- Enhancement of microhardness and cytocompatibility in Mg-BN nanocomposites for potential biomedical applications
Liwen Wen*
Medical College, Huanghuai University, 463000, Zhumadian, Henan, China
This article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Magnesium (Mg) possesses desirable properties for biomedical applications but suffers from limitations in mechanical strength. This study explores Mg-Boron Nitride (BNNS) nanocomposites as a potential solution. We investigate the microhardness and cytocompatibility of these nanocomposites to assess their suitability for biomedical use. Vickers microhardness testing revealed a significant enhancement (1.5 times) in microhardness with the incorporation of 15 vol.% BNNS nanoparticles. This improvement can be attributed to the presence of BNNS nanoparticles at grain boundaries, hindering dislocation movement and twinning within the Mg matrix. Cytotoxicity evaluation using mouse osteocyte cells demonstrated good cytocompatibility for both bare Mg and Mg-BNNS nanocomposites with low BNNS content. The viability improved with increasing dilution, suggesting a dose-dependent response. These findings highlight the potential of Mg-BNNS nanocomposites for biomedical applications. The increased microhardness offers promise for improved mechanical performance, while the good cytocompatibility at low BNNS content indicates biocompatibility. Future research will focus on optimizing BNNS content, exploring surface functionalization strategies for further cytotoxicity mitigation, and conducting long-term biocompatibility studies.
Keywords: Microhardness, Cytocompatibility.
The demand for biocompatible materials in biomedical applications has driven intensive research into the development of advanced implant materials. Among these, magnesium (Mg) alloys have emerged as promising candidates for temporary implants, offering distinct advantages over traditional materials like titanium and stainless steel. Their ability to eliminate the necessity for a second surgery, owing to their biodegradability, makes Mg alloys particularly attractive for medical device applications. Moreover, Mg alloys exhibit mechanical properties that closely match those of human bone, minimizing the risk of stress shielding and enhancing overall implant performance. The biodegradation of Mg alloys releases Mg2+ ions, which play a pivotal role in promoting tissue growth and healing without adverse effects on the body [1].
However, the widespread adoption of Mg alloys in biomedical implants is hindered by their susceptibility to rapid corrosion in bodily fluids. This corrosion compromises the mechanical integrity of the implants and hampers the tissue-healing process, necessitating the development of effective corrosion mitigation strategies [2-4]. One promising approach is the incorporation of boron nitride nanosheets (BNNS) as a reinforcing agent in Mg composites. By integrating h-BNNS, researchers aim to enhance the corrosion resistance of Mg-based implants, thereby prolonging their functional lifespan and ensuring successful tissue healing outcomes. The utilization of magnesium (Mg) alloys in orthopedic implant applications holds tremendous potential for addressing the shortcomings of traditional implant materials [5]. To enhance the performance of Mg alloys, various strategies such as surface modifications, alloying, and heat treatments have been explored. Among these, Mg composites reinforced with nanoparticles have emerged as a promising avenue, offering significant improvements in strength and ductility without compromising biocompatibility. Particularly noteworthy are Mg nanocomposites reinforced with rare earth oxide nanoparticles, which exhibit remarkable strength and corrosion resistance. Incorporating boron into Mg-based biomaterials presents an intriguing opportunity to further enhance their properties. Boron, recognized as a micronutrient essential for osteogenesis and bone maintenance, has demonstrated pro-angiogenic effects and the ability to promote osteogenic differentiation. BNNS, a boron-containing compound, has also shown promise in enhancing bone fracture healing and stimulating osteogenic differentiation in vitro [6, 7].
Our study focuses on investigating the synergistic effects of incorporating BNNS into Mg matrices to develop Mg-BNNS nanocomposites for orthopedic implant applications. We hypothesize that the addition of BNNS will impart favorable microstructural characteristics to Mg alloys, resulting in improved mechanical properties and enhanced corrosion resistance. Despite extensive research on the degradation and biocompatibility of Mg in physiological environments, there is a notable gap in the literature regarding the characterization of Mg-BNNS nanocomposites in such environments. This work aims to address this gap by comprehensively evaluating the corrosion properties and biocompatibility of Mg-BNNS nanocomposites in a physiological-like solvent relevant to bone implant applications. Through systematic experimentation and characterization, we seek to elucidate the impact of BNNS addition on the performance of Mg alloys in orthopedic settings. The findings of this study will contribute to advancing our understanding of Mg-BNNS nanocomposites and pave the way for their potential translation into clinical orthopedic applications, ultimately benefiting patients in need of advanced implant materials for bone repair and regeneration.
The synthesis of BNNS-Mg composites involved a multi-step process combining powder metallurgy with microwave-assisted sintering method. Initially, BNNS particles (< 1 µm) were incorporated into Mg powder to produce BNNS-Mg nanocomposites with varying volume fractions, specifically Mg with 5% BNNS, Mg with 10% BNNS and Mg with 15% BNNS. The BNNS nanoparticles, provided by Sigma Aldrich, served as the reinforcing agent due to their known properties. Following mechanical mixing, the powders underwent cold compaction under uniaxial pressure at 800 psi before being subjected to sintering at 700 oC in a microwave oven. This rapid sintering process facilitated the bonding of the BNNS nanoparticles with the Mg matrix. In addition to synthesizing BNNS-Mg composites, a control group consisting of pure Mg with no BNNS was also synthesized using the same methodology to facilitate mechanical testing. This synthesis approach offers a systematic and reproducible method for producing BNNS-Mg composites with controlled compositions, paving the way for further investigation into their mechanical properties, corrosion resistance, and biocompatibility for potential orthopedic implant applications.
The microhardness evaluation of Mg-BNNS nanocomposites revealed promising results for their potential use in applications requiring improved mechanical strength. This section will delve into the observed trends and the underlying mechanisms responsible for the increased microhardness. The Vickers microhardness test serves as a valuable tool for assessing the bulk mechanical properties of materials, particularly their resistance to plastic deformation. Our findings demonstrate a clear correlation between the BNNS content and the microhardness of the Mg-BNNS nanocomposites. The microhardness of the synthesized Mg-BNNS nanocomposites and bare Mg samples was evaluated to assess their mechanical properties. The Vickers microhardness test yielded average values of 38 HV for bare Mg (0% BNNS), 47 ± 1.5 HV for Mg with 5% BNNS, and 51 ± 2 HV for Mg with 10% BNNS, and 58 ± 1.4 HV Mg with 15% BNNS (Fig. 2). Interestingly, the addition of only 15 vol.% of BNNS nanoparticles did not result in a significant enhancement in microhardness, suggesting a minimal impact on mechanical properties. However, a notable increase in microhardness was observed with the addition of 15 vol.% BNNS, representing approximately a 1.5 times increase compared to Bare Mg. The BNNS nanoparticles are strategically located at the grain boundaries of the Mg matrix. These interfaces play a crucial role in material deformation. By dispersing at these boundaries, the BNNS nanoparticles act as roadblocks for dislocations, which are line defects within the crystal structure that allow for plastic deformation. This effectively hinders the movement and activity of dislocations, thereby impeding the overall deformation of the Mg matrix grains. Twinning is another deformation mechanism observed in some metals. It involves the formation of mirrored atomic arrangements within a portion of the crystal lattice. The presence of BNNS nanoparticles at grain boundaries can also hinder the propagation of twins, further contributing to the enhanced hardness of the Mg-BNNS nanocomposites [8-11]. Fig. 1, 3
While the addition of 15% BNNS nanoparticles resulted in a substantial increase in microhardness, the results suggest a potential saturation effect. Further addition of BNNS might not yield significant additional benefits. Identifying the optimal BNNS content that maximizes the strengthening effect while maintaining other desirable properties like ductility and fracture toughness. Moreover, investigating the influence of BNNS nanoparticle size and distribution patterns within the Mg matrix on the overall mechanical response. In addition, exploring methods to tailor the interface between the Mg matrix and BNNS nanoparticles to enhance load transfer and further improve the strengthening effect.
Cytotoxicity study
A critical consideration before implementing any nanomaterial for biomedical applications is its biocompatibility which refers to the material’s ability to coexist with living tissues without inducing adverse effects. BNNS, with their unique properties like excellent thermal conductivity and chemical inertness, have emerged as attractive candidates for various biomedical applications. However, a concerning lack of consensus exists regarding their biocompatibility. Studies investigating the biocompatibility of BNNS paint a complex picture. While some report promising results, with minimal cytotoxicity observed, others highlight potential dangers. This inconsistency underscores the dependence of BNNS toxicity on several factors. Research on Cell Type and specificity suggests that the impact of BNNSs on cells can vary significantly depending on the specific cell line used in the study. This implies that a material deemed safe for one cell type might be detrimental to another. Moreover, the concentration of BNNSs administered seems to play a crucial role in determining their toxicity. Lower doses might be well-tolerated, while higher doses could trigger cell death or disrupt cellular processes [12-16].
The cytotoxicity assessment of Mg-BNNS nanocomposites compared to bare Mg sheds light on their biocompatibility, a crucial factor for biomedical applications. The study employed a mouse osteocyte cell line to evaluate the potential cytotoxic effects of the Mg-BNNS nanocomposites. A reduction in cell viability exceeding 30% served as the benchmark for cytotoxicity. The cytotoxicity experiments revealed a generally positive trend, with cell viability increasing across an 8-fold dilution range for all materials tested. This indicates a dose-dependent response, where lower concentrations of the materials exhibited improved cytocompatibility. A critical finding is the lack of a statistically significant difference in cytotoxicity between bare Mg and Mg-BNNS nanocomposites containing low BNNS volume percentages. This suggests that the incorporation of BNNS nanoparticles at low levels does not adversely affect the cytocompatibility of the material. While the initial findings regarding cytocompatibility are encouraging, there is always room for improvement. By introducing biocompatible molecules onto the BNNS surface, researchers could potentially enhance their interaction with biological systems and further mitigate any potential cytotoxic effects. The current study likely focused on short-term exposure. Long-term in vitro and in vivo studies are essential to comprehensively understand the chronic effects of Mg-BNNS nanocomposites on cell viability and function. Moreover, elucidating the underlying mechanisms by which BNNS nanoparticles interact with cells would provide valuable insights for optimizing cytocompatibility. This could involve investigating factors like cellular uptake, surface interactions, and potential degradation products.
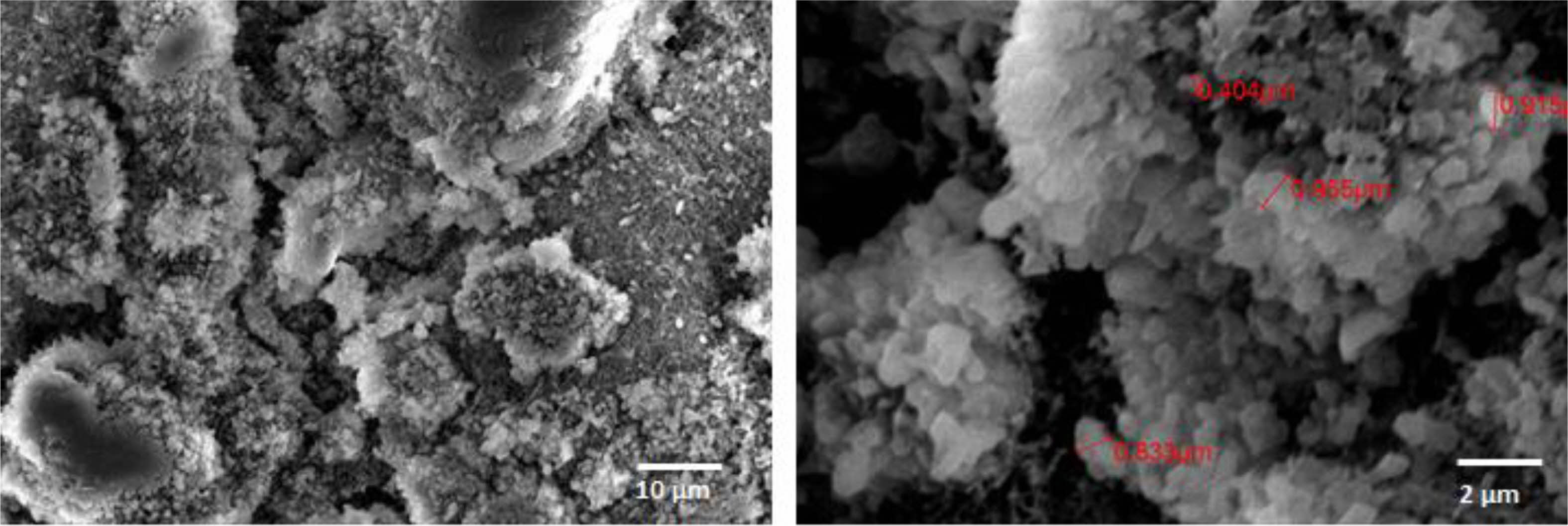
|
Fig. 1 SEM image of Boron Nitride used in this study |
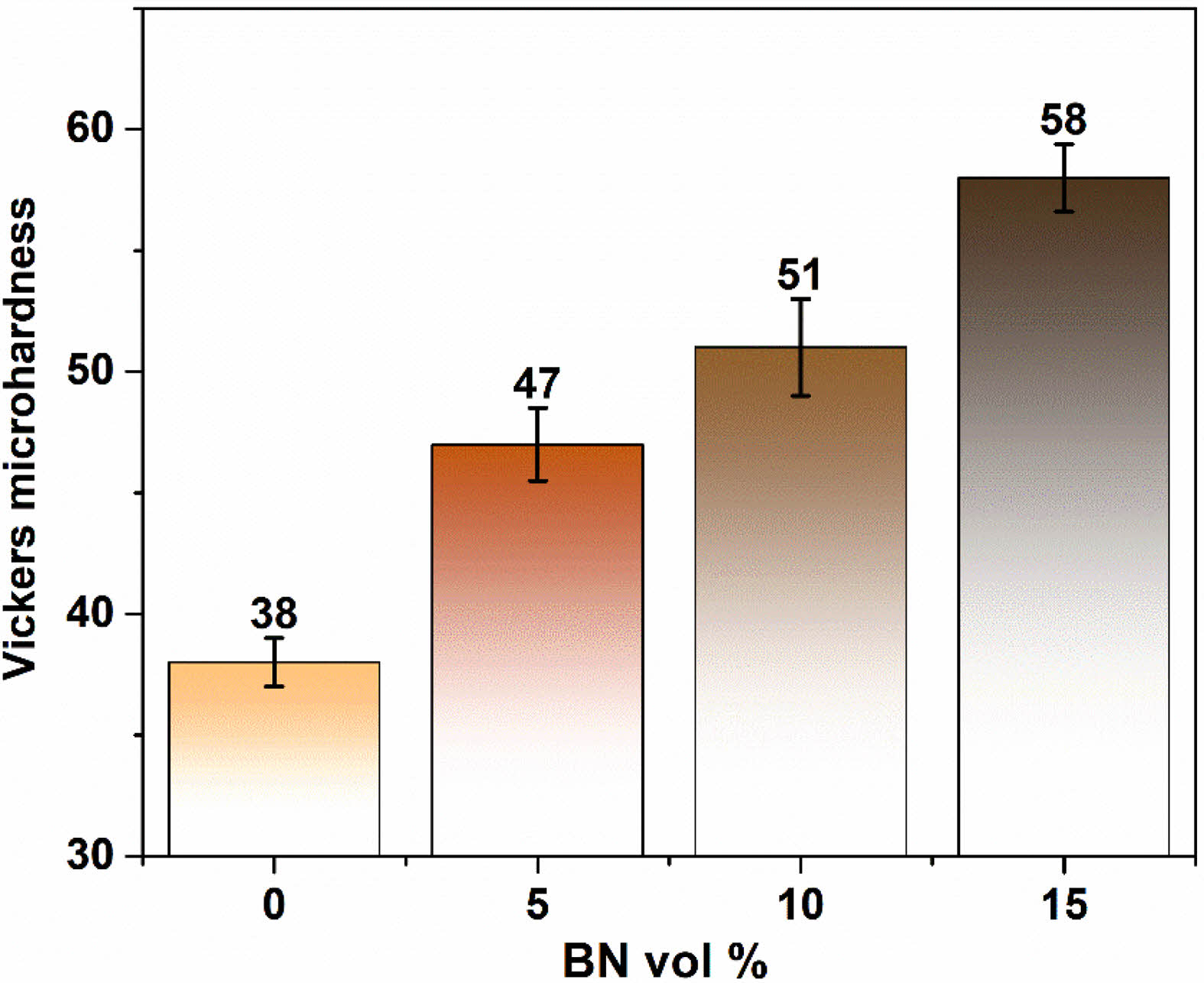
|
Fig. 2 Vickers micro hardness test of the samples synthesized |
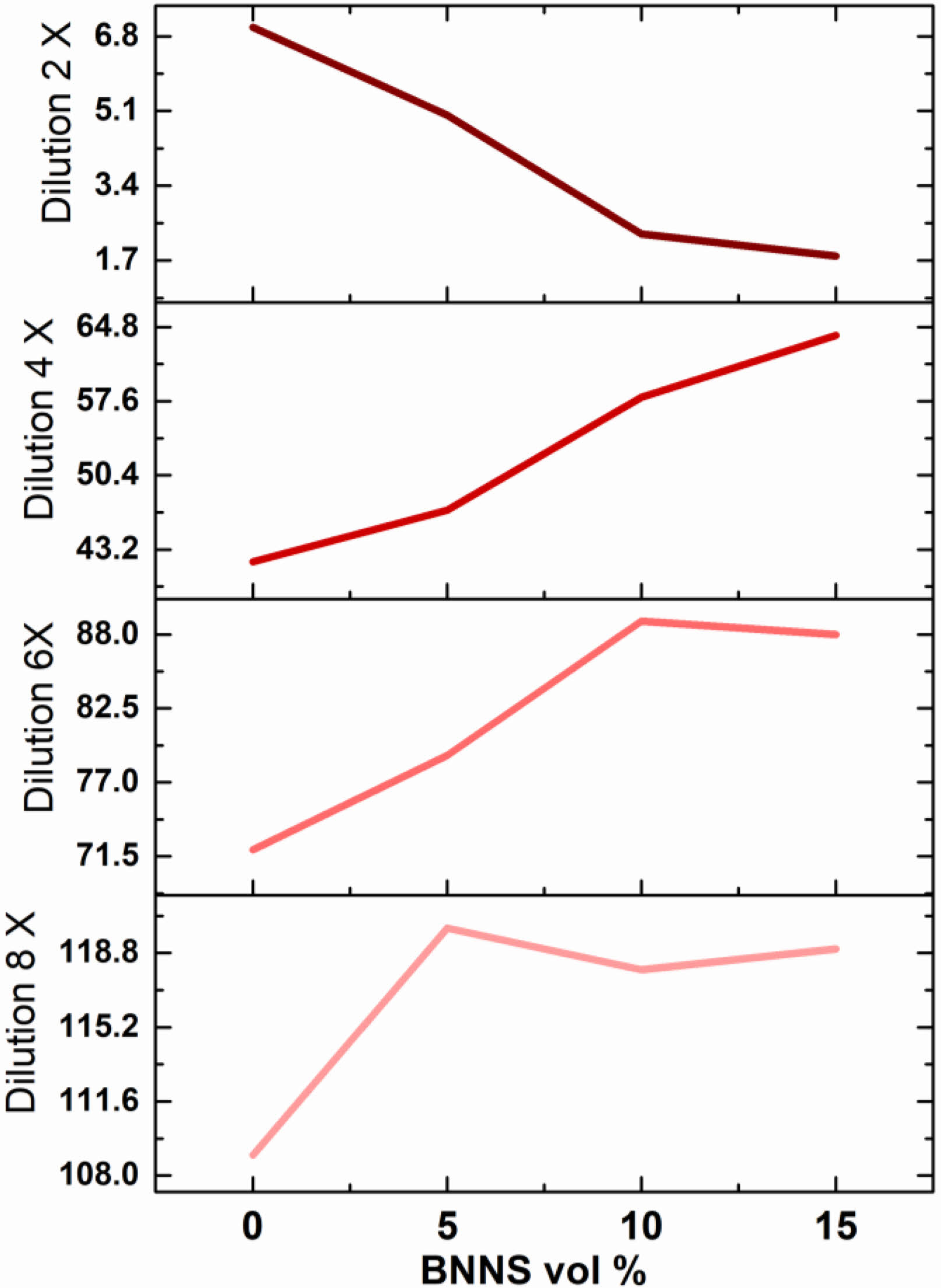
|
Fig. 3 Cytocompatibility of Mg-BNNS composite. |
The incorporation of BNNS nanoparticles demonstrably enhances the microhardness of Mg-BNNS nanocomposites. This phenomenon can be attributed to the strategic positioning of BNNS nanoparticles at grain boundaries, hindering dislocation movement and twinning within the Mg matrix. By optimizing BNNS content, size, distribution, and interface properties, these nanocomposites hold promise for applications demanding superior mechanical performance. The cytotoxicity evaluation of Mg-BNNS nanocomposites demonstrates their potential for biomedical applications, particularly at lower BNNS concentrations. However, the discussion emphasizes the importance of further research, particularly focusing on surface functionalization strategies, long-term studies, and mechanistic understanding. By addressing these aspects, researchers can ensure the development of biocompatible Mg-BNNS nanocomposites for safe and effective use in medical devices and implants.
- 1. Y. Chen, J. Zou, S.J. Campbell, and G. Le Caer, Appl. Phys. Lett. 84 (2004) 2430-2432.
-

- 2. M. Korkmaz, E. Uzgoren, S. Bakırdere, F. Aydın, and O. Y. Ataman, Toxicol. 22 (2007) 17-25.
-

- 3. D. Lahiri, V. Singh, A.P. Benaduce, S. Seal, L. Kos, and A. Agarwal, J. Mech. Behav. Biomed. Mater. 4[1] (2011) 44-56.
-

- 4. A.M.P. Dupraz, S.A.T. Meer, J.R.D. Wijn, and J.H. Goedemoed, J. Mater. Sci. Mater. Med. 7 (1996) 731-738.
-

- 5. L. Chetibi, D. Hamana, and S. Achour, Mater. Chem. Phys. 144[3] (2014) 301-309.
-

- 6. L. Choudhary, R.R.K. Singh, J. Hofstetter, and P.J. Uggowitzer, Mater. Sci. Eng. C 42[1] (2014) 629-636.
-

- 7. T. Kraus, S.F. Fischerauer, A.C. Hanzi, P.J. Uggowitzer, J.F. Loffler, and A.M. Weinberg, Acta Biomater. 8[3] (2012) 1230-1238.
-

- 8. M.S. Jia, S. Hash, W. Reynoso, M. Elsaadany, and H. Ibrahim, Bioengineering 10[7] (2023) 757.
-

- 9. M.S. Kujur, V. Manakari, G. Parande, S. Prasadh, R. Wong, A. Mallick, and M. Gupta, Mater. Today Commun. 26 (2021) 102171.
-

- 10. M.S. Kujur, V. Manakari, G. Parande, S. Prasadh, R. Wong, A. Mallick, and M. Gupta, J. Mech. Behav. Biomed. Mater. 114 (2021) 104162.
-

- 11. X. Ying, S. Cheng, W. Wang, Z. Lin, Q. Chen, W. Zhang, D. Kou, Y. Shen, X. Cheng, F.A. Rompis, L. Peng, and C.Z. Lu, Biol. Trace Elem. Res. 144 (2011) 306-315.
-

- 12. T. Lu, L. Wang, Y. Jiang, Q. Liu, and C. Huang, J. Mater. Chem. B. 36 (2016) 6103-6110.
-

- 13. T. Mohan, P. Verma, and D.N. Rao, Indian J. Med. Res. 138[5] (2013) 779-795.
- 14. M. Alrwashdeh and S.A. Alameri, Energies 15[21] (2022) 8008.
-

- 15. K. Peters and F.M. Richards, Annu. Rev. Biochem. 46 (1977) 523-551.
-

- 16. M. Alrwashdeh, S.A. Alameri, and A.K. Alkaabi, Nucl. Sci. Eng. 194[2] (2020) 163-167.
-

 This Article
This Article
-
2024; 25(3): 457-460
Published on Jun 30, 2024
- 10.36410/jcpr.2024.25.3.457
- Received on May 3, 2024
- Revised on May 13, 2024
- Accepted on May 16, 2024
 Services
Services
Shared
 Correspondence to
Correspondence to
- Liwen Wen
-
Medical College, Huanghuai University, 463000, Zhumadian, Henan, China
Tel : +86 18539616981 Fax: +86 0396-2639859 E-mail: wlw20171792@126.com - E-mail: wlw20171792@126.com






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.