- Analysis and preventive measures for spangle formation in antiveiling photoelectric glass
Ke Wanga, Meilun Zhanga, Zhenbo Caoa,b,*, Shengyun Yanga, Yu Hana, Qiao Wanga,b, Shaohua Lia, Yang Zhanga,b, Haifeng Lva, You Zhoua,b and Jinsheng Jiaa,b
aChina National Building Material Photonics Technology Co., Ltd., Zaozhuang, Shandong, 277000, PR China
bChina Building Materials Academy Co., Ltd., Beijing, 100000, PR ChinaThis article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
The effective region of Antiveiling Photoelectric Glass (AVG) has high transmittance for ultraviolet, visible and near-infrared light. At the same time, a high-efficiency light absorption layer can be generated from the substrate outside the effective region to eliminate stray light and improve the clarity of optical imaging. It is widely used in the fields of low-light night vision and space optical imaging. However, the spangle in AVG will affect its optical properties and reduce the transmittance and optical uniformity of the glass. In this paper, AVG was prepared by platinum crucible, and the morphology and size of spangle in AVG were observed by scanning electron microscope (SEM). The surface of the glass substrate containing the spangle was sprayed with carbon. The composition of the spangle and the glass substrate was analyzed by Energy Dispersive Spectroscopy (EDS) energy spectrum. The results showed that the main component of the spangle was Pt element. The valence state of platinum in the spangle was analyzed by X-ray photoelectron spectroscopy (XPS). The results showed that the Pt element in the spangle was mainly in the form of 0 valence and +2 valence. The mechanism and preventive measures of AVG platinum spangle were proposed.
Keywords: Anti vignettiong glass, Spangle, Platinum, Mechanization.
Optical glass is a kind of glass material specially used in optical equipment, which generally requires high purity, high transparency, low scattering and high refractive index. In the manufacturing process, the purity of optical glass is much higher than that of ordinary glass. Platinum crucible is an ideal reaction vessel for preparing optical glass. At present, the melting of high-quality optical glass is carried out in platinum crucible [1-3]. When melted in air or oxygen atmosphere, platinum will inevitably be oxidized to platinum ions and dissolved in the glass, causing pollution to the glass [4]. Spangle is a common defect in the preparation of optical glass by platinum crucible, which is generally in particle or irregular shape. The spangle in glass will cause irregular refraction and scattering of light, reduce its optical uniformity and internal quality, and affect the strength, refractive index and density of optical glass [5]. Therefore, how to control the spangle in optical glass has attracted extensive attention from researchers in the industry. Wiraseranee et al. [6] analyzed the solubility of different oxides for platinum. The results show that the solubility of platinum decreases sharply with the increase of Al2O3, Fe2O3 and MgO content, but decreases slightly with the increase of CuOx content. Acidic oxides significantly inhibit the dissolution of platinum, and alkaline oxides increase the activity coefficient of PtO. Li et al. [7] studied the spangle of heavy barium crown optical glass. The results show that the flash point of heavy barium crown glass is formed by the chemical reaction of platinum crucible of melting glass with barium oxide, one of the glass components, in the air. Ginther [8] studied the platinum contamination in the melting process of silicate glass. In a non-oxidizing environment, the platinum contamination produced when using platinum crucible to melt silicate glass is due to the oxidation of platinum crucible by volatile alkali and alkaline earth oxides separated from the glass melt. Wang et al. [9] studied the structural characteristics and formation mechanism of platinum spangle in special dispersion glass. For SiO2-B2O3-Ta2O5-ZrO2-Na2O system glass, due to the high content of Ta5+ and Zr4+ and the low viscosity of the glass, the erosion of platinum by the melt increases. When the melt is cooled, Ptn+ ions precipitate, agglomerate and grow to form spangle. Li et al. [10] found that the erosion effect of low viscosity glass on platinum crucible is more obvious, and it is easier to form spangle in the glass. For the glass with low viscosity, the diffusion ability of oxygen atoms in the glass liquid is enhanced, and the oxygen atoms are easy to react with platinum to form platinum spangle. At the same time, the platinum spangle content has a linear relationship with the melting temperature and time of the glass liquid. It can be seen from the above research that the mechanism of spangle formation of optical glass with different components will be different. Alumino-silicate glass has excellent chemical durability and mechanical stability due to the excellent glass forming ability of Al3+ and Si4+ cations among constituentions [11]. As a special key material for low-light-level night vision instrument, AVG belongs to alummino-silicate glass, and it is easy to form spangle due to its high viscosity and melting in air environment [12]. However, there are few systematic studies on the formation mechanism of spangle, and there are no relevant reports.
In this paper, AVG was prepared by melting annealing method. The morphology and size of spangle were analyzed by SEM. The elemental composition and valence state of AVG spangle were analyzed by EDS and XPS. The mechanism and preventive measures of AVG spangle formation were proposed.
The AVG powder was mixed evenly and melted using a platinum crucible. After feeding, melting, clarification of the glass liquid, cooling in the glass liquid furnace, forming, and rough annealing in a segmented mesh belt annealing furnace, the AVG was placed in an annealing furnace that warmed up in advance for further fine annealing. After the fine annealing is completed, the sample is processed into the size required for the test.
The high temperature viscosity-temperature curve of 200 g AVG fragment sample was tested by Orton high temperature viscometer in the United States, and the test temperature range was 900°C-1450°C.
The microstructure and EDS scanning analysis of AVG samples with flash point were carried out by Zeiss Sigma360 thermal emission electron microscope combined with Oxford energy spectrometer, and the acceleration voltage was 20 kV.
XPS spectrum testing using AXIS SUPRA photoelectron spectrometer. The energy scanning range was 1-1400 eV, and the sample size was less than 25×25×10 mm3. The element type, content and valence state of the glass sample were qualitatively analyzed. Test conditions: analysis chamber vacuum: less than 5.0×10-9 Torr, X-ray source: Al Ka (1486.6 eV), scanning mode: Spectroscope, element scanning range: all elements between Li3-U92 in the periodic table.
Spangle morphology and composition analysis
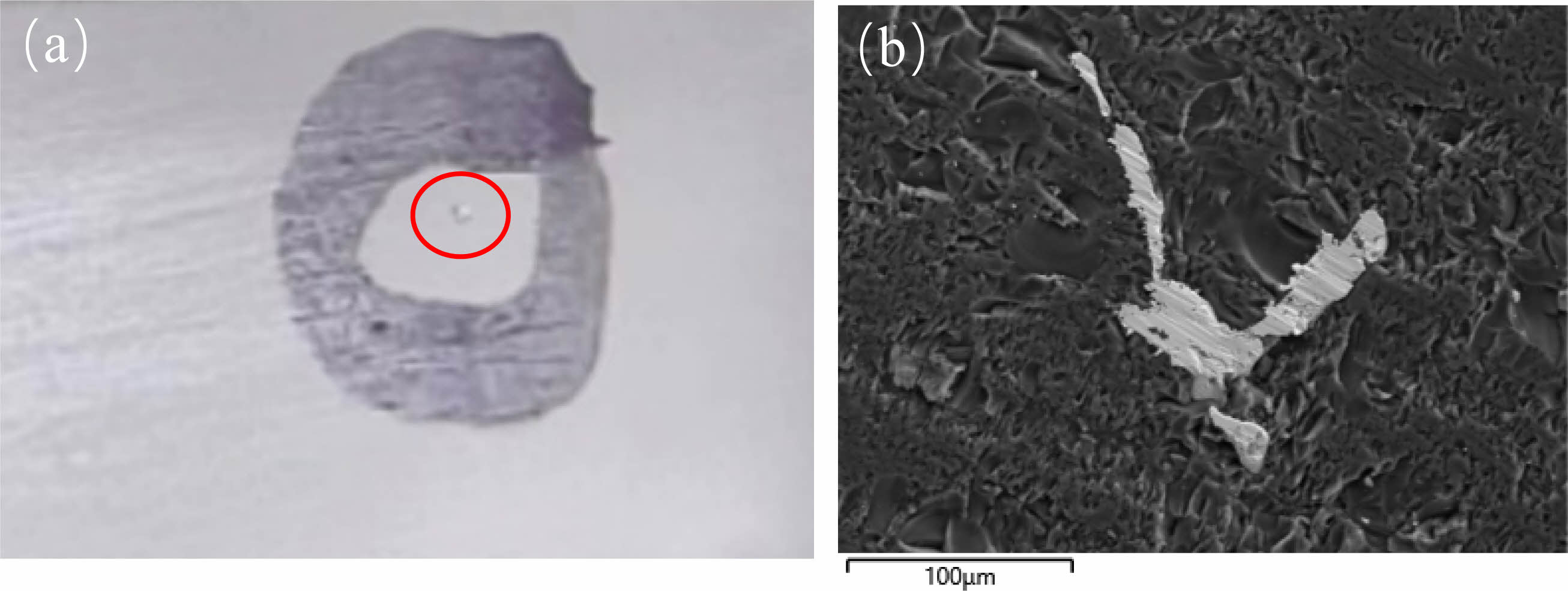
Fig. 1(a) is the surface morphology image of AVG glass substrate with spangle under the microscope. The magnification is 20 times. The red circle mark in the picture is the AVG spangle. It can be seen from the picture that the size of the spangle is large and visible to the naked eye under the microscope.
The size and morphology of the AVG spangle were characterized by SEM. The SEM image is shown in Fig. 1(b). It can be seen from the diagram that the spangle in the glass matrix is an irregular strip with a size of about 100 μm.
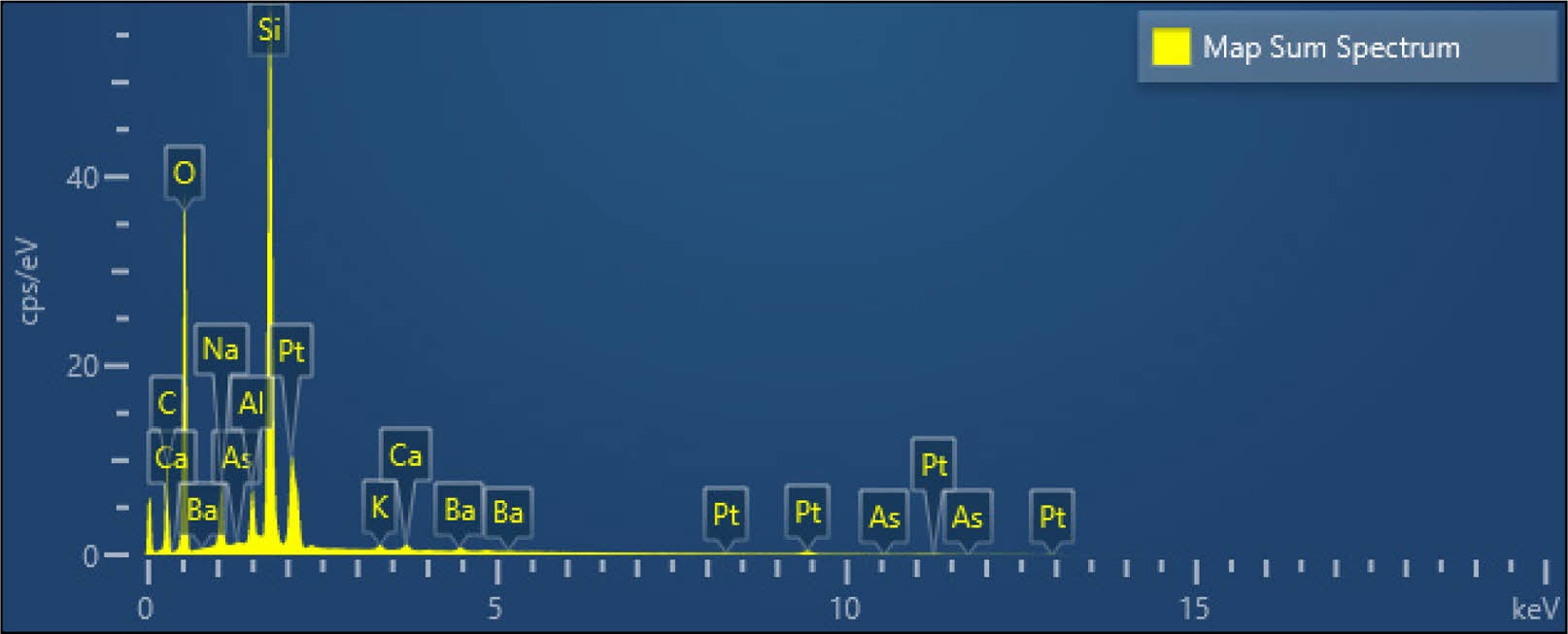
In order to further explore the composition and causes of AVG spangle, EDS composition analysis of AVG glass matrix and spangle was carried out. Fig. 2 shows the EDS analysis spectrum of AVG glass matrix and spangle. It can be seen from the spectrum analysis that the main component element of AVG matrix is Si, and the content of Al element is also high. The addition of Al2O3 into the glass enhances the glass forming capacity and at the same time reduces the non-bridging oxygen [13]. The introduction of Al element will increase the viscosity of glass liquid, which can play a good role in protecting platinum crucible, but the bubbles are not easy to discharge. Therefore, composite clarifying agents such as Sb2O3, SnO2, CeO2, NaCl and As2O3 are introduced in the glass melting process to accelerate the discharge of bubbles in the glass liquid. The main component of the composite clarifying agent is As2O3.
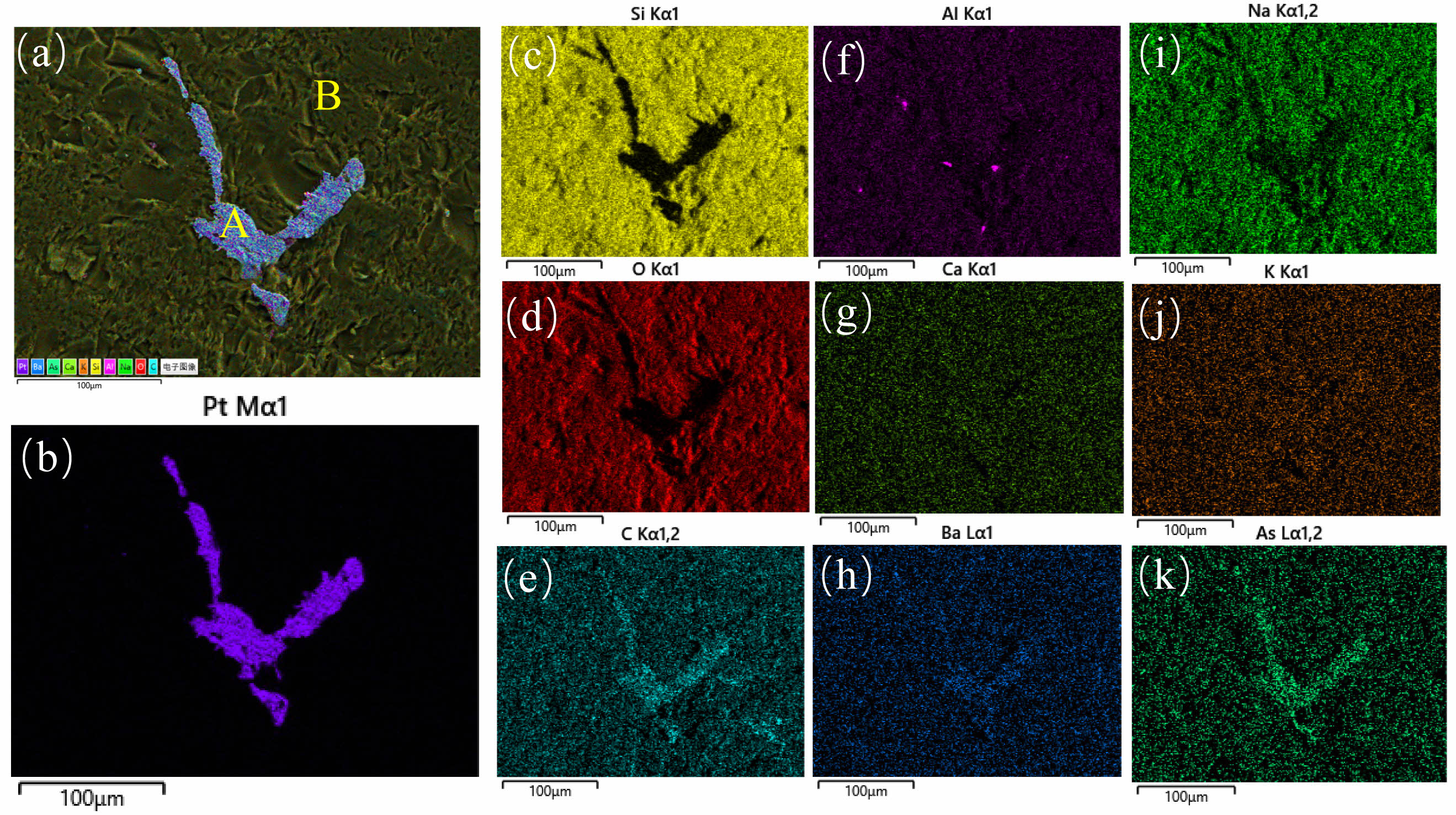
Fig. 3 is the EDS element distribution map of AVG matrix and spangle, and the scanning mode is surface scanning. Fig. 3(a) is the distribution image of each element in the AVG matrix and the spangle, in which A is the AVG spangle and B is the AVG matrix. Fig. 3(b) is the distribution image of Pt element. It can be seen that Pt element is mainly concentrated in the spangle position, and there is no Pt element distribution in the AVG matrix. Fig. 3(c)~(k) is the distribution image of several other elements in the AVG matrix and spangle.
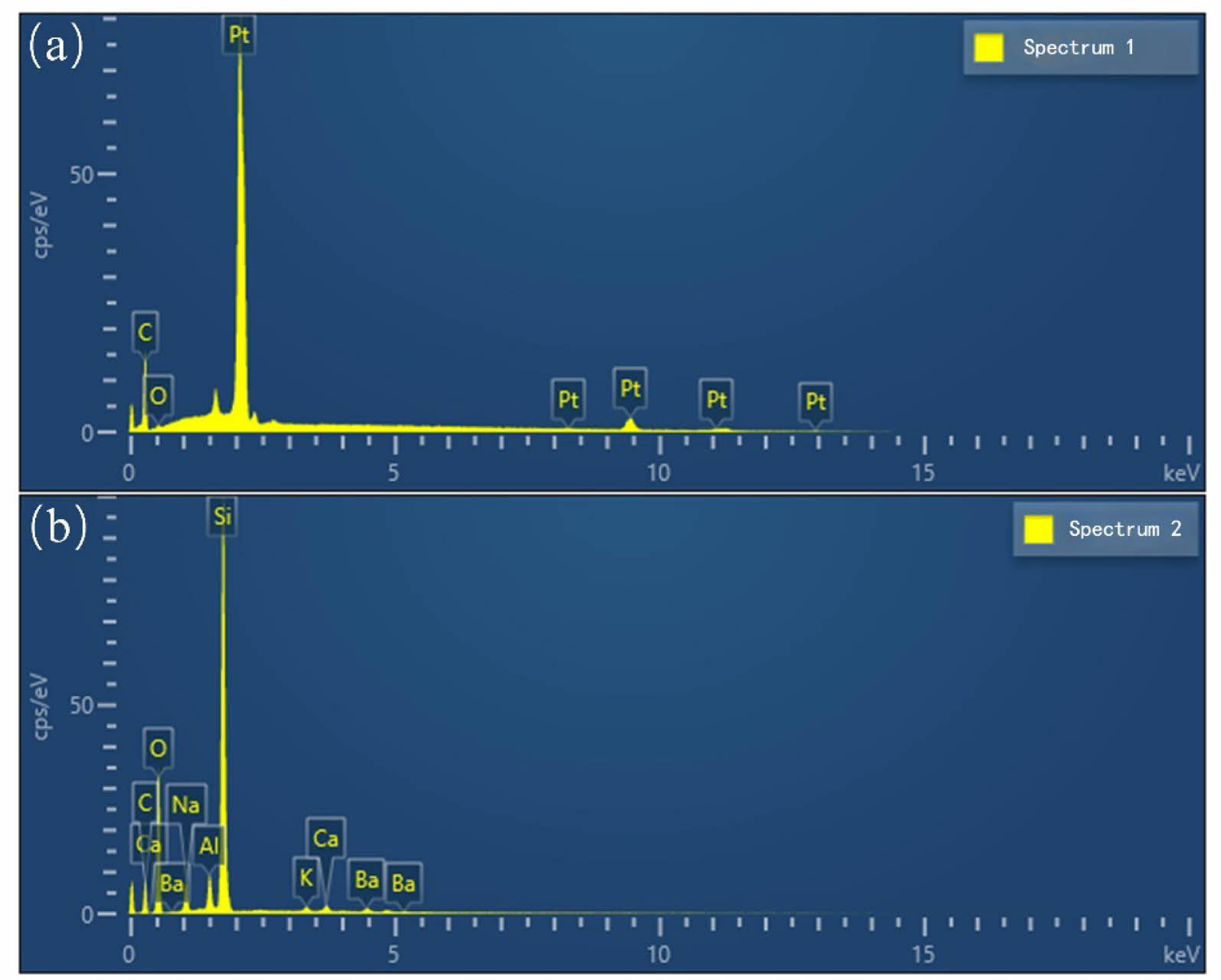
In order to further explore the composition of the spangle, the spangle and the glass matrix were subjected to EDS point scanning analysis. The point scanning position is shown in Fig. 4, and Fig. 5 shows the energy spectrum scanning results of two points. The spangle EDS analysis spectrum of Fig. 5(a) shows that the components of the spangle are Pt element and C element, and the C element is introduced by the carbon spraying treatment on the surface of the spangle. It can be seen that the composition of the spangle is mainly Pt element. Fig. 5(b) is the EDS analysis spectrum of the AVG matrix. The test results show that the glass matrix is composed of Si, O, Na, Al, Ba and other elements, and Pt element is not detected, which is consistent with the surface scanning results of the AVG matrix and spangle. The content of each element in the AVG spangle and matrix is shown in Table.1.
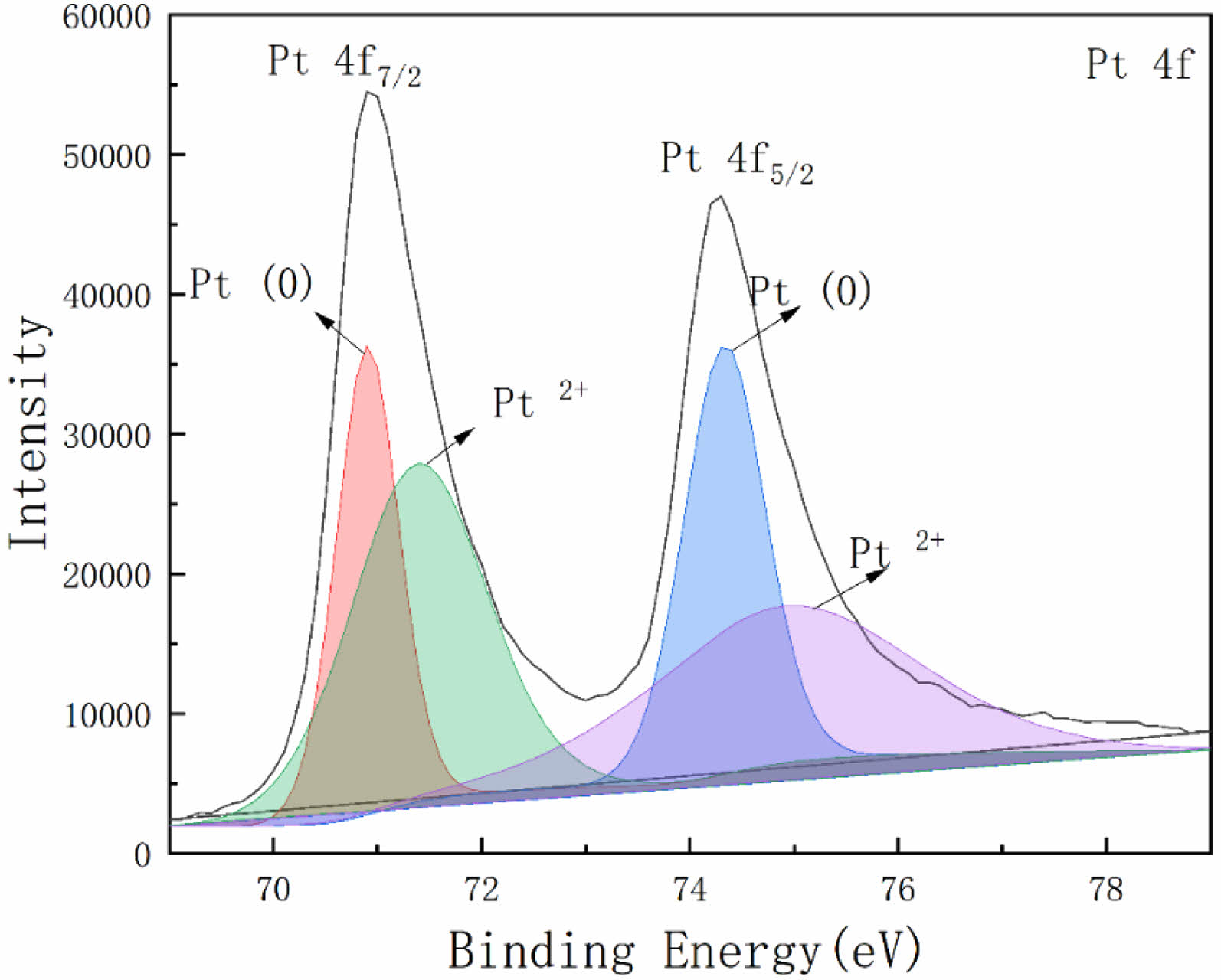
Fig. 6 is the high-resolution XPS spectrum of Pt 4f in the spangle. The analysis shows that the two main peaks of the high-resolution XPS spectrum of Pt 4f are located at 71.3 eV and 74.7 eV, corresponding to Pt 4f7/2 and Pt 4f5/2, respectively. The Origin software is used to fit the XPS spectrum. The fitting method is Gaussian multi-peak fitting. The Gaussian multi-peak fitting curve is the superposition of Gaussian multi-peak functions at different positions to fit the required approximate curve [14]. The function expression of Gaussian multi-peak fitting is
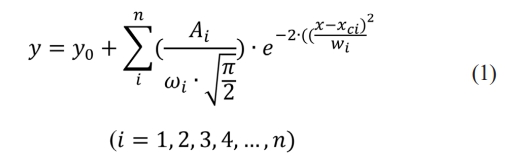
After peak processing, it can be seen that the positions of the sub-peaks are located at 70.9 eV, 71.6 eV, 74.3 eV and 75.2 eV, respectively. Among them, 70.9 eV and 74.3 eV correspond to 0 valent platinum, and the sub-peak convolution integral area is 44034. The 71.6 eV and 75.2 eV correspond to the +2 valent platinum element [15], and the sub-peak convolution integral area is 55960.
The sub-peak convolution integral area ratio corresponding to +2 valence Pt and 0 valence Pt is 1.27:1. Since the sub-peak convolution integral area ratio corresponds to its content ratio, the ratio of +2 valence Pt content to 0 valence Pt content is also 1.27: 1. Among them, Pt2+ exists in the form of PtO compound.
Analysis of the causes of spangle formation of AVG
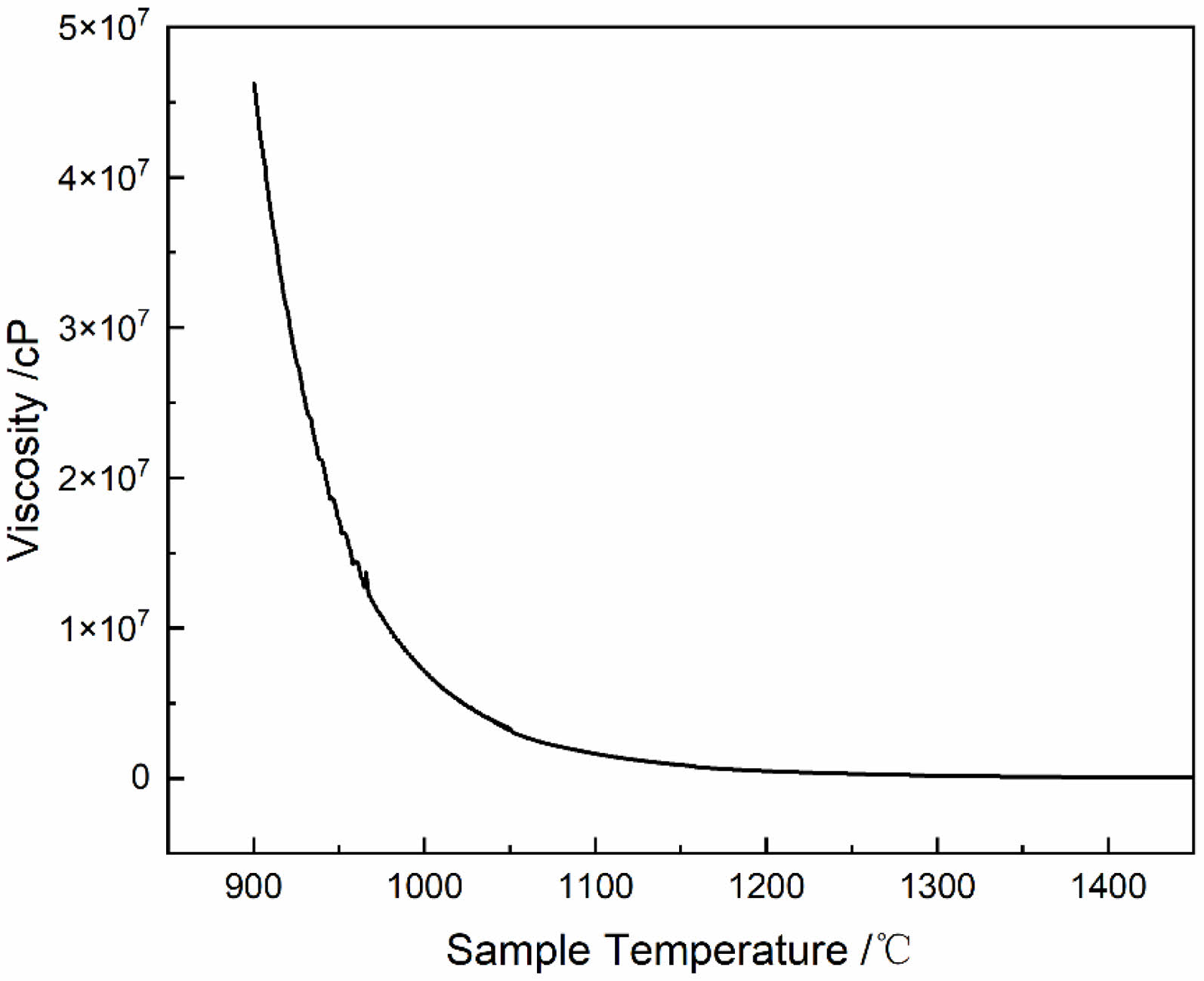
In order to further explore the cause of spangle formation of AVG, the high temperature viscosity-temperature curve of the glass sample was tested, as shown in Fig. 7. It can be seen from the diagram that the viscosity of the AVG is higher and the curve is steeper. When the melting temperature is 1050°C, the corresponding viscosity is 3.2×103 Pa·s. When the melting temperature is 1460°C, the corresponding viscosity is 50 Pa·s, which is a typical high viscosity glass [16].
The formation mechanism of AVG spangle is different from other optical glass. The viscosity of AVG is larger. According to the Stoke's law:

where V is the rising velocity of the bubble, r is the radius of the bubble, η is the viscosity of the liquid glass, ρ and ρ' are the density of the liquid glass and the gas in the bubble. It can be seen from the above formula that the speed of bubble rising is inversely proportional to the viscosity of the glass liquid. The greater the viscosity, The gas inside the glass is not easy to escape [17]. When the glass raw materials contain clarifier As2O3 and nitrate, the reaction of As2O3 in the glass liquid mainly goes through two stages, as shown in formulas (3) and (4). In the first stage, As2O3 reacts with nitrate in the glass solution to form As2O5, and O2 is also released. In the second stage, As2O5 decomposes into As2O3 and O2 under high temperature [18]. The combination of O2 and bubbles in the glass liquid increases the bubble diameter and accelerates the bubble rising rate, thus accelerating the elimination of AVG bubbles.
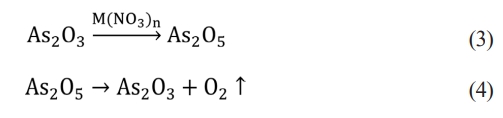
Therefore, the introduction of the clarifying agent As2O3 will produce more O2 in the glass liquid. After a long time of high temperature stirring, the diffusion of oxygen is accelerated. The oxygen in the glass liquid reacts with the platinum crucible to form platinum ions and diffuses into the glass melt. Platinum is more active above 750°C, and it is easy to react with oxygen in the glass liquid to form platinum oxides PtO2 and PtO. The reaction formulas are shown in formulas (5) and (6). The connection structure of platinum atoms and oxygen atoms in PtO2 is loose, and the properties are stable below 1000°C. When the temperature exceeds 1000°C, the properties are unstable, and it is easy to decompose into Pt and oxygen to form platinum particles, which pollute the glass matrix. The reaction is shown in formulas (7)[19, 20]. The properties of PtO are relatively stable, and it is not easy to decompose at high temperature, and it continues to remain in the glass in the form of PtO.
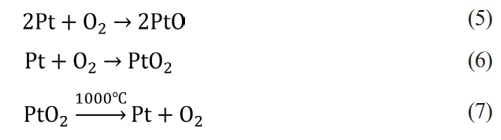
In addition, if the platinum crucible is subjected to high temperature for a long time, the erosion of the glass liquid and the long-term mechanical action of the stirring paddle, the platinum will be aged, the platinum structure will be loose, the platinum strength will be reduced, and the corrosion resistance of the glass liquid will be weakened. It will also cause platinum pollution to the optical glass and form a spangle.
In order to reduce the spangle in AVG glass and improve its transmittance and optical uniformity, the following measures can be taken: (1) Reduce the amount of As2O3 in the composite clarifier, increase the content of clarifier such as SnO2 and NaCl, and reduce the oxygen generated during the glass melting process; (2) Shorten the melting time, reduce the temperature of the clarification section, and avoid the long-term high-temperature melting to aggravate the reaction of platinum and oxygen; (3) Reduce the amount of nitrate, use carbonate instead of nitrate, reduce the oxygen generated during the glass melting process, and reduce the oxygen partial pressure; (4) Platinum crucible is regularly maintained to avoid the platinum structure loose due to the aging of platinum, so that the corrosion resistance of platinum is weakened; (5) Reduce the impeller speed, slow down the oxygen flow in the glass liquid.
|
Fig. 1 AVG spangle image: (a) Microscopic images; (b) SEM images. |
|
Fig. 2 EDS analysis spectrum of AVG substrate and spangle. |
|
Fig. 3 Image of EDS element distribution in AVG substrate and spangle. |

|
Fig. 4 AVG substrate and spangle EDS scanning position image. |
|
Fig. 5 EDS analysis spectrum of AVG: (a) AVG pangle EDS analysis spectrum; (b) EDS analysis spectrum of AVG matrix. |
|
Fig. 6 AVG spangle XPS analysis spectrum. |
|
Fig. 7 High temperature viscosity temperature curve of AVG |
In this paper, AVG was prepared by melting annealing method through platinum crucible at high temperature. The morphology of flash point in AVG was observed by scanning electron microscope. The shape of spangle was irregular strip, and the size was about 100 μm. The results of XPS analysis show that the composition of the spangle is Pt element. Gaussian multi-peak fitting shows that Pt element mainly exists in the form of +2 valence and 0 valence. The ratio of +2 valence to 0 valence Pt element content is 1.27: 1, and +2 valence Pt exists in the form of PtO. The reason for the spangle of AVG is related to the As2O3 in the composite clarifier and the high-temperature clarification stirring time. The introduction of As2O3 will produce a large amount of O2 in the glass solution. Long-term high-temperature stirring accelerates the flow of O2 in the glass solution. O2 is transferred to the surface of the Pt crucible by diffusion. O2 and Pt react to form platinum oxides PtO2 and PtO. PtO2 is easy to decompose at high temperature to form Pt, while the nature of PtO is relatively stable and not easy to decompose. PtO and Pt are dissolved in the AVG glass solution to form Pt spangle. In addition, in order to reduce and control the occurrence of AVG spangle, measures such as replacing the clarifying agent, shortening the melting time, reducing the amount of nitrate, reducing the rotation speed of the stirring paddle, and regularly maintaining the platinum crucible can be taken to effectively prevent.
This manuscript is funded by the special fund of Zaozhuang Talent Gathering Project (Cao Zhenbo), completed with the support of “202350400012” Shandong enterprise technology innovation project and China Building Materials Group Co., Ltd.
- 1. S. Konoshita, F. Sato, S. Yoshihara, and A. Sakamoto, Opt. Mater. 34[4] (2012) 600-603.
-

- 2. M. Kubliha, V. Trnovcová, I. Furár, M. Kadlečíková, J. Pedlíková, and J. Greguš, J. Non-cryst. Solids 355[37-42] (2009) 2035-2039.
-

- 3. J.H. Campbell, J.S. Hayden, and A. Marker, Int. J. Appl. Glass Sci. 2[1] (2011) 3-29.
-

- 4. G.E. Rindone and J.L. Rhoads, J. Am. Ceram. Soc. 39[5] (1956) 173-180.
-

- 5. R.J. Landry and J.T. Fournier, Office. of. Naval. Research, Washington, DC (1969).
- 6. C. Wiraseranee, T. Yoshikawa, T.H. Okabe, and K. Morita, Mater. Trans. 55[7] (2014) 1083-1090.
-

- 7. S.N. Li and G.L. Jin, J. Metall. 01 (1982) 581-587.
-

- 8. R.J. Ginther, J. Non-cryst. Solids 6[4] (1971) 294-306.
-

- 9. Y.H. Wang, X.D. Xu, C.K. Zu, K. He, and P. Zhou, J. Funct. Mater. 48[03] (2017) 3078-3082.
- 10. S.N. Li and G.L. Jin, J. Non-cryst. Solids 52[1-3] (1982) 47-52.
-

- 11. T. Ha and S. Kang, J. Ceram. Process. Res. 23[2] (2022) 237-242.
-

- 12. X.L. Lv, China Building Materials 08 (2016) 80-81.
- 13. D. Kim and S. Kang, J. Ceram. Process. Res. 24[6] (2023) 926-934.
-

- 14. W. Xie, X.S. Jiang, J.N. Xu, Y. Zhou, and D. Wang, J. Vib. Shock 37[24] (2018) 201-207.
- 15. H. Li, Y. Han, H. Zhao, W. Qi, D. Zhang, Y. Yu, W. Cai, S. Li, J. Lai, B. Huang, and L. Wang, Nat. Commun. 11[1] (2020) 5437.
-

- 16. K. Li, X. Lv, Z. Li, J. Zheng, Y. Sun, Z. Bo, T. Bo, S. Hong, Z. Kong, T. Na, and C. Wang, AOPC. 11567 (2020) 932-938.
-

- 17. D. Shiratori, Y. Isokawa, H. Samizo, M. Koshimizu, N. Kawaguchi, and T. Yanagida, J. Ceram. Process. Res. 20[4] (2019) 301-306.
-

- 18. L.F. Wang and M.Y. Zhao, in “Optical Glass Technology” (Beijing: Ordnance Industry Press, 1995) p. 98.
- 19. M. Hujova and M. Vernerova, Ceram. -Silik. 61[2] (2017) 119-126.
-

- 20. M. Zhang and Y.F. Ma, Glass 50[01] (2023) 30-38.
 This Article
This Article
-
2024; 25(4): 607-612
Published on Aug 31, 2024
- 10.36410/jcpr.2024.25.4.607
- Received on Mar 16, 2024
- Revised on Jul 18, 2024
- Accepted on Jul 18, 2024
 Services
Services
- Abstract
introduction
sample preparation and structure performance test
results and discussion
conclusion
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Zhenbo Cao
-
aChina National Building Material Photonics Technology Co., Ltd., Zaozhuang, Shandong, 277000, PR China
bChina Building Materials Academy Co., Ltd., Beijing, 100000, PR China
Tel : 0632-3026007 Fax: 0632-3026006 - E-mail: czb824@163.com






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.