- Quasi-nanocrystalline ZrB2 powder synthesized by salt-assisted combustion and its formation mechanism
Faqi Zhana,*, Ru Baia, Xiao Liua, Ke Xua, Hua Zhanga, Min Zhua, Yuehong Zhenga, Shipeng Xub, Jie Shenga and Peiqing Laa,*
aState Key Laboratory of Advanced Processing and Recycling of Nonferrous Metals, Lanzhou University of Technology, Lanzhou 730050, China
bGansu Key Laboratory of Solar Power System Engineering, Jiuquan Vocational and Technical College, Jiuquan 735000, ChinaThis article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Nanoscale ceramic powders are very important for sintering densification and deviceization. A new salt-assisted combustion synthesis process was used to prepare pure quasi-nanocrystalline zirconium diboride (ZrB2) powder on a large scale. The ZrO2-B2O3-Mg system with single NaCl salt and NaCl-KCl mixed salts as diluents was adopted to prepare ZrB2 powders. The results show that high-purity ZrB2 quasi-nanocrystalline powder with average size of ~105 nm and purity of 99.24% was obtained by adding molten salts diluent. The impurity mainly existed in the form of Mg0.2Zr0.8O1.8 phase and Mg3B2O6 phase. The effects of diluent content and type on the adiabatic temperature, powder purity, particle size and microstructure of the system were studied. This work will provide theoretical basis and technical support for large-scale preparation of boride nano-ceramic powders.
Keywords: Salt-assisted combustion, ZrB2 nanopowders, Molten salt diluent, Formation mechanism.
The boride ceramic materials, known as ultra-high temperature ceramics, exhibit exceptional physical and mechanical properties characterized by high melting points and hardness. They also demonstrate remarkable impact and wear resistance, excellent resistance to chemical agents, as well as a high capacity for neutron absorption [1-3]. Zirconium diboride (ZrB2) is an ultra-high temperature ceramic material with great development prospect. It has high melting point, high hardness and strength, high thermal conductivity and electric conductivity [4-6], good oxidation resistance, wear resistance and corrosion resistance [7, 8]. Owing to these excellent properties, ZrB2 is widely used in propulsion materials, electrodes, thermocouples, high temperature structural ceramic materials, anti-corrosion and wear-resistant coating materials and cutting tools in aerospace [9-13]. However, the development and application of most high melting point compounds such as ZrB2 are limited by the poor sintering performance [14]. The main reason is that the strong covalent bond, low self-diffusion coefficient and large particle size in the material jointly inhibit the diffusion and degradation of grains [15]. However, the preparation of ZrB2 powder with smaller particle size and higher surface energy can well solve this problem [16, 17].
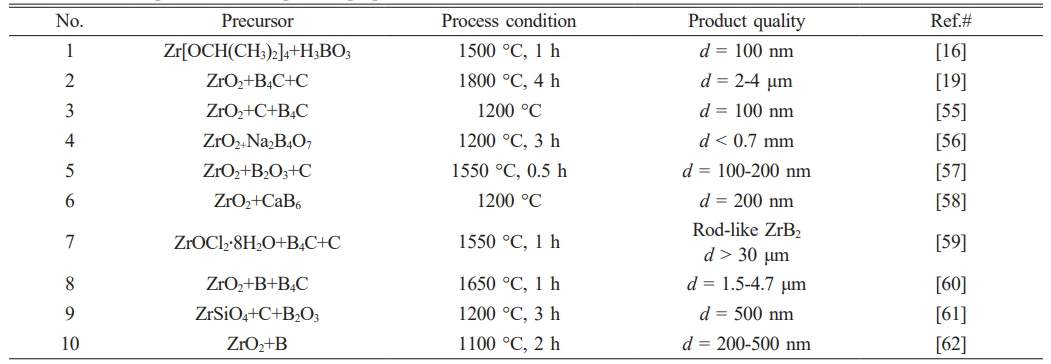
At present, the commonly used preparation methods of ZrB2 are carbothermal reduction method [18, 19], sol-gel method (Sol-Gel) [20, 21], self-propagating high temperature synthesis method (SHS) [22, 23], salt-assissed combustion synthesis [24, 25], mechanical alloying (MA) [26, 27], etc. In recent years, Gye et al. [28] used ZrO2 and B4C as raw materials to synthesize submicron ZrB2 powder with minimum particle size of 245 nm by carbothermal reduction method. Hossein et al. [29] prepared ZrB2 powders with average particle size less than 250 nm by sol-gel method using ZrCl4 and H3BO3 as raw materials. Mustafa et al. [30] used ZrO2, B2O3 and C as raw materials to prepare ZrB2 powder by mechanical alloying. The crystallite size was 0.5~1.0 μm. Wang et al. [31] added a diluent NaCl into the raw materials of ZrO2 and amorphous B, and synthesized ZrB2 powder with an average particle size of 500 nm by salt-assisted combustion. According to recent study, most of the reported ZrB2 powder particle size is above 250 nm, which does not really reach the nanometer level. Therefore, large-scale preparation of ZrB2 nanopowder (~100 nm) is technical bottleneck.
In this paper, ZrO2-B2O3-Mg system was adopted by salt-assisted combustion synthesis. By regulating thermodynamic [32, 33] and kinetic conditions [34, 35] via adding single NaCl and mixed NaCl-KCl salt as diluents, high purity quasi-nanocrystalline ZrB2 powder with the average particle size of ~100 nm was obtained. The effect of the inert diluent on the purity and particle size of ZrB2 and its mechanism were investigated.
Material
The experimental materials were shown in Table 1.
The total chemical reaction equation is as follows:
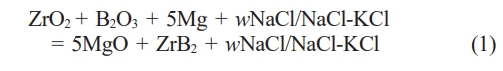
ZrO2, B2O3 and Mg powders were mixed at the molar ratio of 1:1:6.25 (with 25% excess of Mg). The mass percent w of the diluent was 0 wt.%, 50 wt.%, 60 wt.%, 70 wt.% of the total mass of the material (excluding excess Mg). The mixed powder was ball milled for 8 h. ZrB2 powder was synthesized by self-propagating reaction in a self-designed combustion synthesis furnace under the protection of 2.0 MPa argon [36, 37]. After natural cooling of sample in the reactor, the black sample was taken out and crushed. The reaction products need to be purified to completely remove MgO and residual salts by washing with 3.0 mol/L hydrochloric acid and distilled water. Finally, the product was dried in a vacuum oven at 70 °C for 12 h.
Characterization methods
The products before and after leaching were identified by D/MAX-2400 X-ray Diffractometer (XRD) with copper target Kα as radiation source. The particle size and microstructure of ZrB2 powder were analyzed by JSM-6700 Scanning Electron Microscope (SEM) and JEM-2010 Transmission Electron Microscope (TEM). The particle size distribution of the samples was analyzed by Image-Pro Plus 6.0 image statistical software. The specific surface area of sample was measured by JW-BK200C specific surface and pore size analyzer (BET). The surface element content and chemical valence of the samples were determined by Shimadzu AXIS SUPRA X-ray Photoelectron Spectrometer (XPS).
Thermodynamic calculation of system
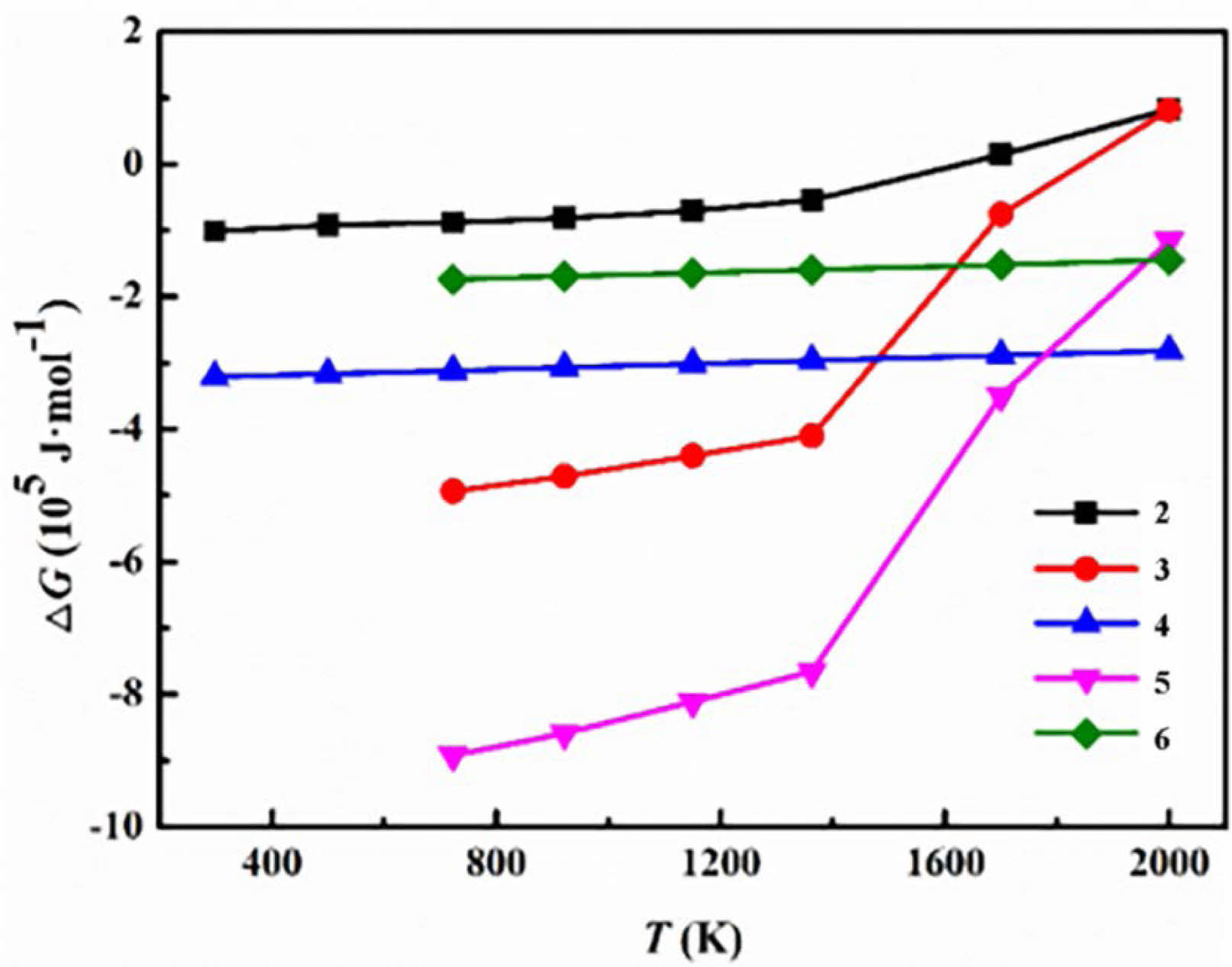
The Gibbs energy ∆G of the possible reactions (Table 2) in ZrO2-B2O3-Mg system was calculated, and the relationship curve of ∆G-T of each reaction was presented in Fig. 1. The relevant data were referred to the Handbook of thermodynamics [38].
Fig. 1 showed the change trend of ∆G in each reaction with temperature. It could be seen that the ∆G was less than zero in the temperature range of 298 K to 1800 K, which indicated that the reaction could be spontaneous. In these reactions, ∆G of reaction (5) was the most negative. This indicated that the positive trend of reaction (5) was the largest, and ZrB2 and MgO formed by the reaction were the most stable phases. In addition, the three stages of reaction (2), (3) and (4) which constitute reaction (5) were spontaneous.
In addition, the ∆G of reaction (2) and (3) was greater than zero as the temperature exceeded 1800 K, and it was likely that the inverse reaction would occur. Inadequate magnesium thermal reduction resulted in residual ZrO2 or the formation of intermediate product ZrxMgyOz [39, 40]. Further, impurity phases were generated in reactions (6), and ∆G was slightly higher than that of reaction (3). So the Mg3B2O6 was inevitably generated in the reaction [41]. In conclusion, it was necessary to consider that the reaction temperature was too high, which leaded to reverse magnesium thermal reduction and even the formation of impurities in the early stage of experimental design.
The adiabatic temperature Tad refers to the maximum temperature that the system can reach under ideal adiabatic conditions. There is no heat exchange between the system and the outside world, the reaction is strictly in accordance with the reaction equation and there is no leftover of the reactants at standard atmospheric pressure, which is the ideal adiabatic conditions [42]. According to experience, spontaneous reactions can be maintained at Tad ≥ 1800 K. If Tad < 1800 K, preheating, chemical furnace and thermal explosion can be used for heating system.
The adiabatic temperature calculation formula of the system [43]:
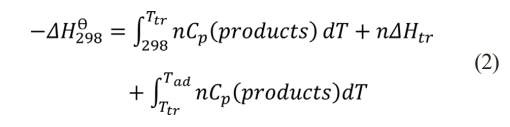
Where ∆H q298 is the standard molar enthalpy of formation of the product at 298 K; Cp is the molar constant pressure heat capacity of the product; Ttr is the melting point of the product, and ∆Ht is the melting heat of the product. Cp can be calculated as:
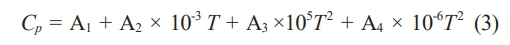
Where Al, A2, A3 and A4 are the coefficients of each temperature term in the constant pressure molar heat capacity calculation formula of matter, which are obtained by querying the thermodynamic manual [38]. Thus, the adiabatic temperature of the system with different diluent additions was calculated by Formula (4):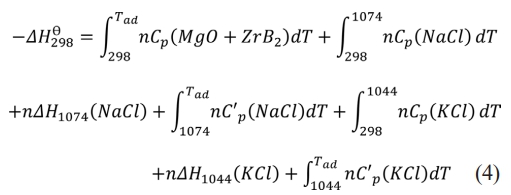
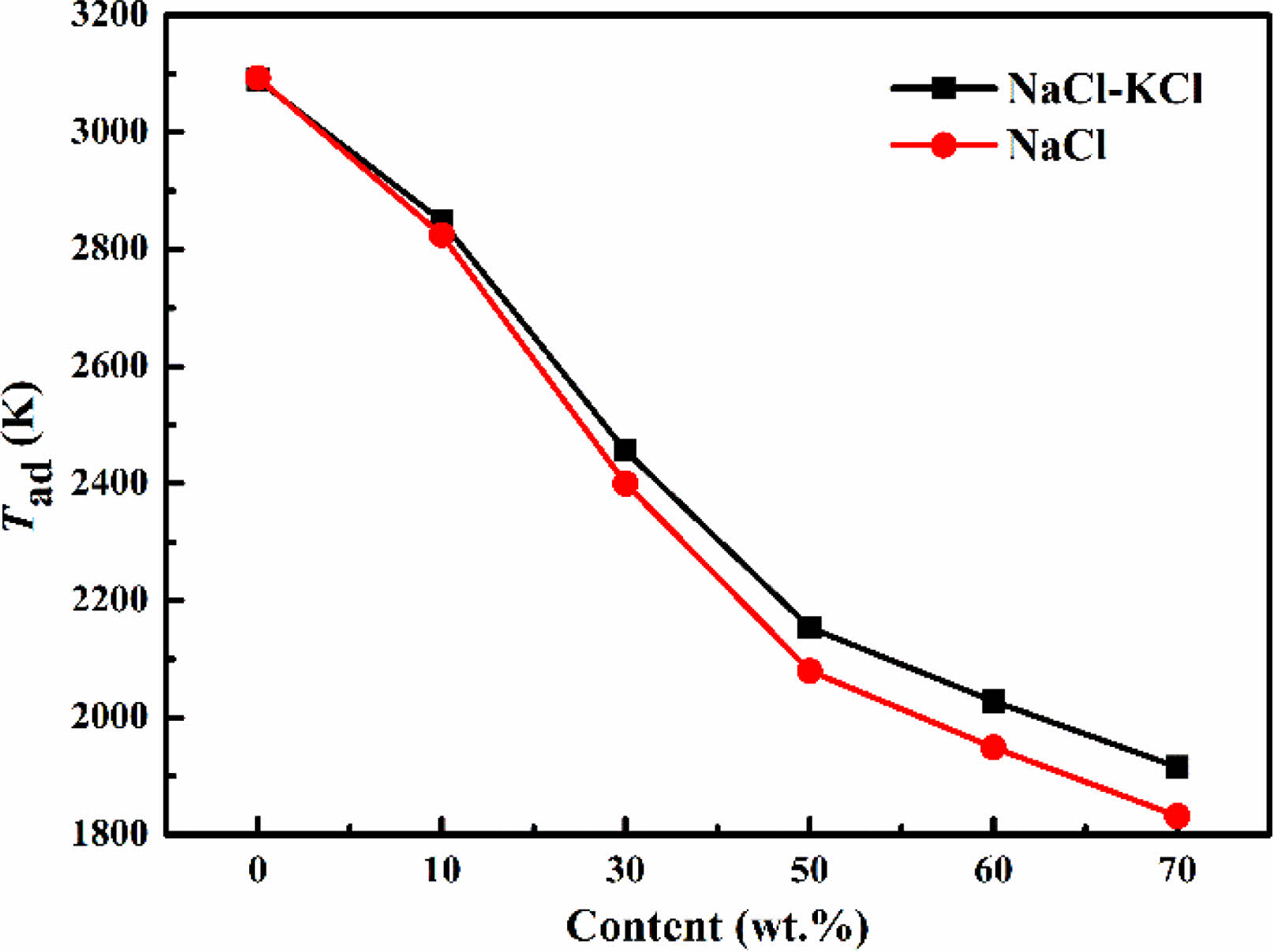
Fig. 2 showed the effect of NaCl and NaCl-KCl as diluents on the adiabatic temperature of ZrO2-B2O3-Mg system. The adiabatic temperature of the system gradually decreased with the increase of the amount of diluent added. The Tad reduced from 3090 K to 1949 K with 60 wt.% NaCl and 2028 K with 60 wt.% NaCl-KCl as the diluent. In comparison of the two diluents, NaCl had a better effect on reducing the adiabatic temperature for the same addition amount. When the amount of diluent was 70 wt.%, the reaction didn’t occur in the actual experiment.
Phase composition of ZrB2 powder
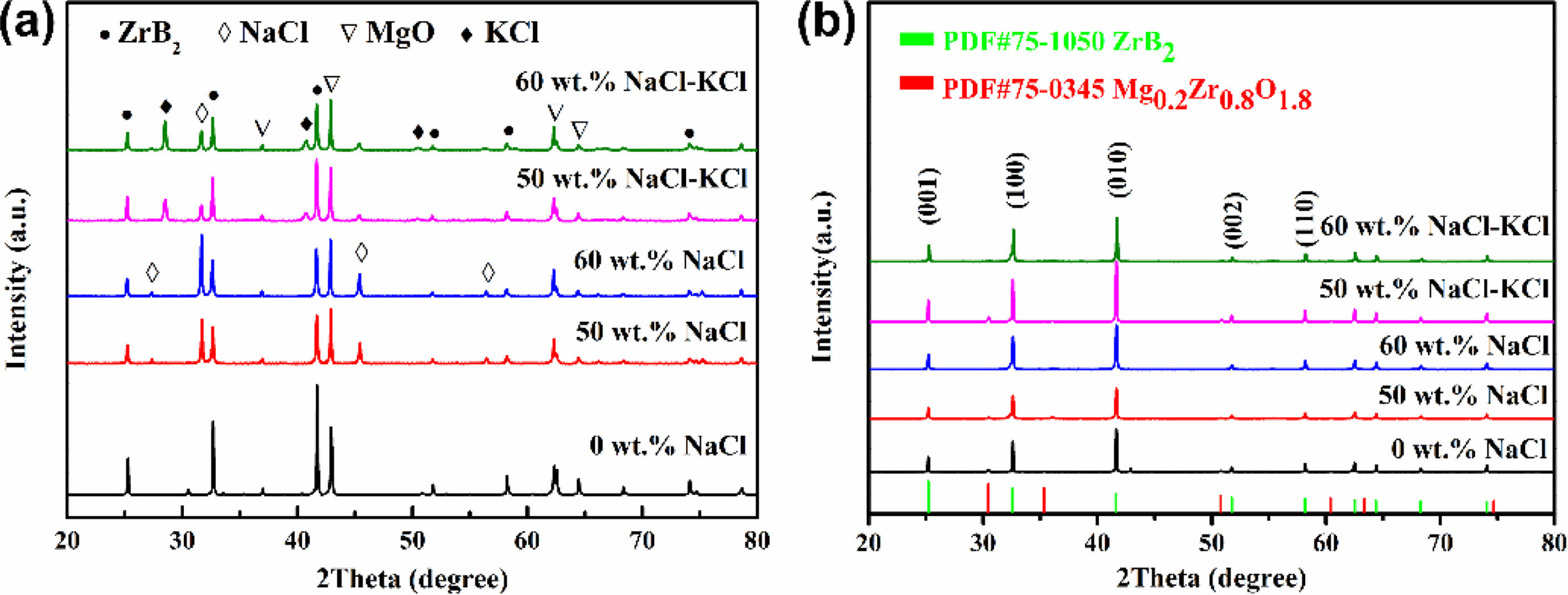
Fig. 3 was the XRD images of ZrB2 prepared by adding different diluents. In Fig. 3a, the main phases before washing were ZrB2, MgO and NaCl with addition of NaCl. And the main phases were ZrB2, MgO, NaCl and KCl with addition of NaCl-KCl. No obvious reactant phase was observed in each group of samples, which indicated that the reaction was sufficient. When the addition amount of NaCl and NaCl-KCl was 0~60 wt.%, the combustion synthesis reaction of ZrO2-B2O3-Mg system could be carried out normally, the reaction was sufficient and the excess Mg was also completely volatilized. Here, diluent salt phase appeared in each group, indicating that the diluent was not completely volatilized during the reaction.
In Fig. 3b, the final product after washing was pure ZrB2 only when the NaCl addition was 60 wt.%, while slight Mg0.2Zr0.8O1.8 phase appeared in other samples. According to the relative peak intensity of impurity phase and main phase, the content of impurity phase was low. After the initial product obtained by the salt-assisted combustion synthesis was washed, the by-product MgO and residual salt were completely removed, but the impurity phase Mg0.2Zr0.8O1.8 couldn’t be removed by hydrochloric acid [41]. The reason for the formation of Mg0.2Zr0.8O1.8 phase was the intermediate product of insufficient reduction of ZrO2 by Mg. When the NaCl addition was 60 wt.%, a large amount of molten salt provided liquid mass transfer for the reaction, which was beneficial to the full reaction of Mg and ZrO2.When the NaCl-KCl addition was 60 wt.%, a small amount of impurity phase appeared. This was because the viscosity of the mixed molten salt was higher than that of the single molten salt [44, 45], resulting in a slower mass transfer rate and a part of Mg0.2Zr0.8O1.8 phase remained.
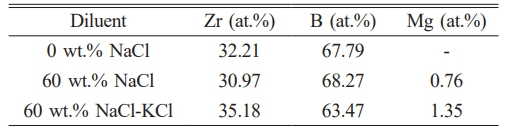
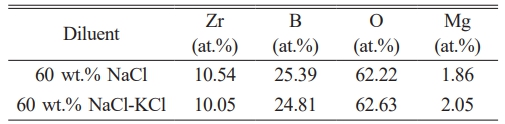
Electron probe X-ray microanalyser (EPMA) was selected to detect the element composition of ZrB2 in each group. The test results in Table 3 indicated that the samples contained Zr, B and Mg elements. The ratio of Zr to B in other samples was close to 1:2, indicating that the phase was ZrB2. From the XRD results, it is found that the samples with high Mg content exhibit high peak strength of Mg0.2Zr0.8O1.8 phase in the XRD patterns, which indicated that Mg exists in the form of Mg0.2Zr0.8O1.8. The content of Mg was the lowest with addition of 60 wt.% NaCl, and the purity of ZrB2 was 99.24%, while the purity of ZrB2 was 98.65% with addition of 60 wt.% NaCl-KCl mixed salt.
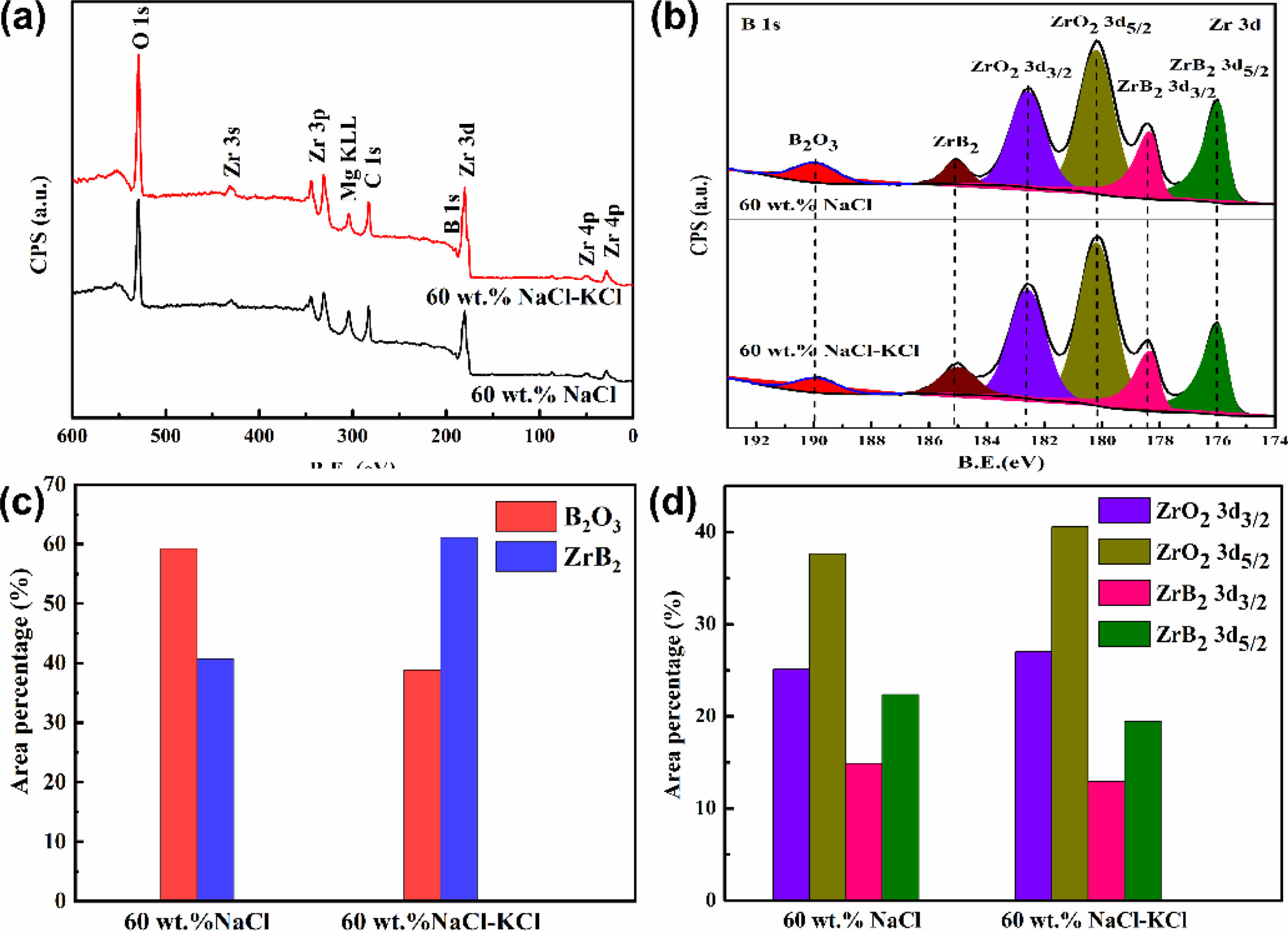
XPS was used to analyze the surface elements of ZrB2 prepared with different diluents. Fig. 4a showed the XPS full spectrum analysis. It could be seen that the surface elements of ZrB2 include Zr, B, O and Mg. This indicated that Zr and B were the main elements of the product, O and Mg were impurity elements. The specific atomic ratio of each element was listed in Table 4. It could be found that the ratio of Zr and B was close to 1:2, and B was slightly excessive. It showed that the main chemical state of these two elements was ZrB2, but there was still a small amount of B enriched on the surface under other chemical states. It was also found that the content of Mg was relatively small. According to the previous analysis, it was inferred that Mg existed in the product in the form of Mg0.2Zr0.8O1.8 or Mg3B2O6. According to the literature findings, the presence of MgZrOx and Mg3B2O6 does not exert any detrimental impact on the utilization of ZrB2 in high temperature ceramic applications [6, 46]. The content of O element was the highest. Except for the impurity phase formed by the reaction with other elements, a large part of it comes from the surface oxidation of ZrB2 particles exposed to air.
Fig. 4b was the relative content of high resolution XPS spectra and chemical states of B1s and Zr3d peaks after ZrB2 washing treatment. Fig. 4b showed that the 3d level of Zr shows typical 3d5/2 and 3d3/2 spin orbital splitting, while the B 1s showed typical single peak, which clearly indicated that there were two different bonding states between Zr and B atoms. In the product ZrB2 powder, the binding energies of a group of Zr3d5/2, 3d3/2 doublet states and B1s singlet state were 176 eV, 178.4 eV and 185.1 eV, respectively [47]. Another group of doublet and singlet states appeared at higher binding energies (180.2 eV, 182.6 eV and 189.9 eV), which were caused by ZrO2 and B2O3 [47]. The content of B2O3 in B1s peak was higher when NaCl was added as diluent in Fig. 4c. According to the calculated adiabatic temperature and Gibbs energy, the adiabatic temperature of the system with addition of 60 wt.% NaCl was 1949 K, This was 100 K lower than adding NaCl-KCl. At this temperature, the Gibbs energy of the magnesium thermal reduction reaction was greater than zero, and the inverse reaction trend increased. Moreover, it was not conducive to the binding of free B and Zr to generate ZrB2 at a lower temperature. Therefore, when NaCl was used as diluent, the B2O3/ZrB2 ratio of the product was larger. Fig. 4d showed that the ZrO2/ZrB2 of both samples were greater than 1.0, and the ZrO2/ZrB2 was larger when NaCl-KCl was added. This indicated that the content of ZrO2 was higher, which was closely related to the oxidation of ZrB2 particles.
Particle size and microstructure of ZrB2 powder
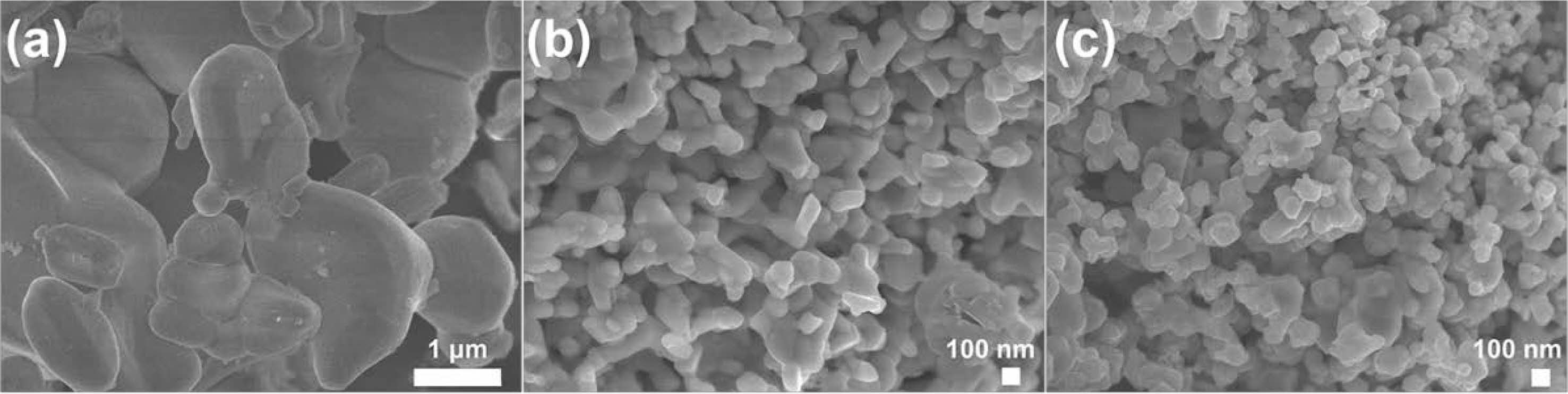
Fig. 5 was the SEM image of ZrB2 powder prepared by adding different diluents after immersion treatment. It could be seen from Fig. 5a that ZrB2 particles prepared without diluents were coarse, and the particle size was generally above 1.0 μm. The particle shape were irregular and agglomeration was serious. In Fig. 5b, the ZrB2 sample with addition of 60 wt.% had spherical and rectangular particles, and particle size was small, but there were adhesion and agglomeration between particles. It could be seen from Fig. 5c, the ZrB2 particles with addition of 60 wt.% NaCl-KCl were finer and more uniform, most of the particle size were about 100 nm, the morphology was mainly spherical with good dispersion.
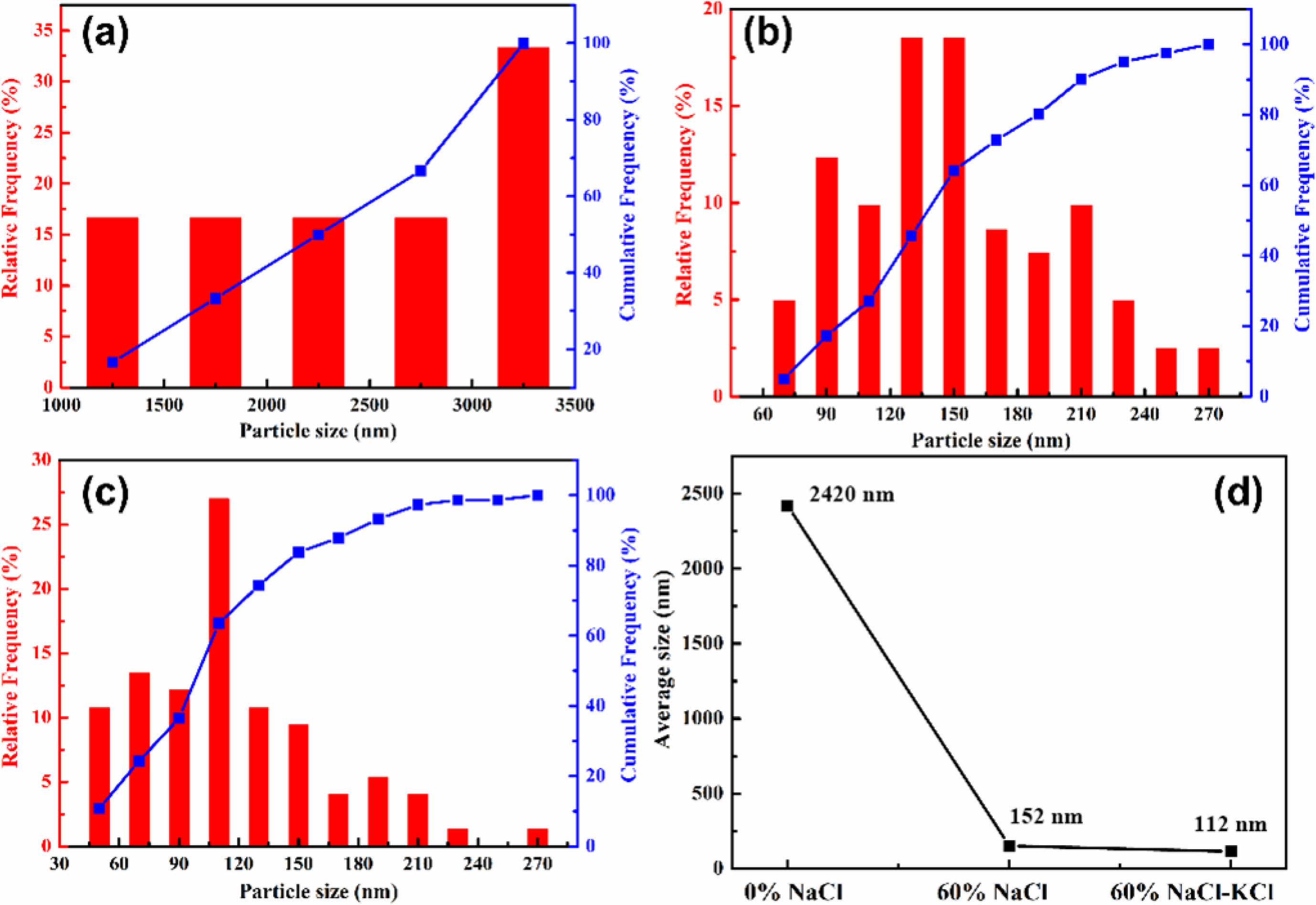
The particle size distribution was showed in Fig. 6. It could be found more intuitively that the particle size of ZrB2 powder decreased with the increase of the amount of diluent. The product had the smallest particle size, more uniform distribution and more regular morphology with addition of 60 wt.% NaCl-KCl. According to the thermodynamic calculation, the adiabatic temperature of the system without diluent was 3090 K, but it decreased to 1949 K and 2028 K with addition of 60 wt.% NaCl and NaCl-KCl, respectively. This resulted in the reaction at a lower temperature and reduced the growth rate of ZrB2 particles. At the same addition amount, NaCl-KCl had higher specific heat and viscosity than NaCl [48, 49], which was beneficial to slow down the heating rate of the system and reduce the crystal growth rate. Therefore, adding NaCl-KCl as diluent could effectively reduce particle size.
The specific particle size indexes of each group of
samples were listed in Table 5. The particle size of ZrB2 powder could be controlled from 2.0 μm to below 100 nm by adding diluents. When 60 wt.% NaCl-KCl was added, the D10, D50 and D90 of ZrB2 particles were 50 nm, 100 nm, and 179 nm, respectively. The minimum average particle size is 105 nm. According to the literature findings, for ZrB2 and its composite ceramic materials, a reduction in grain size leads to enhanced sintering densification of the powder, thereby improving the mechanical properties and high-temperature thermal properties of the material [50, 51].
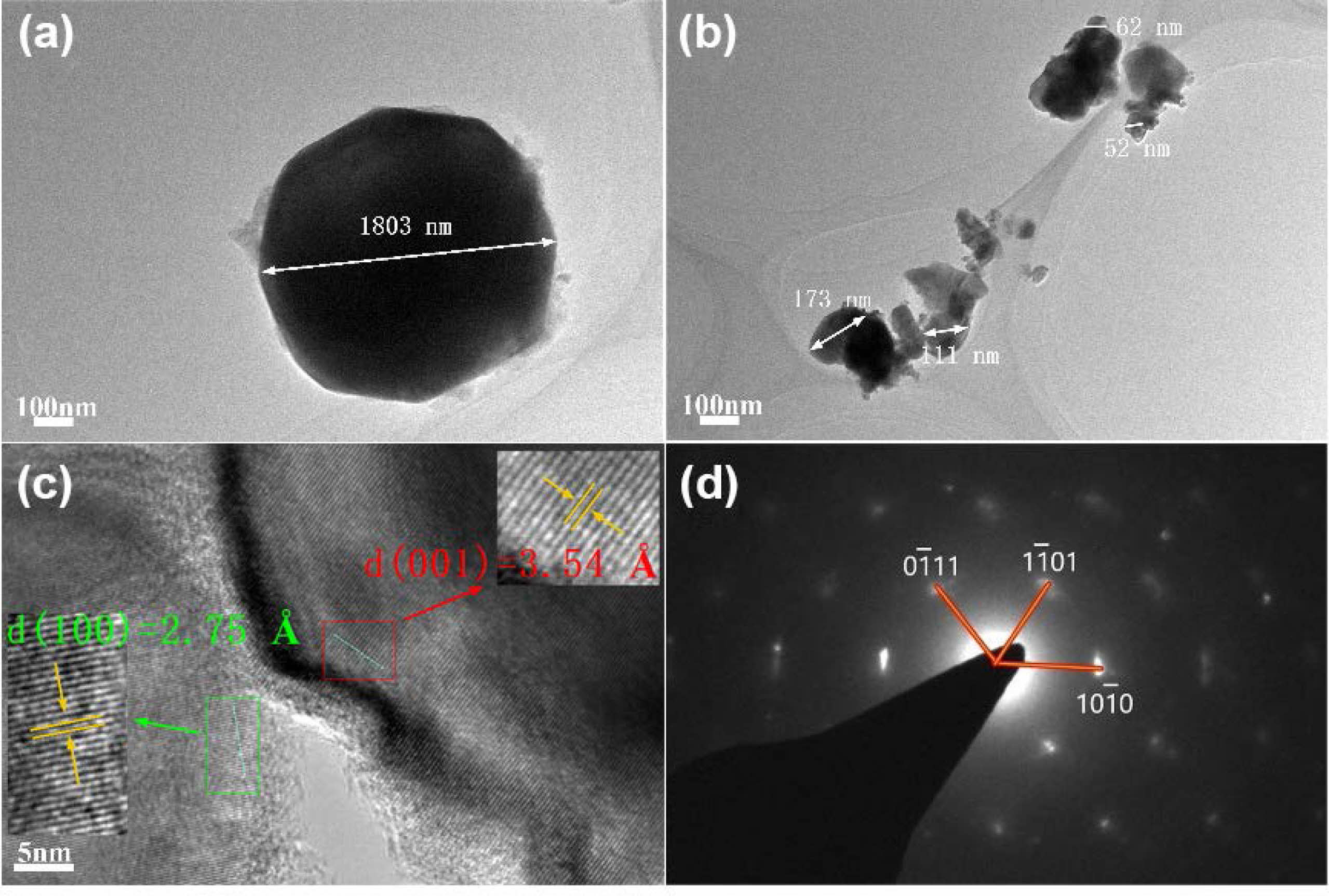
Fig. 7 showed the TEM images of ZrB2 with different diluents. In Fig. 7a, particle size of ZrB2 without salt was large, about 2.0 μm, and the morphology was extremely irregular. When 60 wt.% NaCl-KCl was added, ZrB2 particles with particle size less than 150 nm were obtained, and the minimum particle size was 52 nm (Fig. 7b). The TEM results were consistent with the SEM particle size statistics. The crystal plane spacing of (001) and (100) planes measured from the high-resolution image in Fig. 7c was 3.54 Å and 2.75 Å, respectively, corresponding to the standard ZrB2 crystal [53]. In addition, combined with selected area electron diffraction (SAED) diagram of Fig. 7d, it was determined to be hexagonal ZrB2 particle [54].
In addition, the ZrB2 powder prepared in other literatures was also compared in Table 6, and it was found that the ZrB2 prepared in this paper possessed the smallest average particle size (~105 nm). Importantly, compared with the method reported in other literatures (high-temperature synthesis requires more than 1200 oC), the self-spreading technology only needs to be ignited at ~250 oC to complete the preparation reaction, and the energy cost is lower. Some methods use organic raw materials, which are expensive and difficult to synthesize ZrB2 powder on a large scale. In a comprehensive comparison, the technology in this paper has the advantages of low cost, low energy consumption and suitable for large-scale production, which provides convenience for nano-powders used in the device sintering of boride ceramics.
Formation mechanism of ZrB2 nanopowder in diluent medium
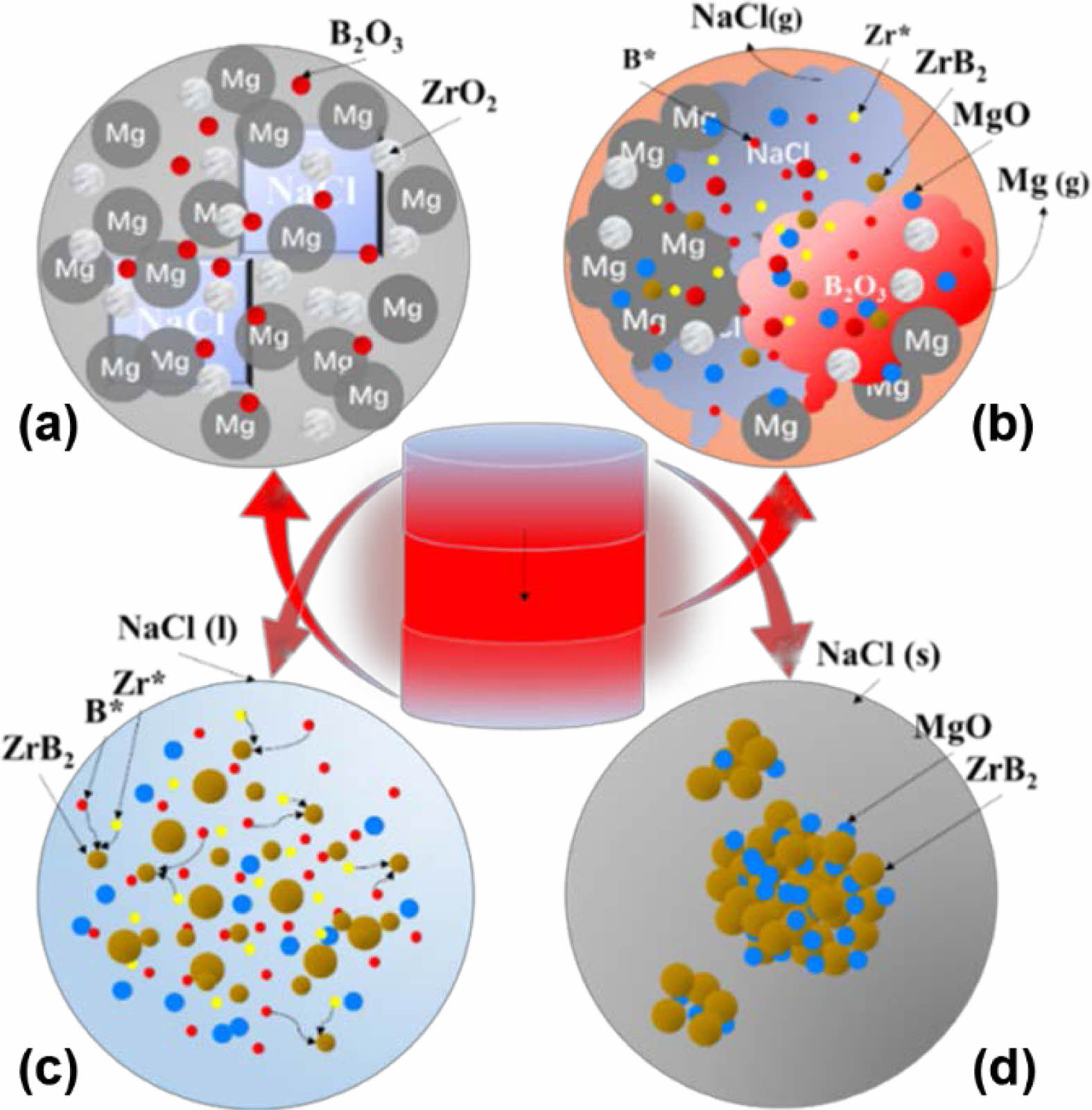
The reaction process of ZrO2-B2O3-Mg system was presented in Fig. 8. The initial mixture was placed in the reactor and preheated to the combustion temperature (533 K) of the ignition agent. The combustion of the ignition agent induced the materials to conducted magnesium thermal reduction reaction, and the self-propagating combustion heating stage began (Fig. 8a). As the temperature of the system increased, the melting point of B2O3 (723 K), Mg (923 K), and NaCl (1074 K) were reached successively. Liquid phase continuously appeared and the reaction degree was constantly intensified [63]. Liquid Mg reacted with ZrO2 and liquid B2O3 respectively, and the heating rate of the system was accelerated (Fig. 8b). The micro reaction region became the heat source of the system and possessed the highest temperature. At the same time, NaCl phase transition absorbed heat and slowed down the heating rate of the system, making it the lowest temperature point in the system. In this case, the temperature field of the system exhibited a maximum temperature gradient [64]. When the heat release rate of the reaction and the heat absorption rate of NaCl reached equilibrium, the system reached the highest temperature [65]. After that, the system kept constant temperature at the NaCl crystallization point. During the constant-temperature process, NaCl continuously melted to provide sufficient liquid phase to coat the reactants and products, as shown in Fig. 8c. At this time, the free Zr* and B* migrated and diffused in molten salt, and combined to form ZrB2 crystal nuclei after contact with each other. The small crystal nuclei continued to grow. However, due to the dilution and dispersion of NaCl, the molten salt surrounded the nucleated grains and inhibited the crystal growth [63]. The special molten salt environment was conducive to the nucleation and growth of ZrB2 crystals into fine and uniform particles. Finally, at the cooling stage, the molten salt crystallized, the crystals stopped nucleating and growing [66], and all reactions completed, as shown in Fig. 8d.
|
Fig. 1 Relationship of ∆G-T in the ZrO2-B2O3-Mg system. |
|
Fig. 2 Effect of different diluent additions on adiabatic temperature. |
|
Fig. 3 XRD patterns of ZrB2 with different diluents: (a) before washing, (b) after washing |
|
Fig. 4 (a) XPS full spectra, High resolution XPS spectra (b) and relative chemical content of B1s (c) and Zr3d (d) peaks of ZrB2 with different diluents. |
|
Fig. 5 SEM images of ZrB2 with different diluents: (a) 0 wt.% NaCl, (b) 60 wt.% NaCl, (c) 60 wt.% NaCl-KCl. |
|
Fig. 6 The particle size distribution of ZrB2 with different diluents: (a) 0 wt.% NaCl, (b) 60 wt.% NaCl, (c) 60 wt.% NaCl-KCl, (d) average particle size of ZrB2 samples. |
|
Fig. 7 The particle size distribution of ZrB2 with different diluents: (a) 0 wt.% NaCl, (b) 60 wt.% NaCl-KCl, (c, d) HRTEM and SAED images of 60 wt.% NaCl-KCl. |
|
Fig. 8 Formation mechanism of ZrB2 powder synthesized by salt-assisted combustion: (a) initial state, (b) temperature-rise period, (c) constant temperature stage and (d) cooling stage. |
|
Table 5 Particle size index of ZrB2 with different diluents |
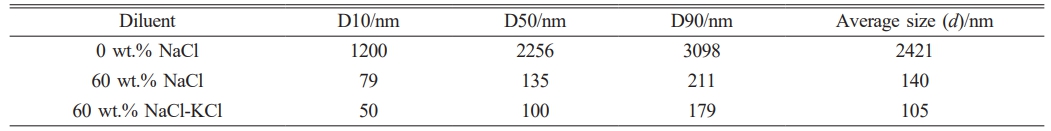
Note: D10, D50 and D90 correspond to the particle size of the sample whose cumulative particle distribution reaches 10%, 50%, and 90%, respectively [52]. |
In this paper, a new salt-assisted combustion synthesis process was proposed to prepare quasi-nanocrystalline ZrB2 ultrafine powders by adding different diluents into ZrO2-B2O3-Mg system. The two different diluents were single salt NaCl and mixed salt NaCl-KCl. The effects of the two diluents on the purity, particle size and microstructure of the product ZrB2 were investigated as well as its mechanism. The quasi-nanocrystalline ZrB2 powders with purity of 99.24% and 98.65% were obtained by adding 60 wt.% NaCl and NaCl-KCl, respectively. The impurity elements mainly existed in the form of Mg0.2Zr0.8O1.8 phase and Mg3B2O6 phase. Morphology and particle size analysis showed that both of two diluents could effectively reduce ZrB2 powder particle size and obtain quasi-nanocrystalline powder with complete morphology and uniform particle size distribution. ZrB2 power particle size could be controlled from 2.0 μm to below 100 nm by adding various diluents. Particle size of the product was the smallest with addition of 60 wt.% NaCl-KCl, and the D10, D50 and D90 of ZrB2 particles were 50 nm, 100 nm, and 179 nm, respectively. The minimum average particle size is 105 nm. The salt-assisted combustion reaction degree, the mass transfer rate, crystal nucleation and growth rate were all affected by the phase transition temperature, specific heat and viscosity of diluents. This work will provide theoretical basis and technical support for large-scale preparation of boride nano-ceramic powders, which has broad application prospect.
This work was supported by the College
Industry Support Plan of Gansu Province (2023CYZC-29), the Gansu Provincial Department
of Education young doctor support project (2023QB-036), the China Postdoctoral
Science Foundation (2022MD723787), the Open Fund of Gansu Key Laboratory of
Solar Power System Engineering Project (2022SPKL04), and the Tamarisk
Outstanding Young Talents Program of Lanzhou University of Technology (062202).
- 1. F.Q. Zhan, H. Zhang, K. Xv, M. Zhu, Y.H. Zheng, and P.Q. La, J. Ceram. Process. Res. 23[5] (2022) 694-708.
-

- 2. Y. Zhang and H. Sun, J. Ceram. Process. Res. 19[4] (2018) 355-359.
-

- 3. J.H. Kimb, J.S. Choia, J.U.K. Hura, S.C. Choia, and G.S. Ana, J. Ceram. Process. Res. 21[3] (2020) 351-357.
-

- 4. G.M. Yuan, X.H. Zuo, Z.J. Dong, Z.W. Cui, and X.K. Li, J. Ceram. Process. Res. 22[1] (2021) 31-38.
-

- 5. E.P. Aa, J. GRa, V. Sb, and A.B.J. Sc, J. Ceram. Process. Res. 21[5] (2020) 524-532.
-

- 6. M.H. Bagherabadi, R. Naghizadeh, H.R. Rezaie, and M.F. Vostakola, J. Ceram. Process. Res. 19[3] (2018) 218-223.
-

- 7. S. Gadakary, M.J. Davidson, R. Veerababu, and A.K. Khanra, J. Min. Metall. B. 52[1] (2016) 69-76.
-

- 8. M. Li and J. Zhang, J. Solid. State. Chem. 289 (2020) 121529-121532.
-

- 9. T. Gui, X.M. Wang, L. Yang, Y.Y Liu, X. Bai, L.J. Wang, and B. Song, Rare Metals. 37[12] (2018) 1076-1081.
-

- 10. H. Jin, S.H. Meng, C.H. Xu, J.H. Niu, and W.H. Xie, Ceram. Int. 42[14] (2016) 16354-16358.
-

- 11. X. Liu, C. Wei, L. Feng, J. Niu, and Z. Yang, Ceram. Int. 43[8] (2017) 6612-6617.
-

- 12. A.M. Stolin, P.M. Bazhin, A.S. Konstantinov, A.P. Chizhikov, and E.V. Kostitsyna, Ceram. Int. 44[12] (2018) 13815-13819.
-

- 13. R.Z. Wang, D.Y. Li, A. Xing, B. Jia, and W.G. Li, Compos. Struct. 162 (2017) 39-46.
-

- 14. L. Bai, Y. Ouyang, and F. Yuan, Nanomaterials. 11[9] (2021) 2345-2362.
-

- 15. V. Zamora, A.L. Ortiz, F. Guiberteau, and M. Nygren, J. Eur. Ceram. Soc. 32[10] (2012) 2529-2536.
-

- 16. M. Rahmani-Azad, A. Najafi, N. Rahmani-Azad, and G. Khalaj, J. Sol-Gel. Sci. and Techn. 103[1] (2022) 87-96.
-

- 17. B.Y. Yang, J.P. Li, B. Zhao, Y.Z. Hu, and T.Y. Wang, Powder Technol. 256 (2014) 522-528.
-

- 18. J.H. Liu, S. Du, and F.L. Li, J. Wuhan. Univ. Technol., 33 (2018) 1062-1069.
-

- 19. B.L. Liu, C.Q. Li, and Y.N. Wang, Eur. Phys. J-Spec. Topics. (2022) 1-8.
-

- 20. J.S. Han, G.S. An, and S.C. Choi, Mater. Today. Commun. 33 (2022) 104646-104651.
-

- 21. C.Q. Liu, X.J. Chang, Y.T. Wu, X. Li, and Y.L. Xue, Ceram. Int. 46[6] (2020) 7099-7108.
-

- 22. S. Cordova and E. Shafirovich, J. Mater. Sci. 53[19] (2018) 13600-13616.
-

- 23. C.L. Yeh and Y.H. Wang, Ceram. Int. 47[5] (2021) 11202-11208.
-

- 24. J. Ma, S. Cao, T. Li, Q. Chen, and D. Zhang, Mater. Sci. Eng. B-Adv. 261 (2020) 114698-114701.
-

- 25. P.Q. La, Y.J. Ou, S.B. Han, X.F. Lu, and Y.P. Wei, J. Mater. Eng. Perform. 43[7] (2015) 14-20.
- 26. W.W. Wu, G.J. Zhang, and Y. Sakka, J. Asian. Ceram. Soc. 1[3] (2013) 304-307.
-

- 27. L. Yuan, C. Wang, M. Bi, S.D. Ma, and X.L. Weng, Adv. Appl. Ceram. 118[7] (2019) 395-402.
-

- 28. G.S. An, J.S. Han, J.U. Hur, and S.C. Choi, Ceram. Int. 43[8] (2017) 5896-5900.
-

- 29. H. Moayyeri, R.M. Aghdam, Ghelich, and F. Golestani-fard, Adv. Appl. Ceram. 117[3] (2018) 189-195.
-

- 30. M. Baris, T. Simsek, T. Simsek, S. Ozcan, and B. Kalkan, Adv. Powder. Technol. 29[10] (2018) 2440-2446.
-

- 31. Y. Wang, Y.D. Wu, K.H. Wu, S.Q. Jiao, K.C. Chou, and G.H. Zhang, Int. J. Min. Met. Mater. 26[7] (2019) 831-838.
-

- 32. M.B. Lee, J. Ceram. Process. Res. 19[2] (2018) 150-153.
-

- 33. A. Masoudian, M. Karbasi, F. SharifianJazi, and A. Saidi, J. Ceram. Process. Res. 14[4] (2013) 486-491.
-

- 34. X. Zheng, Q. Wang, X. Li, C. Zheng, and C. Shen, J. Ceram. Process. Res. 24[5] (2023) 874-883.
-

- 35. J.H. Lee, J.C. Park, B.S. Park, and H.K. Park, J. Ceram. Process. Res. 24[2] (2023) 216-221.
-

- 36. C. Kaemkit, S. Niyomwas, and T. Chanadee, J. Ceram. Process. Res. 21[4] (2020) 460-464.
-

- 37. V. Balouchi, F.S. Jazi, and A. Saidi, J. Ceram. Process. Res. 16[5] (2015) 605-608.
-

- 38. I. Barin, O. Knacke, and O. Kubaschewski, J. Chem. Thermodyn. 2013.
- 39. K.R. Kumar, K.K. Dama, and V.V. Satyanarayana, J. Ceram. Process. Res. 24[3] (2023) 439-445.
-

- 40. N. Ravikumar, R. Vijayan, and R. Viswanathan, J. Ceram. Process. Res. 24[1] (2023) 142-152.
-

- 41. B. Akgün, H.E. Çamurlu, Y. Topkaya, and N. Sevinç, Int. J. Refract. Met. H. 29[5] (2011) 601-607.
-

- 42. P.G. Ovcharenko, T.M. Makhneva, A.Y. Leshchev, V.V. Tarasov, and N.A. Balobanov, Met. Sci. Heat Treat 63[11] (2022) 629-633.
-

- 43. M. Sharifitabar, J. Vahdati khaki, and M. Haddad Sabzevar, Int. J. Refract. Met. H. 47 (2014) 93-101.
-

- 44. C. Koop-Santa, A. Sanchez-Martinez, E.R. López-Mena, J.L.A. Ponce-Ruiz, and E. Orozco-Guareño, Ceram. Int. 47[23] (2021) 33315-33321.
-

- 45. S. Zhang and Y. Yan, Case. Stud. Therm. Eng. 25 (2021) 100973-100984.
-

- 46. E.A. A.S¸ . Demirkıran, Surf. Coat. Tech. 116-119 (1999) 292-295.
-

- 47. A.L. Ortiz, V. Zamora, and F. Rodríguez-Rojas, Ceram. Int. 38[4] (2012) 2857-2863.
-

- 48. H.X. Liu, W.H. Song, Q. Xu, W. Ma, and Y. Bai, Int. J. Electrochem. Sci. 15[7] (2020) 6238-6248.
-

- 49. Q. Yan, Q. Fan, C. Liu, and Z. Jing, Thermochim. Acta 690 (2020) 178689-178706.
-

- 50. M. Thompson, W.G. Fahrenholtz, and G. Hilmas, J. Am. Ceram. Soc. 94[2] (2010) 429-435.
-

- 51. J.-H. Yuan, Q.-Y. Liu, Y. You, L.-Y. Zeng, M.-W. Bai, L.R. Blackburn, W.-M. Guo, and H.-T. Lin, Ceram. Int. 47[11] (2021) 15843-15848.
-

- 52. J. Miller, P. Mulligan, and C.E. Johnson, Powder Technol 375 (2020) 28-32.
-

- 53. C. Zhibo, Z. Xiaotong, L. Mingliang, W. Hailong, and L. Qiang, Ceram. Int. 45[11] (2019) 13726-13731.
-

- 54. M. Velashjerdi, M. Soleymani, H. Sarpoolaky, and A. Mirhabibi, Ceram. Int. 46[18] (2020) 28639-28651.
-

- 55. Y. Wang, Y. Li, X.-H. Yang, and G.-H. Zhang, J. Nanopart. Res. 24[12] (2022) 1-13.
-

- 56. M. Li, C. Ke, and J. Zhang, J. Alloy. Compd. 834 (2020) 155062-155067.
-

- 57. H. Shen, X. Li, C. Hu, Z. Wang, X. Hu, Y. Li, and J. Yan, Surf. Interfaces 25 (2021) 101162-101167.
-

- 58. Z.-B. Li, Y. Wang, H. Zhang, B. Chen, G.-H. Zhang, and K.-C. Chou, Mat. Sci. Eng. A-Struct. 825 (2021) 141870.
-

- 59. Z. Chen, T.S. Suzuki, and H. Wang, Ceram. Int. 49[17] (2023) 28030-28035.
-

- 60. J.-H. Yuan, Q.-Y. Liu, Y. You, L.-Y. Zeng, M.-W. Bai, L.R. Blackburn, W.-M. Guo, and H.-T. Lin, Ceram. Int. 47[11] (2021) 15843-15848.
-

- 61. F. Li, C. Tan, J. Liu, J. Wang, and Q. Jia, Ceram. Int. 45[7] (2019) 9611-9617.
-

- 62. L. Zhou, Q. Fu, D. Hu, J. Zhang, and Y. Wei, Corros. Sci. 183 (2021) 109331-109340.
-

- 63. D. Ding, B. Bai, G. Xiao, J. Luo, and X. Chong, Ceram. Int. 47[13] (2021) 18708-18719.
-

- 64. X. Li, S. Wang, D. Nie, K. Liu, S. Yan, and P. Xing, Diam. Relat. Mater. 97 (2019) 107458-107463.
-

- 65. X. Yi, Q. Li, S. Suzuki, S. Zhang, T. Nomura, and T. Akiyama, Ceram. Int. 45[8] (2019) 10021-10027.
-

- 66. S. Song, J. Sun, J. Zhou, C. Guan, and Z. Hu, CrystEngComm. 23[3] (2021) 671-677.
-

 This Article
This Article
-
2024; 25(4): 490-498
Published on Aug 31, 2024
- 10.36410/jcpr.2024.25.4.490
- Received on Oct 8, 2023
- Revised on Dec 14, 2023
- Accepted on Jan 3, 2024
 Services
Services
- Abstract
introduction
material and methods
results and discussion
conclusion
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Faqi Zhan, and Peiqing La
-
State Key Laboratory of Advanced Processing and Recycling of Nonferrous Metals, Lanzhou University of Technology, Lanzhou 730050, China
Tel : +86 15209310025 Fax: +86 15209310025 - E-mail: zhanfaqi@lut.edu.cn (Faqi Zhan), pqla@lut.edu.cn (






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.