG-85S bioactive glass, aiming to enhance its potential as a scaffold material in tissue engineering applications. Compressive strength and porosity were analyzed, revealing a significant enhancement in compressive strength and a reduction in porosity with increasing ZrO₂ content. Specifically, compressive strength increased from approximately 210 MPa at 0 wt% ZrO₂ to about 300 MPa at 6 wt% ZrO₂, while porosity decreased from around 50 vol% to 30 vol% over the same range. These improvements are attributed to the reinforcing effect of ZrO₂, which enhances structural integrity and resistance to deformation, and promotes a denser material structure through liquid phase sintering and vitrification. Additionally, increases in hardness and Young's modulus with higher ZrO₂ content were observed, further supporting the potential of ZrO₂-doped bioactive glasses for biomedical applications requiring enhanced mechanical performance. The observed improvements in mechanical properties, including increased compressive strength, reduced porosity, and enhanced hardness and Young's modulus, highlight the suitability of ZrO₂-doped bioactive glass for load-bearing applications in tissue engineering. These findings underscore the importance of optimizing ZrO₂ content to maximize mechanical performance while maintaining bioactivity and biocompatibility. Future research should focus on further optimizing ZrO₂ content for specific applications, assessing the long-term stability and bioactivity of these materials in physiological conditions, and exploring the potential of ZrO₂-doped bioactive glasses in various biomedical applications.
Keywords: Artificial bone scaffold, Bioabsorbable ceramics, Tissue engineering.
Bioabsorbable synthetic polymers have gained increasing interest as scaffold structures for tissue engineering, largely due to their numerous practical advantages. The ability to precisely control the composition and structure, including porosity, of these materials makes them highly suitable for various biomedical applications, such as artificial bone scaffolds. However, the use of synthetic polymers comes with significant drawbacks [1]. Upon implantation, these polymers degrade, releasing byproducts that lower pH levels, potentially triggering inflammatory responses. Furthermore, these materials are often bioinactive, hydrophobic, and lack the mechanical strength required for orthopedic applications, presenting considerable challenges for their use in such demanding environments. [2, 3].
Numerous biomaterials have been studied and applied clinically for bone repair and regeneration purposes (Fig. 1). A common issue with these materials is that their degradation leads to a reduction in mechanical properties. Nevertheless, if the degradation process is controlled and gradual, it facilitates the transfer of load from the implants to the bone and soft tissues, thereby preventing the stress shielding effect. Recently, significant advancements have been made in the development of biodegradable rods, plates, pins, screws, and suture anchors for use in tissue engineering and treating injuries such as sprains [4-8].
In contrast, ceramic materials such as hydroxyapatite (HA), tricalcium phosphate (TCP), and certain silicate and phosphate glasses, including glass-ceramics and various compositions of bioactive glasses, offer a different set of benefits [9]. These ceramics can interact with body fluids and form direct bonds with bone tissue, making them more suitable for applications requiring strong biological integration [10]. Bioactive glasses, in particular, exhibit high surface reactivity, which facilitates their interaction with biological environments. However, this same reactivity poses a challenge for in vitro studies, as the release of alkaline ions can significantly increase local pH levels, leading to cytotoxicity [11]. Bioactive glasses (BGs) have emerged as a pivotal material in biomedical applications, particularly in bone regeneration and tissue engineering, due to their excellent biocompatibility and ability to bond with bone tissue [12-16]. Among the various formulations, BG-58S has gained attention for its promising bioactivity and structural properties. However, enhancing the mechanical strength and durability of bioactive glasses remains a critical challenge, especially for load-bearing applications.
In this research, we explore the effects of incorporating zirconium dioxide (ZrO₂) into BG-58S bioactive glasses. The addition of ZrO₂ is hypothesized to improve the
mechanical properties of the glasses without compromising their bioactivity. By varying the ZrO₂ composition, we aim to systematically investigate its impact on the chemical and mechanical properties of the resultant bioactive glasses. To comprehensively assess these effects, we measured key mechanical properties including compressive strength, porosity, Vickers hardness, and Young’s modulus. Compressive strength provides insight into the material’s ability to withstand loads, while porosity is crucial for understanding the material's potential for cell infiltration and nutrient transport. Vickers hardness measures the material's resistance to deformation, and Young’s modulus assesses its stiffness, both of which are vital for applications requiring mechanical resilience.
This study not only aims to enhance the understanding of how ZrO₂ incorporation affects BG-58S bioactive glasses but also seeks to identify optimal compositions that balance bioactivity with mechanical strength. The findings have the potential to advance the development of bioactive glass materials for a variety of biomedical applications, particularly those requiring robust mechanical performance.
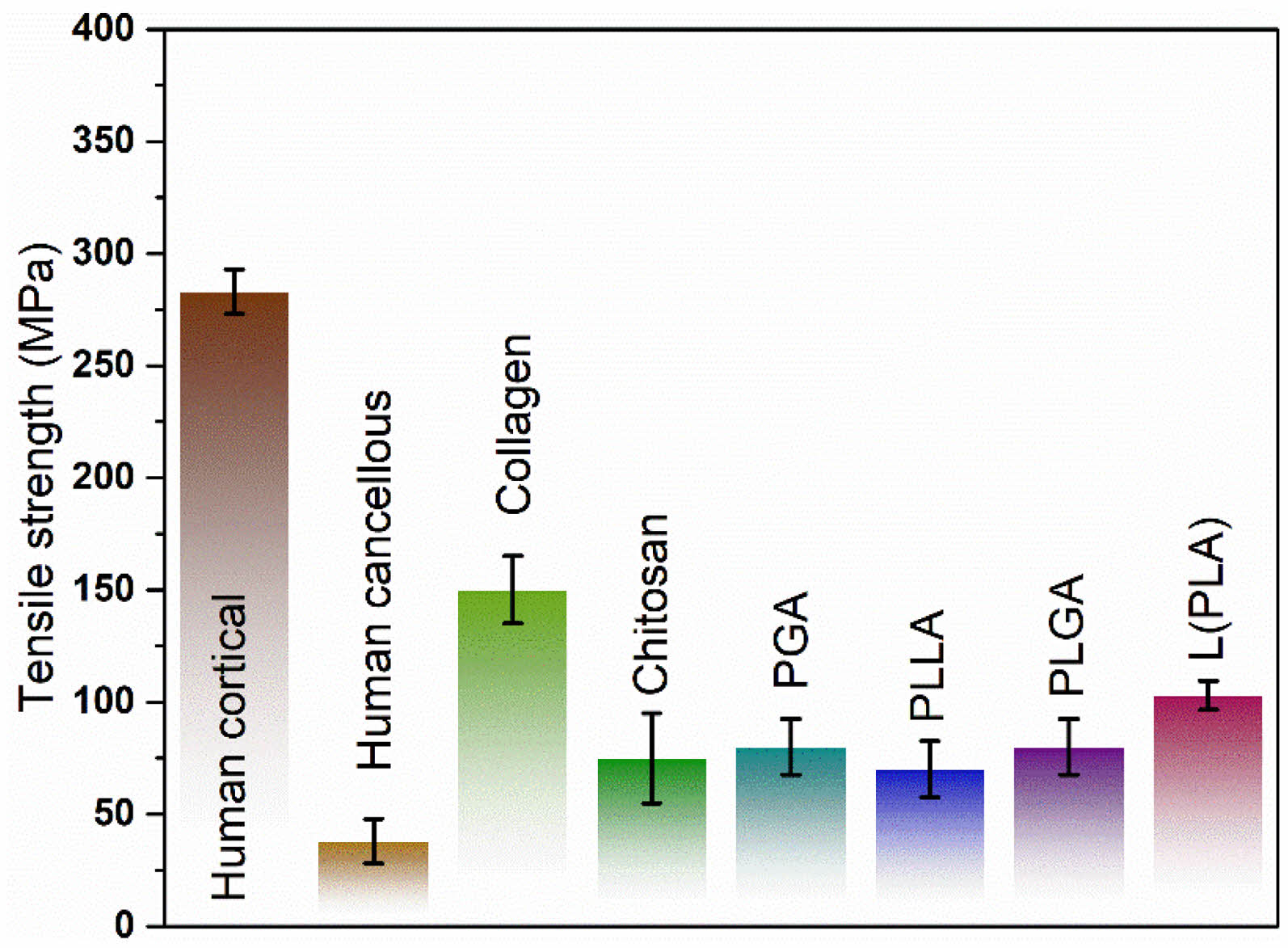
|
Fig. 1 Tensile strength of some degradable and non-degradable materials. |
The BG-58S powder was prepared through hydrolysis and polycondensation reactions using stoichiometric amounts of Tetraethyl orthosilicate, Ca(NO₃)₂, and H3PO4, based on the nominal composition and 2 M HNO3. These components were added to a plastic beaker and stirred constantly at room temperature. The resulting gel was mixed with a glass rod and then placed in an oven at 65°C for 24 hours to remove any residual water and ethanol. The dried powders were then ground using a mortar and subjected to thermal treatment in a muffle furnace at 600°C, with a heating rate of 2°C/min. After this, the material was milled in a high-energy mill at 500 rpm for 5 minutes and then sieved through a 400-mesh sieve to obtain a powder with a median particle size (d50) of 10 μm. This fine powder was subsequently incorporated into a polymeric matrix for scaffold production [17].
The graphs provide insight into the effects of increasing ZrO₂ content on the compressive strength and porosity of bioactive glass. The graph shows a clear trend of increasing compressive strength with higher ZrO₂ content (Fig. 2a). Specifically, At 0 wt% ZrO₂, the compressive strength is approximately 210 MPa. Adding 2 wt% ZrO₂ increases the compressive strength to around 240 MPa. At 4 wt% ZrO₂, the compressive strength further rises to about 270 MPa. The maximum compressive strength, approximately 300 MPa, is observed at 6 wt% ZrO₂. This trend indicates that the incorporation of ZrO₂ significantly enhances the compressive strength of bioactive glass. The improvement in compressive strength can be attributed to the reinforcing effect of ZrO₂, which likely imparts additional structural integrity and resistance to mechanical deformation. As ZrO₂ content increases, its role in hindering crack propagation and providing a tougher matrix becomes more pronounced, thereby enhancing the overall mechanical properties.
Figure 2b, shows that porosity decreases with increasing ZrO₂ content, At 0 wt% ZrO₂, the porosity is around 50 vol%, with 2 wt% ZrO₂, the porosity decreases to approximately 43 vol%. At 4 wt% ZrO₂, porosity further reduces to about 37 vol%. The lowest porosity, around 30 vol%, is observed at 6 wt% ZrO₂. The reduction in porosity with higher ZrO₂ content suggests that the addition of ZrO₂ leads to a denser material structure. This decrease in porosity can be linked to the liquid phase sintering and vitrification processes induced by ZrO₂, which promote a more amorphous and less porous glass matrix. The reduction in porosity is beneficial for enhancing the mechanical properties and ensuring the structural stability of the bioactive glass, making it more suitable for load-bearing applications.
The observed increase in compressive strength and decrease in porosity with higher ZrO₂ content are interrelated. As the porosity decreases, the material becomes denser and more capable of withstanding higher compressive loads. This densification effect, combined with the inherent toughness imparted by ZrO₂, contributes to the improved mechanical performance. The balance between these two properties is crucial for developing bioactive glasses that are not only strong but also sufficiently porous to support biological functions such as cell infiltration and nutrient transport. The addition of ZrO₂ to bioactive glass enhances its compressive strength and reduces its porosity, indicating a significant improvement in mechanical properties. This makes ZrO₂-doped bioactive glass a promising candidate for biomedical applications, particularly in areas requiring high mechanical strength and structural integrity. Future research could further optimize the ZrO₂ content to maximize these benefits while maintaining the bioactive and biocompatible nature of the glass.
The addition of ZrO₂ to bioactive glass shows a clear trend of improving both hardness and Young's modulus. As the ZrO₂ content increases from 0 wt% to 6 wt%, the mechanical properties of the bioactive glass are enhanced, making it more suitable for applications requiring higher strength and stiffness. This enhancement can be attributed to the reinforcing effect of ZrO₂ particles within the glass matrix, which likely impedes the movement of dislocations and contributes to the overall rigidity of the composite material. The observed improvements in mechanical properties with increasing ZrO₂ content highlight the potential of ZrO₂-doped bioactive glasses for use in biomedical applications where enhanced mechanical performance is critical. Further studies could explore the optimal ZrO₂ content for specific applications and investigate the long-term stability and bioactivity of these materials in physiological environments. Fig. 3
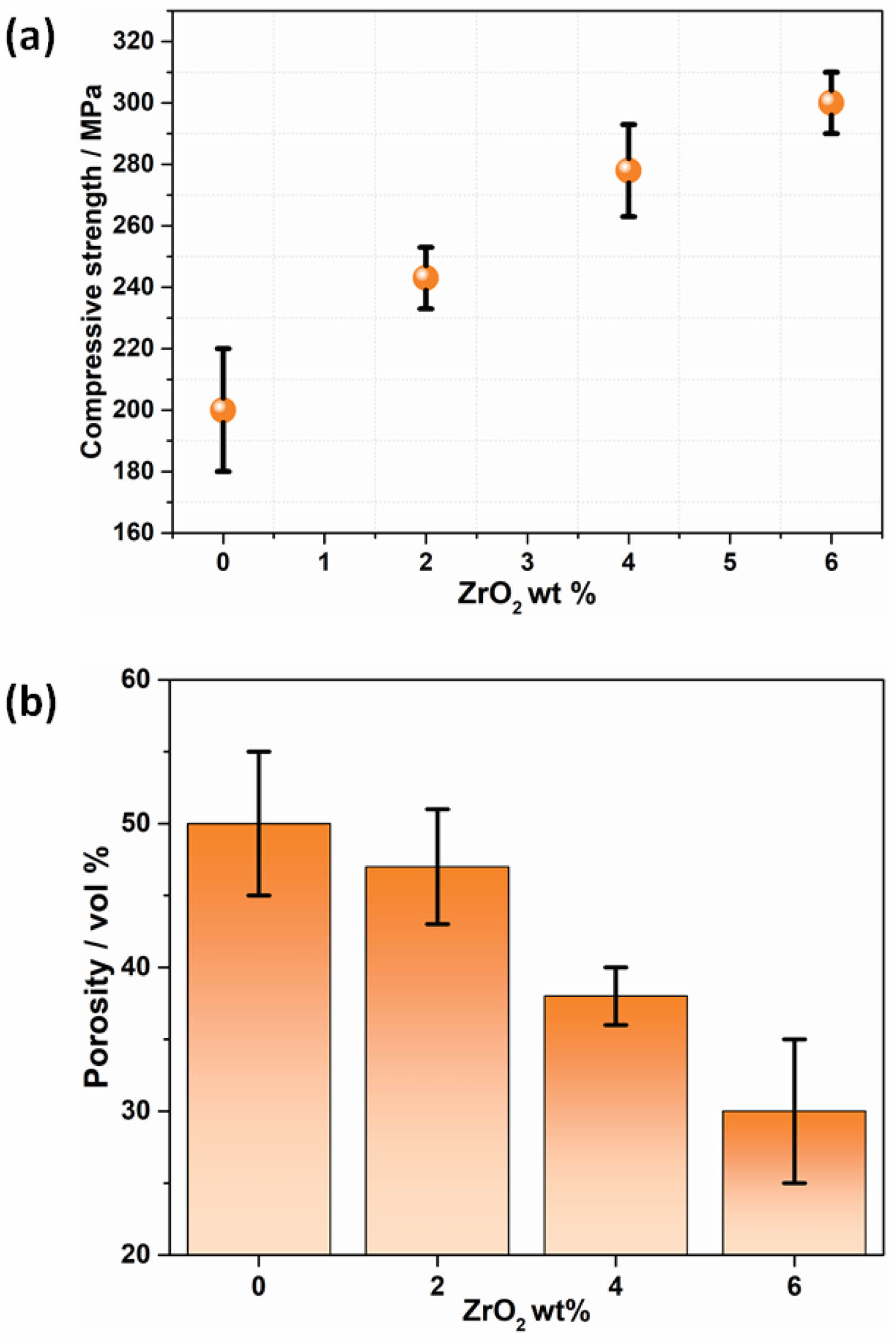
|
Fig. 2 Effect of ZrO2 content in BG-85S with respect to compressive strength and porosity. |
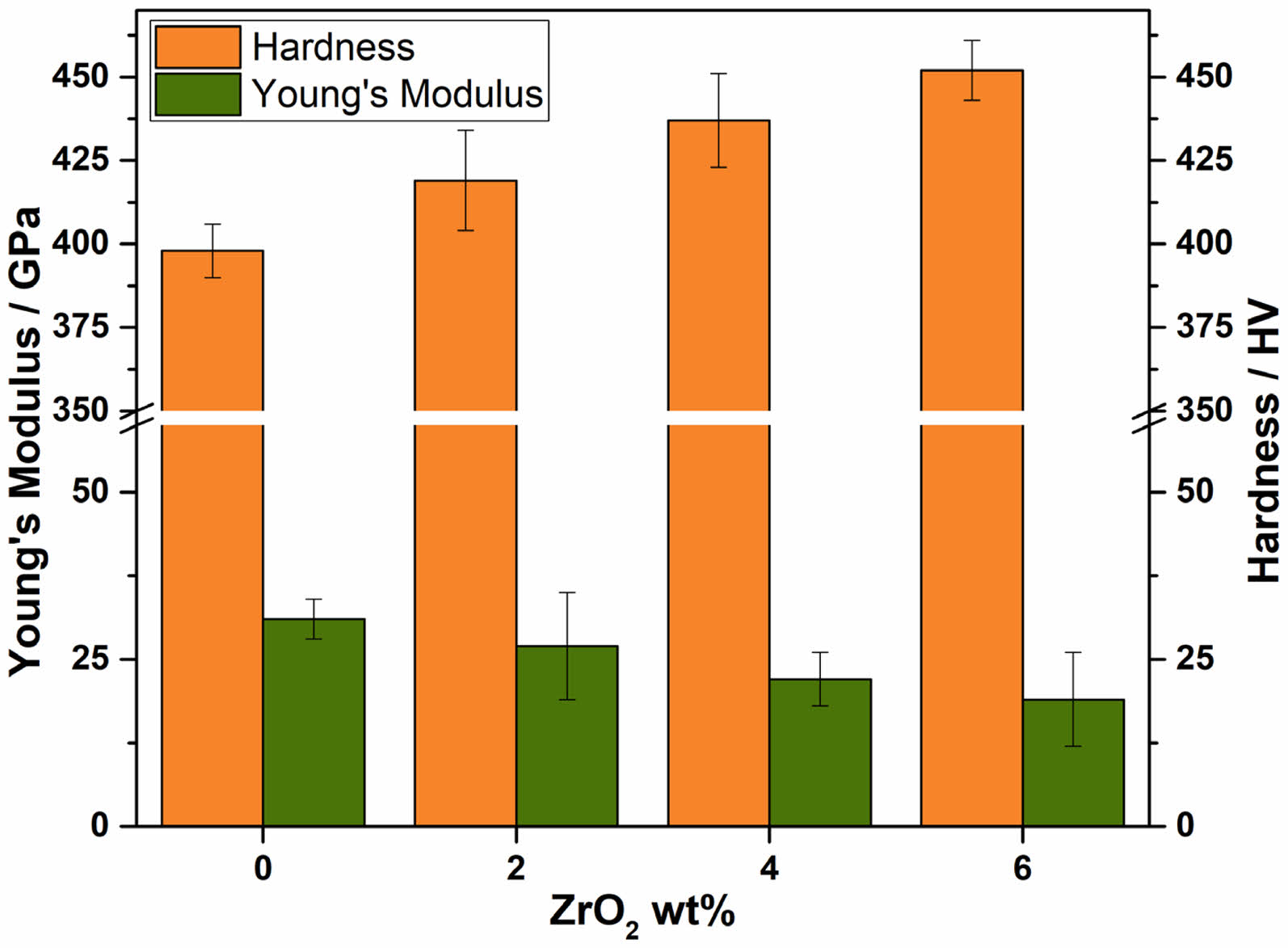
|
Fig. 3 Effect of ZrO2 content in BG-85S with respect to Young’s modulus and Hardness. |
The study demonstrates that the incorporation of ZrO₂ into bioactive glass significantly enhances its mechanical properties, making it a promising candidate for biomedical applications. The compressive strength of the glass increases markedly with higher ZrO₂ content, reaching a maximum of approximately 300 MPa at 6 wt% ZrO₂. This improvement can be attributed to the reinforcing effect of ZrO₂, which provides structural integrity and resistance to mechanical deformation. Concurrently, the porosity of the glass decreases as the ZrO₂ content increases, resulting in a denser material structure that supports higher compressive loads. This densification effect, combined with the toughness imparted by ZrO₂, enhances the overall mechanical performance of the bioactive glass. The addition of ZrO₂ also improves hardness and Young's modulus, further indicating its suitability for applications requiring high strength and stiffness. Future research should focus on optimizing the ZrO₂ content to maximize these benefits while ensuring the bioactive and biocompatible nature of the glass. This optimization could involve detailed studies on the precise balance between ZrO₂ concentration and bioactivity to develop materials that are not only mechanically robust but also supportive of biological functions. Additionally, investigating the long-term stability and performance of these materials in physiological environments will be crucial for their practical application. Research could explore the degradation behavior of ZrO₂-doped bioactive glasses and their interaction with biological tissues over extended periods. Furthermore, expanding the study to include in vivo testing and clinical trials will provide valuable insights into the real-world efficacy and safety of these materials. Overall, the future perspective involves a comprehensive approach to fine-tune the mechanical properties, bioactivity, and long-term reliability of ZrO₂-doped bioactive glasses, paving the way for their successful integration into biomedical devices and tissue engineering scaffolds.
- 1. J. Lian, J.E. Garay, and J. Wang, Scripta Mater 56[12] (2007) 1095-1098.
-

- 2. M.A. Meyers, A. Mishra, and D.J. Benson Prog. Mater. Sci. 51 (2006) 427-556.
-

- 3. M. Cain and R. Morrell, Appl. Organomet. Chem. 15[5] (2001) 321-330.
-

- 4. O.J. Kamigaito, Jpn. Soc. Powder Powder Metall. 38 (1991) 315-321.
-

- 5. W.A. Soer, K.E. Aifantis, and J.T.M. De Hosson, Acta Mater. 53[17] (2005) 4665-4676.
-

- 6. U. Anselmi-Tamburini, J.E. Garay, Z.A. Munir, A. Tacca, F. Maglia, G. Spinolo, and G. Chiodelli, J. Mater. Res. 19[11] (2004) 3255-3262.
-

- 7. A. A. Elmustafa and D.S. Stone, J. Mechanics Phys. Solids 51[2] (2003) 357-381.
-

- 8. J. Spino, J. Cobos-Sabate, and F. Rousseau, J. Nucl. Mater 322[2-3] (2003) 204-216.
-

- 9. D. Caceres, I. Vergara, R. Gonzalez, and Y. Chen, Nucl. Instrum. Methods Phys. Res. B 191[1-4] (2002) 154-157.
-

- 10. M. Alrwashdeh and S.A. Alameri, Energies 15[21] (2022) 8008.
-

- 11. A.A. Elmustafa, J.A. Eastman, M.N. Rittner, J.R. Weertman, and D.S. Stone, Scripta Mater 43[10] (2000) 951-955.
-

- 12. D. Mondal, S. So-Ra, and B.T. Lee, J. Mater. Sci. 48 (2013) 1863-1872.
-

- 13. P. Sepulveda, J.R. Jones, and L.L. Hench, J. Biomed. Mater. Res. 58[6] (2001) 734-740.
-

- 14. M.R. Ramsheh, A. Behnamghader, and A. Khanlarkhani, Iran Biomed. J. 25[3] (2021) 180-192.
-

- 15. T. Oliveira, G. Botelho, N.M. Alves, and J.F. Mano, Colloid Polym. Sci. 292 (2014) 863-871.
-

- 16. M. Alrwashdeh, S.A. Alameri, and A.K. Alkaabi, Nucl. Sci. Eng. 194[2] (2020) 163-167.
-

- 17. V.C.P.F. Aguiar, R. do N. Bezerra, K.W. dos Santos, I. dos S. Goncalves, K.J.S.G. Costa, D.P. Lauda, T.M.B. Campos, R.F. do Prado, L.M.R. de Vasconcellos, and I.R. de Oliveira, J. Biomater. Sci. Polym. Ed. 35[10] (2024) 1493-1510.
-

 This Article
This Article
-
2024; 25(4): 660-663
Published on Aug 31, 2024
- 10.36410/jcpr.2024.25.4.660
- Received on Apr 24, 2024
- Revised on Aug 12, 2024
- Accepted on Aug 13, 2024
 Services
Services
Shared
 Correspondence to
Correspondence to
- Guangjiu Chen
-
Luzhou Vocational and Technical College, Luzhou, 646000, China
Tel : +86-18881557127 Fax: +86-0830-3151916 - E-mail: chenguangjiu@lzy.edu.cn






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.