- Preparation of low-cost ceramic membrane supports: a comprehensive review
Zhiqiao Lia, Yanli Rena, Jianzhang Haoa, Jie Xua, Panling Yana, Xiang Zhangb,* and Lin Lia
aState Key Laboratory of Vanadium and Titanium Resources Comprehensive Utilization, Pangang Group Research Institute Co., Ltd., Panzhihua 617000, Sichuan, China
bJoint International Research Laboratory of Refractories and Metallurgy, Ministry of Education, Wuhan University of Science and Technology, Wuhan 430081, Hubei, ChinaThis article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
In water treatment applications, ceramic membrane supports have the advantages of excellent thermal stability, stain resistance, and low maintenance costs. However, most commercial ceramic membrane supports are expensive to produce, mainly due to raw materials and molding processes. Therefore, new ceramic membrane supports based on low-cost raw materials and processes are widely studied. This paper introduces different preparation processes of ceramic membrane support, and the low-cost raw materials such as natural materials, industrial solid waste, and agricultural solid waste in the preparation of ceramic membrane supports, and analyzes the existing problems in the current research, which can provide references for the preparation of low-cost ceramic membrane support system and the resource utilization of solid waste.
Keywords: ceramic membrane support, low-cost raw material, solid waste, molding technology.
At present, membrane separation technology is widely employed across various fields, including sewage treatment, food processing, chemical industry, pharmaceuticals industry, and metallurgy industry. This technology offers functions such as separation, purification, concentration, and refining, alongside benefits such as environmental protection, high efficiency, low cost, precise filtration, simple operation, and easy control [1-3]. According to the different membrane materials used, membrane separation technology is classified into organic membranes and inorganic membranes [4]. The main materials for the preparation of organic membranes are cellulose and its derivatives and polysulfones [5]. However, under high temperatures and high acid-base conditions, the organic membrane will corrode and decompose, which greatly shortens its service life [6-8]. At the same time, this process will also lead to the volatilization of toxic substances, causing serious damage to the ecological environment [9, 10]. Compared with organic membranes, inorganic ceramic membranes have significant advantages such as excellent thermal stability, chemical stability, high-temperature resistance, high mechanical strength, high permeability flux, high cleaning efficiency, and low operating costs [11, 12]. Therefore, it is widely used in flue gas filtration, sewage treatment, pulp and paper making, and other fields [13-16].
Generally, the ceramic membrane is composed of support, a transition layer, and a separation layer, where the separation layer provides the required precision for separation, while the support provides mechanical strength, prevents the membrane from breaking, improves the performance of the membrane, and extends the service life. At present, inorganic ceramic membrane support materials are divided into oxide and non-oxide, in which the support prepared by Al2O3, TiO2, ZrO2, and natural minerals as aggregates in the oxide ceramic membrane have been industrialized, while the non-oxide ceramic membrane is mainly silicon carbide [17, 18]. However, the preparation of the above-mentioned raw materials has the problems of high cost and high sintering temperature, which limits the development of the support materials [19]. Therefore, the search for low-cost raw materials for the preparation of ceramic membrane supports has become a research hotspot.
To address the issues above, researchers have been searching for low-cost methods of producing ceramic membrane support materials recently, and their efforts have yielded some success. To create ceramic film supports, a range of inexpensive materials, such as fly ash, steel slag, kaolin, clay, loess, coal gangue, bauxite, etc. have been employed to prepare ceramic membrane supports [20, 21]. This study describes the structure and classification of ceramic membranes, summarizes the types of raw materials used in the manufacturing of ceramic membrane support, and discusses ways to improve the performance of ceramic membrane support. Finally, the research status of ceramic membrane support based on solid waste was summarized and projected.
Classification of ceramic film
Currently, industrial production has severe requirements for high permeability and selective separation of ceramic filtration membranes. Permeability refers to the high permeability flux, and selectivity is influenced by the membranes’ maximum, lowest, and average pore diameters [22]. Many parameters influence the permeability and selective separability of porous ceramic films, including porosity, film thickness, and the pore-bending factor. Membrane technology is classified into four types based on the aperture: microfiltration, ultrafiltration, nanofiltration, and reverse osmosis membranes, with reverse osmosis membranes allowing only water molecules to flow through. Fig. 1 depicts the separation of the first three membranes for various contaminants [23, 24].
Microfiltration (0.1-10 μm) is the first commercial pressure-driven membrane. Microfiltration is based on the principle of physical separation and can separate micron-sized items such as suspended particles, significant viruses, and a wide range of bacteria, proteins, and yeast cells. Furthermore, microfiltration membranes have weak hydrodynamic strength, requiring low hydrostatic pressure to achieve high pollutant removal rates and solvent fluxes [24]. Despite various issues, it remains widely employed in pharmaceuticals, water and wastewater treatment, food, desalination, and biotechnology.
Benchold coined the term “ultrafiltration” in 1907 to describe the process of pushing a solution through a membrane at various pressures [25], produced by hydrostatic pressure. The basic mechanism of an ultrafiltration membrane is the size exclusion of pore size, and the interaction between particle and membrane can limit its passing performance. The nanofiltration membrane has pore sizes ranging from 0.5 to 10 nm with a filtration pressure of more than 1 MPa. It is frequently used for separating small molecular organic materials and inorganic salt. The chemical composition and physical qualities of nanofiltration membranes influence their water permeability, solute selectivity, mechanical/thermal stability, and anti-fouling capabilities, all of which have a substantial impact on separation performance in nanofiltration membrane applications. The reverse osmosis membrane has a hole size of 0.1-0.7 nm and may trap substances larger than 0.0001 μm. It is commonly employed in sectors like saltwater desalination and medical dialysis.
Configuration of ceramic membrane support body
The raw material used to manufacture the support body limits its sintered profile and film microstructure to some extent, and it is also the primary determinant of cost. Furthermore, the geometry of the finished product is determined by the process used to manufacture the support. Currently, there are three types of ceramic membrane supports: flat membrane, tubular membrane, and hollow fiber membrane [11, 26].
Fig. 2 depicts the appearance and operating principles of various support setups. The hollow flat film is frequently used in intrusive units due to its advantages such as high filling density, easy shape control, and ease of cleaning, which is excellent for spray cleaning and film replacement [27]. Compared to other ceramic membranes, multi-channel tubular ceramic membranes offer a bigger membrane area and greater strength [28]. The tubular ceramic membrane comes in a variety of shapes, including square and hexagonal. Furthermore, the number of pores in the tubular film ranges from a few to several hundred, whereas the number of membrane tubes ranges from a few to dozens. The hollow fiber membrane has the maximum volume density when compared to the other two membrane topologies, however, it will cause major issues such as fiber fracture and scale formation during production. Furthermore, tubular films have been widely used because of their superior mechanical strength and resistance to cross-flow pressure [29].
Preparation process of ceramic membrane support
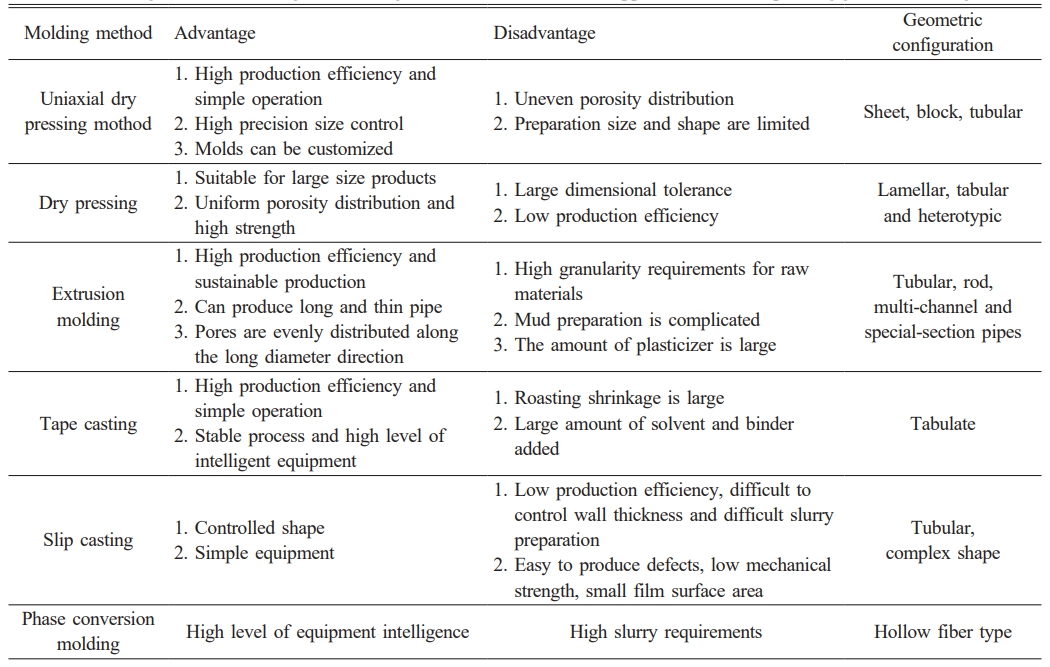
Extrusion forming, grouting forming, casting forming, dry pressing forming, and phase change forming are all popular forming procedures used to create various configurations of ceramic film supports. Fig. 3 and Table 1 depict schematic diagrams of several forming methods, their advantages and limitations, and the appropriate geometric configuration. Currently, the majority of the supports are powder-pressed, primarily through extrusion molding. Because the molding method has a direct impact on the microstructure and qualities of the support [24, 34], appropriate molding procedures should be used for raw materials with varying properties, and this aspect of the research remains to be completed.
Grouting molding
Grouting molding typically involves injecting a slurry into a plaster mold. The slurry solidifies against the inner wall of the mold due to capillary action, resulting in the formation of a green billet upon drying. The density of these billets can vary near the mold wall, the particle packing density is higher, while it decreases further away from the mold wall. This density variation can lead to defects, reduced mechanical strength, and a smaller surface area for the film during firing. Techniques such as centrifugation and vacuum-assisted molding can mitigate these density differences, enabling the fabrication of complex, non-concentric, and irregularly shaped support structures [36].
Dry-press molding
Dry pressing is a traditional farming technique in the ceramic industry and is regarded as the simplest manufacturing method for ceramic-based products due to the lack of slurry preparation [37]. This technique is straightforward and quick to use, and the sintering is minimal. It is a molding process used to produce huge numbers of flat ceramics. The prepared sample typically has a consistent structure and integrity, high mechanical strength, and easy size change.
Extrusion Molding
Extrusion is another frequent ceramic membrane support technique, particularly for tubular structures. The primary step in extrusion molding is to combine equally mixed ceramic raw ingredients. During the mixing process, the moisture content of the slurry is adjusted by combining the powder with the necessary binders, surfactants, flocculants, coagulants, lubricants, plasticizers, and preservatives. Then vacuum refining and aging techniques are used to create plastic mud. The plastic mud is then fed into the extruder, where a ceramic body with a certain shape is extruded through a predetermined nozzle. To avoid the slurry from sticking to the mold and losing its form, the moisture level of the slurry used in the extrusion process can be managed at an average of 20% due to the higher percentage of purified water used in the process. High production efficiency and automated production are possible with this approach. Still, there are a few issues, including the sample’s potential for significant shrinkage following firing, the intricate nozzle structure, and the high equipment precision requirements.
Tape casting
Tape casting is a mature technology for producing thin piezoelectric materials that was invented in the mid-1940s. It is also used to produce flat ceramic membrane structures. It is now widely employed in the manufacturing of thick ceramic substrates and layered materials [38], thanks to ongoing improvements. Its preparation performance is determined by the particle size of the raw material, the amount and kind of organic matter added, and the firing method. The process equipment is simple, and the production efficiency is great. It can be cut into any shape. However, when the raw material is slurry, the preparation time increases. In 2008, Nandi et al. [39] used this process to prepare low-cost support by using kaolin for the first time, and the prepared film has a porosity of 42% and a bending strength of 8 MPa.
Phase Conversion Molding
Phase conversion is the process by which a polymer solution transitions from a liquid to a solid. It can occur in a range of wet and dry conditions [40]. The wet approach involves immersing the polymer solution in a non-solvent coagulant bath, whereas the dry process exposes the polymer solution to a non-solvent environment. Cross-section photographs of ceramic membrane support created using the phase conversion molding method typically show two types of pores: finger-like pores and spongy pores. The finger-like structure is a fault created by the aggregation and dehydration of ceramic particles, which reduces the mechanical strength of the falling material [41]. This approach was originally used to investigate kaolin in low-cost ceramic membrane support [42].
Currently, researchers increasingly prefer phase conversion molding over other molding techniques due to its capability to produce asymmetric ceramic films with distinct finger-like and sponge-like features. The pore structure can be tailored by adjusting various preparation parameters, including the choice of raw material, the shape of the solidification bath, and the sintering conditions. Moreover, this method holds significant potential for creating hollow fiber ceramic membranes characterized by high specific surface area-to-volume ratios. This attribute enhances the efficiency of membrane modules where these membranes are incorporated.
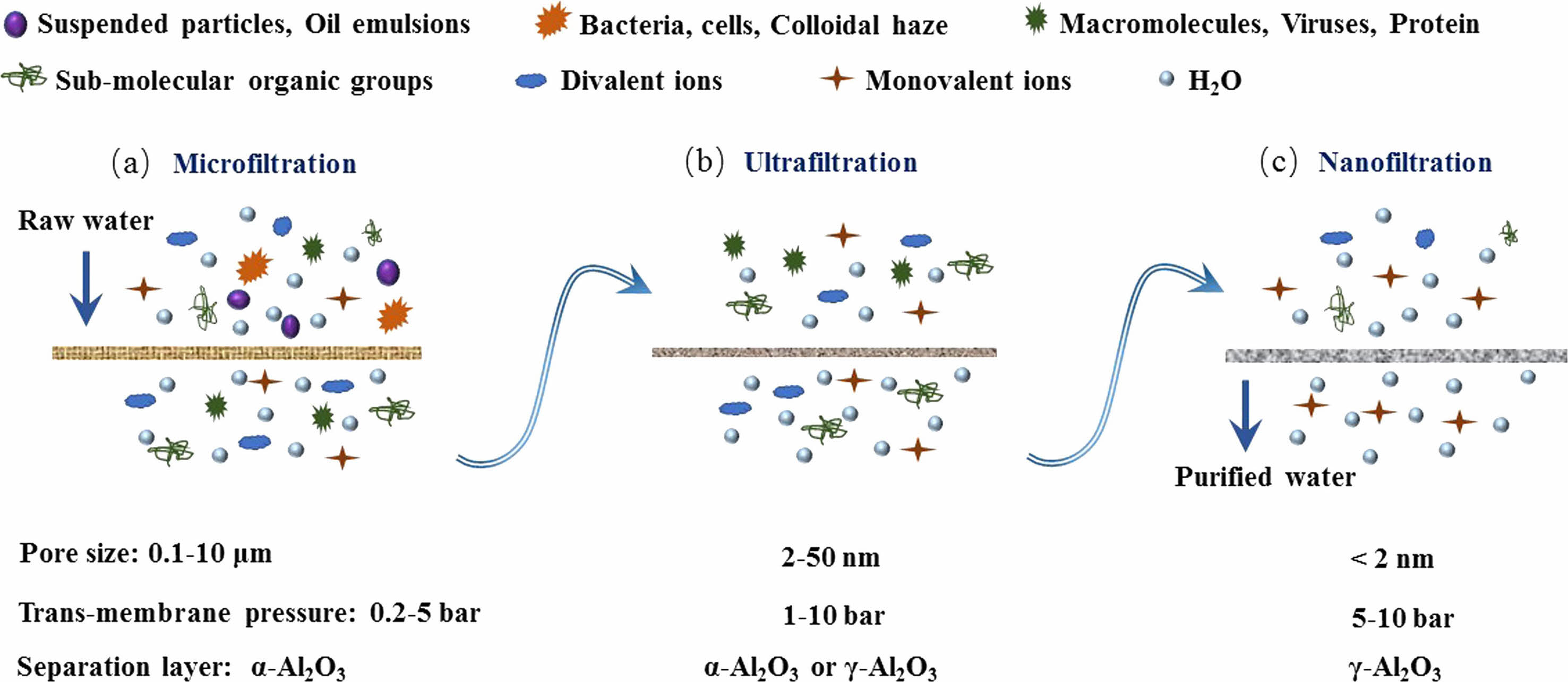
|
Fig. 1 Schematic diagram of membrane filtration process (a) microfiltration, (b) ultrafiltration, (c) nanofiltration [24] |
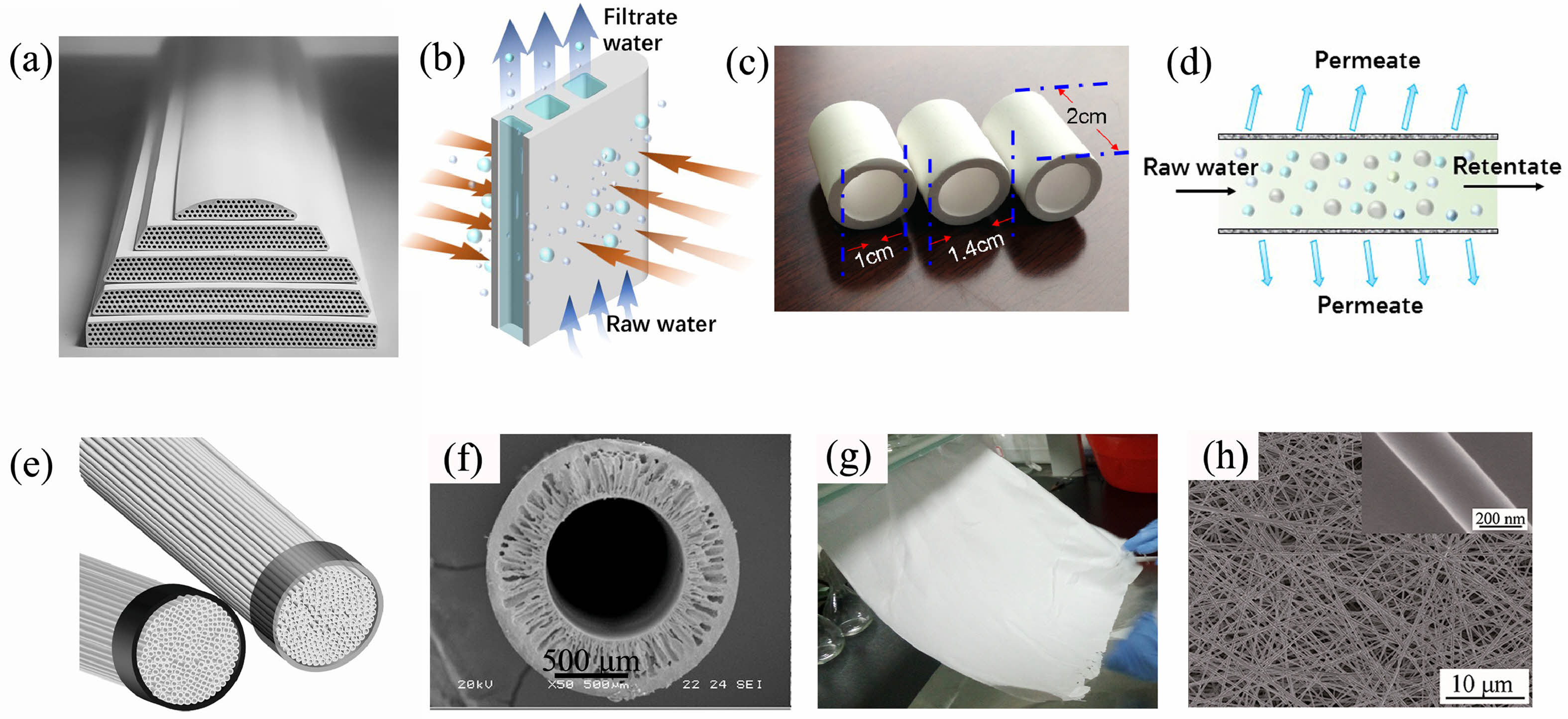
|
Fig. 2 Appearance and working principle of support bodies with different configurations (a) flat film, (b) flat film working principle, (c) tubular film, (d) tubular film working principle, (e) hollow fiber film, (f) hollow fiber film cross-section, (g) flexible nanofiber film, (h) flexible nanofiber film SEM [30-33]. |
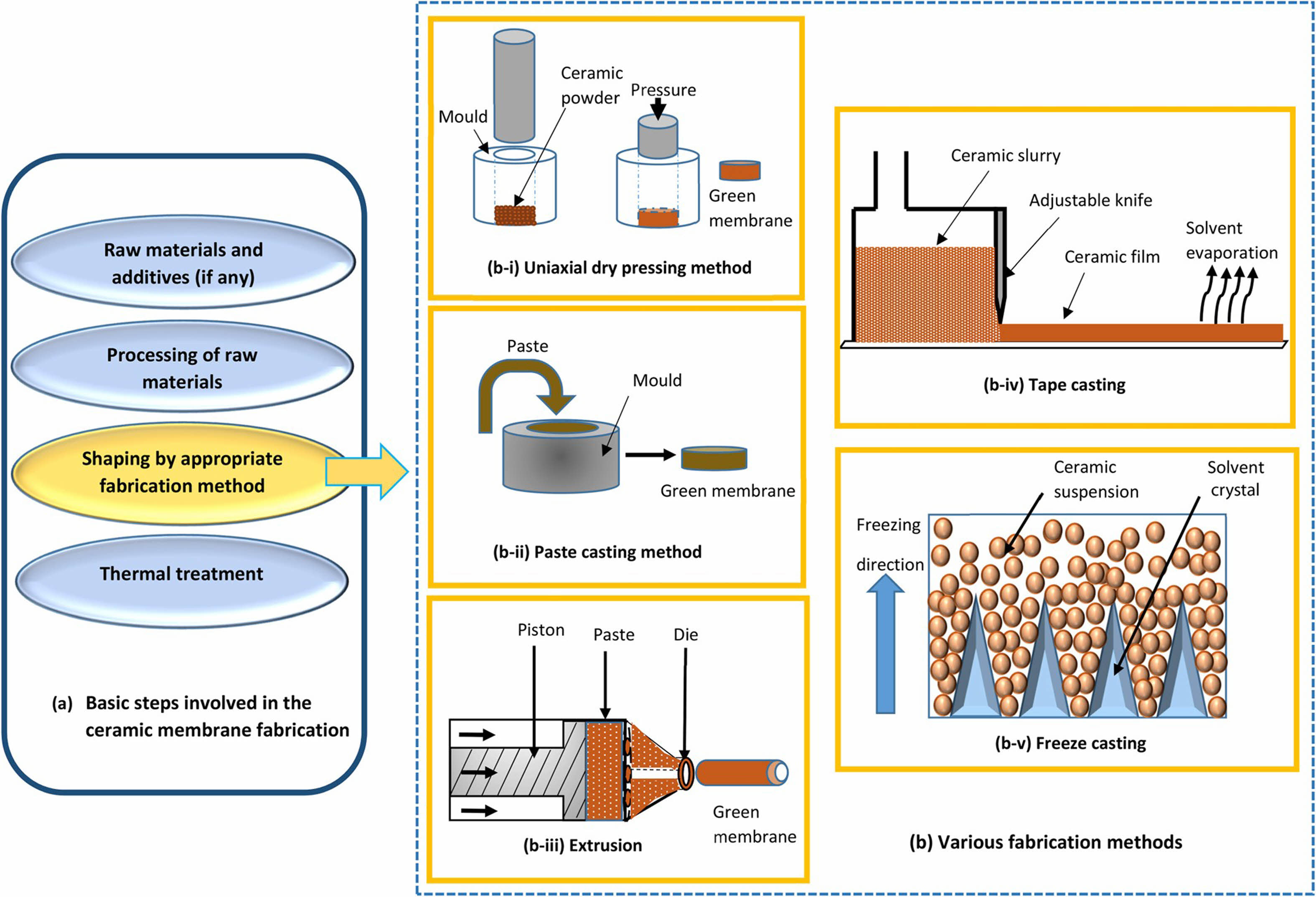
|
Fig. 3 Partial forming method for preparing ceramic film support [34]. |
|
Table 1 Advantages and disadvantages of forming methods for ceramic film support and the corresponding geometric configurations. |
Conventional ceramic membrane supporting aggregate is made of oxide, which results in high raw material costs and sintering temperatures. To lower costs and provide economical, high-performance, versatile, and innovative functional supports, researchers have undertaken extensive studies on the utilization of natural raw materials for preparation [43, 44]. In addition to clay, such as kaolin in natural minerals, researchers concentrated on the utilization of waste materials to manufacture ceramic membrane supports, fulfilling the goal of “turning waste into treasure”. Waste materials are often classified into three types: industrial solid waste, agricultural waste ash, and animal bones. Animal bone preparation difficulties will not be treated here due to their immaturity, the introduction of dangerous compounds, a lack of systematic research, and a lack of industrialization. Fig. 4 depicts a portion of the low-cost production of ceramic membrane support materials.
Natural Materials
Kaolin
Clay minerals such as kaolinite, montmorillonite, and illite require just modest treatment to prepare ceramic film supports. Researchers have undertaken numerous investigations on the production of low-cost ceramic membranes from various types of clay [21]. Kaolin, one of the clay kinds, has become the subject of investigation due to its distinct crystal structure, chemical makeup, and mineralogy. When employed as a film material, it exhibits minimal plasticity, strong fire resistance, and hydrophilicity [45, 46]. Huang et al. [47] created the first low-cost ceramic membranes for nitrogen separation using kaolin (Al2Si2O5(OH)4) in 1999. The disc-shaped ceramic film support was created by combining carboxymethyl cellulose (CMC), dirt, kaolin, and alumina. It was then dried, milled, screened, pressed at 2.76 MPa, and sintered at 1400 ℃. Following that, kaolin was molded into support using a variety of methods, but the specifics of the preparation are lacking in many studies.
Compared with traditional oxide ceramics, kaolin can decompose at a lower thermal treatment temperature and produce spinel and mullite phases. The decomposition reactions are shown as follows [48] :
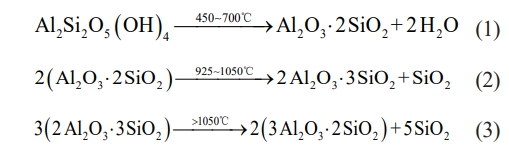
The above equations depict the entire reaction process, from the original layered structure of kaolinite to the residual layered structure of metakaolinite, the cubic spinel phase, and finally the chain structure of mullite. Mullite is a good support material because of its outstanding high-temperature stability, mechanical qualities, low creep rate, low thermal expansion coefficient, and low thermal conductivity [49].
Studies indicate that using kaolin to prepare support without active additives results in a higher preparation temperature of around 1200 ℃ and a phase primarily consisting of SiO2 and mullite (3Al2O3∙2SiO2) [50]. On this basis, increasing the sintering temperature produces a needle-like mullite structure, also known as mullite whisker, which increases mechanical strength [51]. However, if an alumina source is added to kaolin, the cristobalite created during the high-temperature conversion of spinel to mullite reacts with alumina to produce mullite, increasing the support’s performance [52]. In addition to adding aluminum sources to improve performance, the researchers tried adding magnesium or calcium sources such as calcite, dolomite, limestone, and magnesium carbonate to produce a phase of cordierite ((Mg, Fe)2Al4Si5O18) with calcium feldspar (CaAl2Si2O8) that formed at a lower temperature than mullite [53-55].
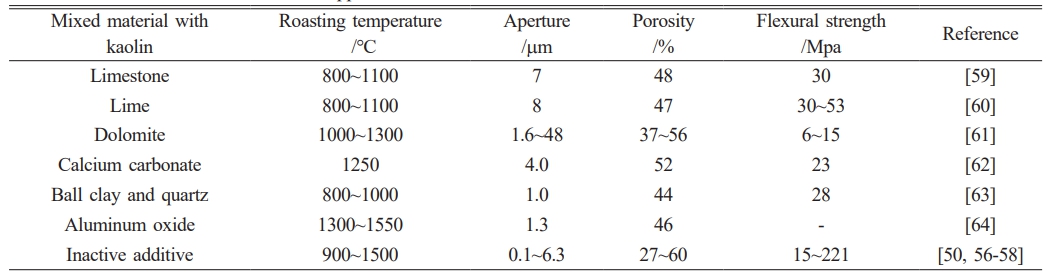
Table 2 shows the properties of ceramic membrane supports prepared partly with kaolin as the main raw material. Most of the active substances added in the research do not participate in the formation of phase but further play the role of pore formation through the evolution of gases, such as the decomposition of CaCO3 to produce CO2. However, some of the added substances can improve the performance of the material, such as quartz to increase mechanical and thermal stability, feldspar as a sintering agent to form a molten glass phase at low temperatures, and the incorporation of spherical clay in the early processing stage to provide plasticity and strength for the body. In addition, the composition gap between the materials selected for preparation will also affect the final performance of the support. The current research is mainly focused on the preparation of support by adding organic pore-forming agents to kaolin [50, 56-58]. The strength of the prepared support body is mainly determined by the porosity and phase. Increasing the sintering temperature is conducive to the formation of the mullite phase, which improves the stability of the film, but also leads to the decrease of its porosity.
Other clays
There are some studies on using other clays besides kaolin (such as bentonite, attapulgite, ball clay, etc.) as raw materials to prepare low-cost ceramic film supports. Table 3 shows some of the research results. As shown in Table 3, there are significant differences in the properties of ceramic membrane supports prepared by clay materials of different sources, particle sizes, and properties, among which the maximum flexural strength can reach 69 Mpa, which is prepared by using sepiolite clay [65]. Among other clays, attapulgite has a large specific surface area, great mechanical qualities, excellent thermal and chemical stability, strong adsorption properties, and the capacity to be made without high-temperature sintering, making it an attractive raw material for support production [66]. Furthermore, due to its abundant reserves and low price, spherical clay has become one of the ideal raw materials for the preparation of low-cost ceramic film support, and the product is mainly composed of SiO2 and Al2O3, while the content of Fe2O3, TiO2, and other metal oxide impurities is low, which will produce a small amount of liquid phase in the sintering process and is conducive to lowering the sintering temperature, but will also lead to shrinkage phenomenon.
Bauxite
Bauxite is a natural mineral composed primarily of Al2O3 and SiO2, with trace amounts of other metal oxides such as TiO2 and Fe2O3. Because of SiO2’s low melting point and the secondary mullite reaction between SiO2 and Al2O3, a stable mullite phase is created, making it one of the best raw materials for producing low-cost ceramic film supports. When bauxite is utilized as a single raw material to create the support, the difference in Fe2O3 composition between raw materials from different origins might greatly affect the performance of the support [73]. Despite this, the bauxite supports have high flexural strength. Zhu et al. [52] found that viscous deformation occurs at 200-300 ℃, combustion loss occurs at 500-620 ℃, and sintering shrinkage occurs at above 800 ℃ when bauxite is used as the raw material and a binder is used. At the same time, increasing the sintering temperature reduces porosity and pore size. The flexural strength is greatly increased.
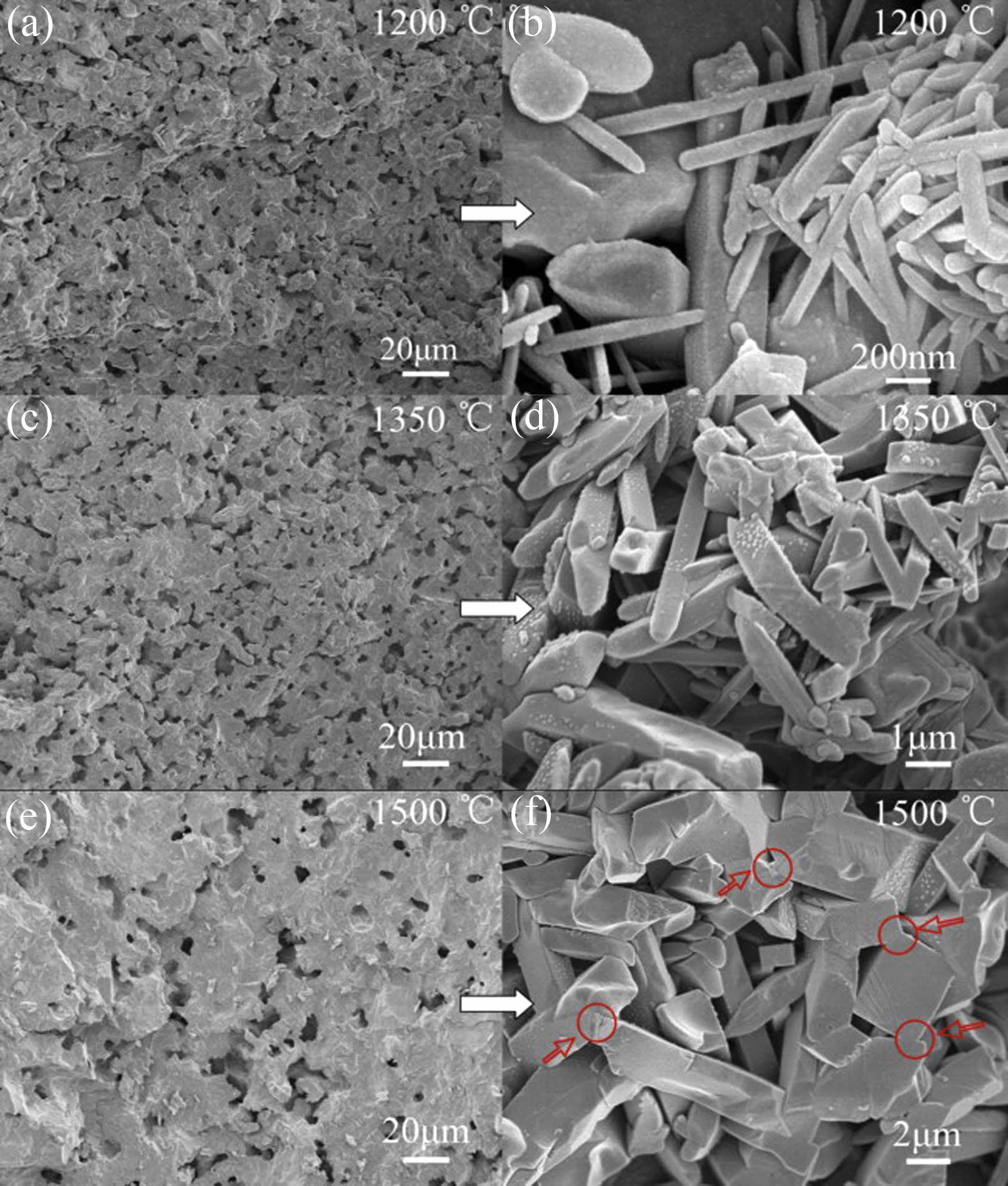
Recently, researchers have focused on the development of bauxite-based ceramic membrane support materials that can work with natural raw materials or solid waste treatment. Fan et al. [74] created a low-cost support by adding bauxite to fly ash. The study found that when the sintering temperature and bauxite content are controlled at 1300 ℃ and 40 wt%, the support body has a high pure water permeability of about 5.36 m3·m−2·h−1·bar−1 and a high bending strength of about 69.6 MPa. At the same time, the support body shows a typical mullite phase and has good acid-alkali resistance. When coal gangue and bauxite are used as raw materials and corn starch is used as a pore-making agent, secondary mullite occurs at 1100 ℃, and mullite is the main phase at 1400 ℃. In the temperature range of 1276~1481 ℃, due to the polycrystalline growth process, porosity increases, and pore size distribution becomes wider [75]. At the same time, the prepared mullite scaffold has a porous microstructure and is composed of sintered glass-like particles embedded with interlocked mullite crystals. With the increase in temperature, Mullite crystals gradually grow from rod-like to block, and its SEM is shown in Fig. 5.
Quartz sand
Quartz sand, a natural sedimentary rock, is primarily made of crystalline SiO2 in the form of quartz. It possesses excellent mechanical resistance but low flexibility. The phase transition from α-type to β-type (573 ℃) is accompanied by a noticeable specific volume change, resulting in the annihilation of quartz particles [76]. When preparing ceramic film supports with quartz, necessary supplementary raw materials must be supplied. The binder is an essential raw element in the manufacturing of ceramic film supports made from quartz sand. It promotes the attachment of quartz sand particles to one another and ensures the support’s performance and mechanical strength. Under certain conditions, a suitable adhesive can be used to create a functioning quartz sand-based film at temperatures as low as 600 °C [77]. Simultaneously, the crystalline oxide of Si(IV) is the determining factor in the preparation of ceramic materials, and the presence of carbonates (calcite, dolomite, aragonite, etc.) and crystalline aluminosilicate (microcline, albite, pyroxene, etc.) causes a decrease in ceramic mechanical strength. In general, additive-assisted sintering of quartz necessitates a temperature of at least 800 °C to achieve the needed mechanical strength [76], whereas sintering without additions necessitates a temperature of more than 1040 °C [78].
Zhu et al. [79] created quartz porous ceramic support using quartz sand as the primary raw material, calcium carbonate and charcoal powder as pore-forming agents, bentonite as a binder, and potassium feldspar and kaolin as sintering additives. The support body has a porosity of 29.33%, a fracture strength of 17.051 MPa, and an average pore size of 12.41 μm after sintering at 1250 ℃ for 30 minutes with a heating rate of 5 ℃/min. The main phases include quartz, mullite, quartzite, and anorthite. In addition, the porosity of the samples with different pore-forming agents is as follows: phosphoric acid > sodium dodecylbenzene sulfonate > charcoal > graphite > starch; The order of fracture strength is: sodium dodecyl benzene sulfonate > starch > graphite > charcoal > phosphoric acid. The above research shows that it is feasible to prepare ceramic film supports with cheap quartz sand, but the effects of different sintering temperatures, particle size, and additives on the pore structure and mechanical properties of the film still need to be further studied in detail.
Other natural materials
Natural zeolite minerals consist of hydrated aluminum silicate [(SiO2)(AlO2)x]M∙yH2O, having a three-dimensional skeletal structure of AlO4 and SiO4 tetrahedra. Grinding, molding, and sintering are required for preparing the support with natural zeolite as the raw material. Roque-Malherbe et al. [80] employed natural zeolite to create porous membrane support. Clinoptilolite is amorphous between 600 and 900 ℃, with siliceous and dense alumino-silicate phases formed between 900 and 1150 ℃. Different particle sizes of zeolite affect the pore size of the supporting body, with the produced membrane ranging from 0.3 to 1.1 μm [81, 82]. When using zeolite to produce the support body, the sintering temperature is low, and the void in zeolite is filled with liquid phase at 800~900 ℃, resulting in the elimination of the void [82]. This is similar to the research findings of Zhou et al. [83]. As the temperature rose, the porosity and nitrogen permeability of the support fell rapidly, while the bending strength increased. The optimal preparation technique involved adding 5 wt% pore-making chemicals and sintering at 800 ℃. The constructed support had a bending strength of 14.75 MPa, 30.99% porosity, and a nitrogen permeability of 312 m3∙m-2∙h-1. Zhang et al. [84] used extrusion to create a natural zeolite ceramic membrane support with 46% porosity and an average pore size of 6.6 μm. The above results suggest that natural zeolite can be utilized to construct low-cost supports at low temperatures and with appropriate pore-forming chemicals, although the parameters influencing the mechanical strength of the supports remain to be investigated.
Natural volcanic ash is composed of ash rock fragments, mineral crystals, and volcanic glass. It has pozzolanic activity and reacts with limestone at room temperature to form a hydrate with hydraulic gelling ability, which is often used in the cement industry. The ceramic support body made of natural volcanic ash is composed of SiO2, Al2O3, Fe2O3, and MgO oxides [85]. Achiou [86] used volcanic ash as the main raw material and starch as the pore-making agent to prepare ceramic membrane supports with 30% porosity and 2-3 μm pore size at 950 ℃ by dry pressing. At present, there are few reports about the use of volcanic ash to prepare ceramic membrane support, lack of systematic research, and lack of research on the phase evolution mechanism, improving pore and mechanical strength properties. The above research results show that natural minerals can be used to prepare low-cost ceramic membrane support materials. In the preparation process, suitable pretreatment methods should be selected for different raw materials, and suitable pore-forming agents and binders should be added.
Industrial solid waste
Fly ash
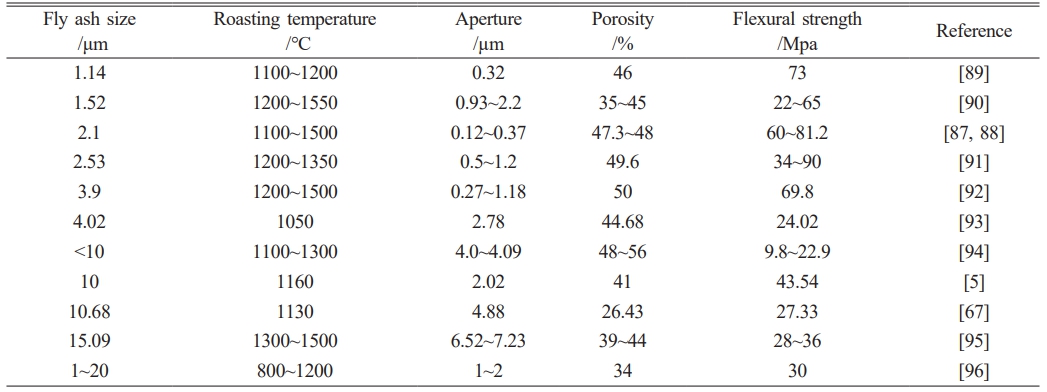
Fly ash is an industrial waste produced by coal-fired power plants, which mainly contains oxides such as Al2O3 and SiO2, and is mostly used to prepare mullite (3Al2O3·2SiO2) ceramic film supports. The general formula of mullite synthesized is Al4+2xSi2−2xO10−x(0 < x < 1) [87]. However, because the composition of fly ash is complex and not fixed, its physical and chemical properties depend on the type of raw coal and combustion conditions, resulting in differences in the performance of prepared supports. Table 4 shows the properties of ceramic membrane supports prepared with different particle sizes of fly ash. As shown in Table 4, with the increase in fly ash particle size, the average pore diameter of the support body increases, the flexion strength decreases, and the porosity increases first and then decreases.
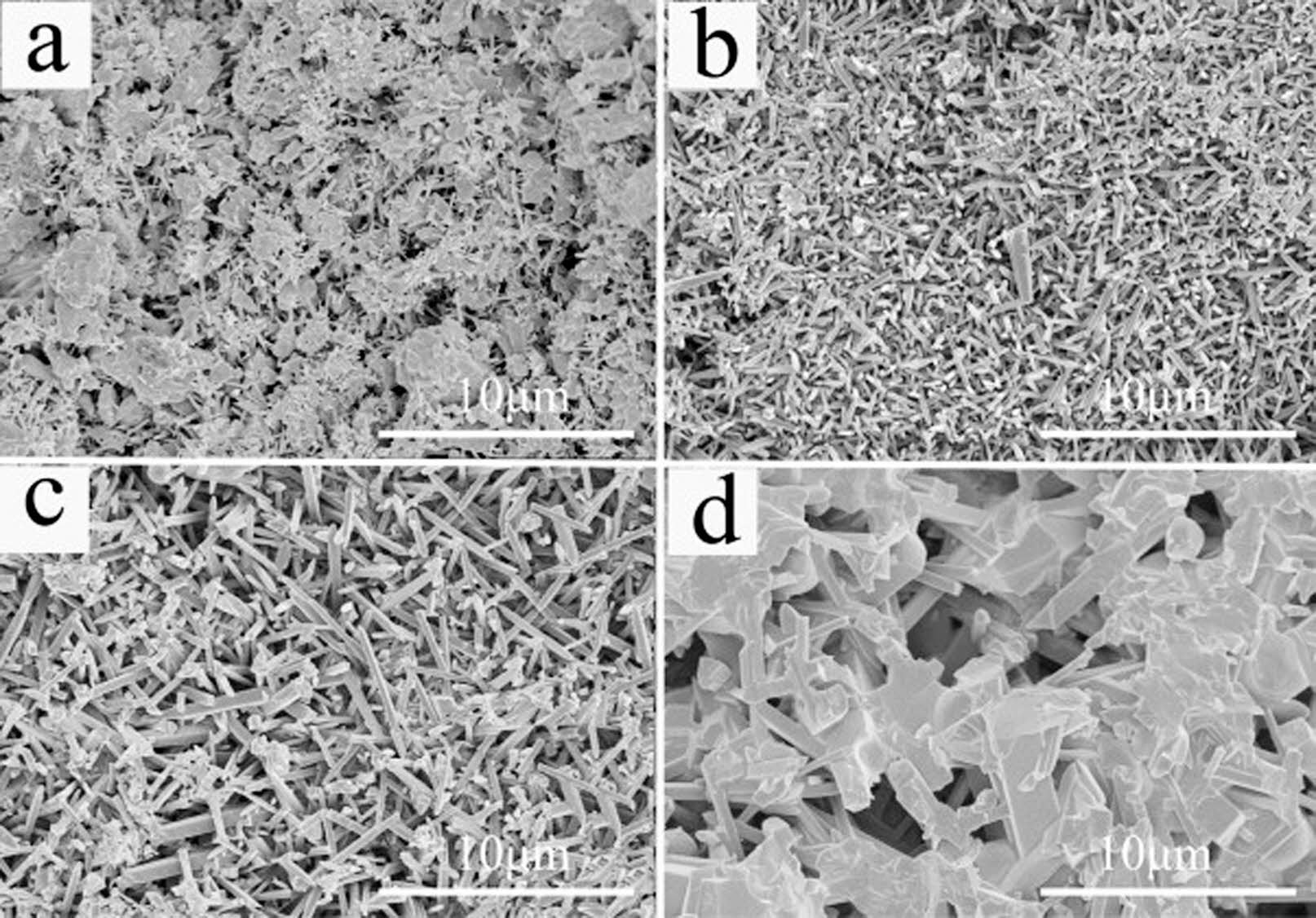
At present, the research on the preparation of mullite base support from solid waste including fly ash is mainly focused on how to improve the strength of mullite and reduce the sintering temperature. The mullite crystal is enhanced by adding AlF3, while the sintering temperature is reduced by adding sintering additives MgO, SrO, TiO2, Fe2O3, CeO2, and YSZ. Fig. 6 shows the SEM images of mullite whisker at different sintering temperatures when fly ash is used to prepare ceramic membrane support. When the temperature is 1200 ℃, the support body forms a uniform porous microstructure, which is completely composed of grown mullite whisker [90].
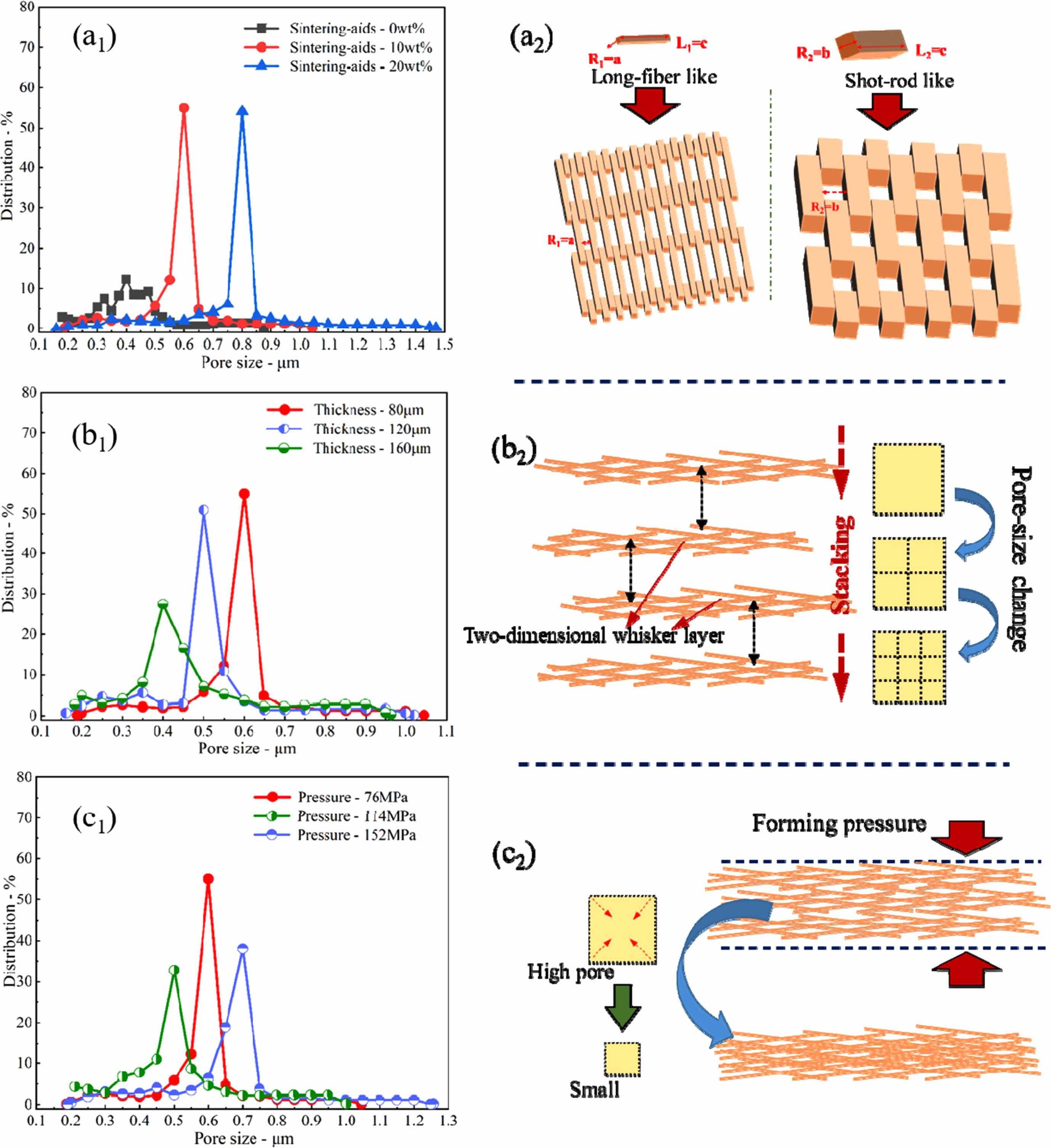
Figure 7 depicts the impact of sintering additives, whisker amount, and molding pressure on the characteristics of mullite-based ceramic membrane whisker support made using fly ash. Moreover, the pore size of the whisker layer is determined not only by the stacking condition of the whiskers but also by their radial size. During the sintering process, the liquid phase generated by the sintering additives is wrapped around the whisker surface, increasing the radial size of the whisker. As a result, increasing the amount of sintering additives increases the pore diameter of the ceramic film to some extent. At the same time, relevant studies show that the pore diameter of the whisker layer decreases with the increase of the thickness of the whisker layer, and when the molding pressure increases, the whisker accumulation will become denser, resulting in a decrease in the pore diameter of the support body. In summary, the pore diameter of the whisker film prepared by the dry pressing method is affected by three adjustment mechanisms, namely, the radial size of the whisker, the pore segmentation mechanism, and the pore compression mechanism [96].
In addition, some investigations concentrate on the manufacture of support by combining fly ash and aluminum-rich compounds. The combination of an extra aluminum source and free SiO2 in fly ash can raise mullite content in ceramics while improving support performance. The degree of secondary mullitization and the liquid glass phase content are the two most important elements influencing the characteristics of
mixed raw materials after sintering. Secondary mullitization is accompanied by volume expansion, which causes higher porosity at elevated temperatures. On the contrary, the liquid glass phase might enhance sintering while decreasing porosity. Therefore, according to the differences in the properties of fly ash, appropriate sintering additives should be used to form a liquid phase to reduce the sintering temperature of mullite film and other properties should not be significantly reduced.
Coal gangue
Coal gangue is a low-carbon rock created during the coal production process and is a frequent byproduct of coal mining and washing. The primary components of coal gangue are SiO2 and Al2O3, with a small quantity of alkali metal oxides such as Fe2O3, K2O, and TiO2, which can lower the sintering temperature. After high-temperature sintering, coal gangue can be changed into mullite [97]. In theory, the molar ratio of Al2O3 to SiO2 in the mullite phase is 3:2, but the content of SiO2 in coal gangue is generally as high as 60%, so aluminum source should be properly added when using coal gangue to prepare ceramic film support. Ji et al. [98] prepared self-strengthening mullite ceramics with interlocking columnar grains by adding La2O3 to enhance anisotropic growth using coal gangue and γ-Al2O3 as raw materials at a temperature of 1400~1550 ℃ and holding time of 4 h. The results show that the addition of La2O3 reduces the formation temperature of secondary mullite by about 50 ℃ and increases the bending strength. Lu et al. [75] took bauxite as the aluminum source and corn starch as the pore-making agent, and the formation of mullite was mainly divided into a contraction stage of 969~1276 ℃ and a volume expansion stage of 1276~1481 ℃. The opening porosity gradually increased in the volume expansion stage, and the recontraction stage occurred when the temperature was higher than 1481 ℃.
In addition to preparing mullite-based supports, Yang et al. [99] prepared cordierite-based ceramic film supports using coal gangue and talc as the main raw materials and held them at 1400 ℃ for 6 h. The bending strength of the supports was 29.1 MPa and the porosity was 39.8%. Li et al. [100] used coal gangue and loess as the main raw materials (mixed mass ratio: 8:2), carbon powder as the pore-making agent, and carboxymethyl cellulose (CMC) as the bonding agent, and prepared the ceramic film support of coal gangue by extrusion forming method. When the sintering temperature was 1125 ℃ and the amount of carbon powder added was 10 wt%, the porosity of the support was 49.9%. The bending strength is 23.57 MPa. However, Fan et al. [101] did not add any aluminum or silicon sources, and the porosity of the prepared support body was 51.23%, but the bending strength was only 4.51 MPa when the sintering temperature was 1100 ℃ for 1 h.
Metallurgical slag
(1) Red mud
Red mud is a polluting waste residue released by the aluminum industry. It has a high alkalinity and is mostly constituted of CaO and SiO2, followed by Al2O3 and Fe2O3. As a result, adequate pretreatment is required before the fabrication of ceramic membrane support with red mud to leach and separate any surplus metal elements present. Man et al. [102] used red mud as the primary raw material and used a two-step leaching method with HCl and H2SO4, resulting in a SiO2 recovery rate of more than 80%. Subsequently, the extracted SiO2 was used as the main raw material and mixed with sodium bentonite, limestone, pulverized coal, and other raw materials according to the mass ratio of 65:25:8:2, to prepare good chemical stability, high strength, and non-toxic support. The increase in the surface activity of the material was mainly attributed to the Si-OH group. Wang et al. [103] took red mud as raw material, added V2O5 and AlF3 as catalysts (to reduce the mullite process temperature), and graphite as a pore-making agent. At the sintering temperature of 1350 °C, the Mullite base support with flexure strength and porosity of 49.4 MPa and 31.4%, respectively, was prepared.
(2) Steel slag
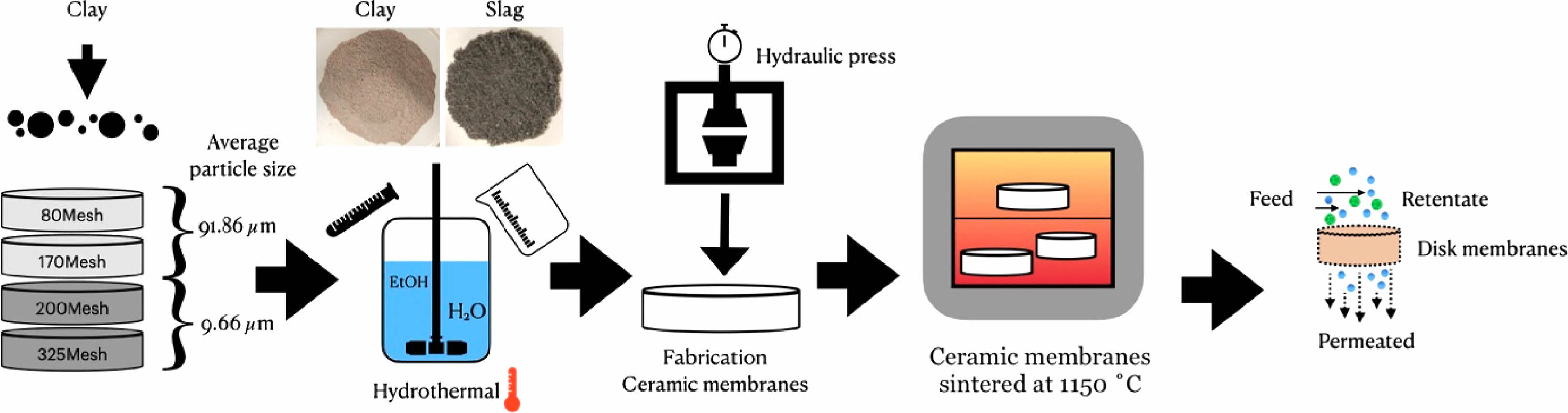
Since steel slag is mainly composed of Al2O3, SiO2, CaO, and Fe2O3, it is one of the available raw materials suitable for preparing ceramic film support. The presence of CaO in the slag contributes to the formation of the feldspar phase, which is also responsible for the mechanical strength of the ceramic film [104]. In the study of using the LD slag to prepare the support body, the LD slag was first modified, and then the modified slag was prepared into a ceramic membrane support body. The bending strength of the membrane was about 10 MPa, and the pure water flux was 341 L·m−2·h−1. In addition, steel slag can also be used as a good additive to increase the porosity of ceramic film and ensure that the ceramic film has good mechanical strength. Xavier et al. [105] used steel slag and pozzolanic clay as raw materials (mass ratio 2:8) to prepare ceramic film supports. The flow chart is shown in Fig. 8. At the sintering temperature of 1150 °C, ceramic film supports with a permeability of up to 5263.2 kg·m−2·h−1·bar−1 were prepared.
(3) Aluminum slag
Aluminum slag is a hazardous industrial byproduct generated in enormous amounts by aluminum smelters. It consists primarily of alumina, metallic aluminum, magnesium spinel (MgAl2O4), magnesium feldspar (MgO), quartz (SiO2), and salt flux, with tiny amounts of aluminum carbide and nitride [106]. Currently, the majority of aluminum slag is disposed of in landfills, and the presence of leachable salts such as NaCl and KCl heightens the risk to human health and the environment. In reality, using aluminum slag to manufacture low-cost ceramic film is one of the viable alternatives for achieving its harmless treatment and value-added utilization, thereby alleviating the aluminum manufacturing industry's significant environmental pressures. Aziz et al. [107] used aluminum slag and spinel as raw materials to successfully prepare ceramic film supports with finger-like and spongy holes through phase-change sintering technology, which has excellent oil removal performance and an oil removal rate of 92.4%.
At present, there is research on the preparation of ceramic membrane support by mining slag, which is mainly limited by the chemical composition characteristics of raw materials. However, if the metal elements contained in the slag are extracted through appropriate technology, and the tailings are further prepared as ceramic membrane support, the environmental pollution pressure can be greatly alleviated, and the high value-added utilization of secondary resources in mining and metallurgy industry can be realized.
Agricultural solid waste
As a large agricultural production country, China produces a large and wide amount of agricultural waste every year, which will cause harm to the environment and also lead to a large amount of waste of resources. Existing research mainly focuses on the use of rice husk ash, straw ash, bagasse, and other agricultural solid waste to prepare ceramic membrane support, which can not only provide a new idea for the huge amount of waste consumption but also alleviate soil and air quality, improve crop growth and human health.
Rice husk ash
Rice husk ash, the outer covering of rice, is one of the main agricultural solid wastes in the process of rice production. In the preparation of rice husk ash with high value, changing the burning temperature of the rice husk is considered an effective method. When the temperature is lower than 700 ℃, amorphous silica can be formed, and when the temperature is higher than 700 ℃, crystalline silica can be formed [40]. The research results of Serra et al. [108] showed that when rice husk ash and alumina were mixed to prepare mullite ceramic films, the film strength and porosity would be greatly increased. At present, the sintering temperature of ceramic membrane support prepared by rice husk ash is moderate, generally in the range of 1200~1400 ℃, and the porosity is about 40%, which is one of the promising raw materials [73,109].
Straw ash
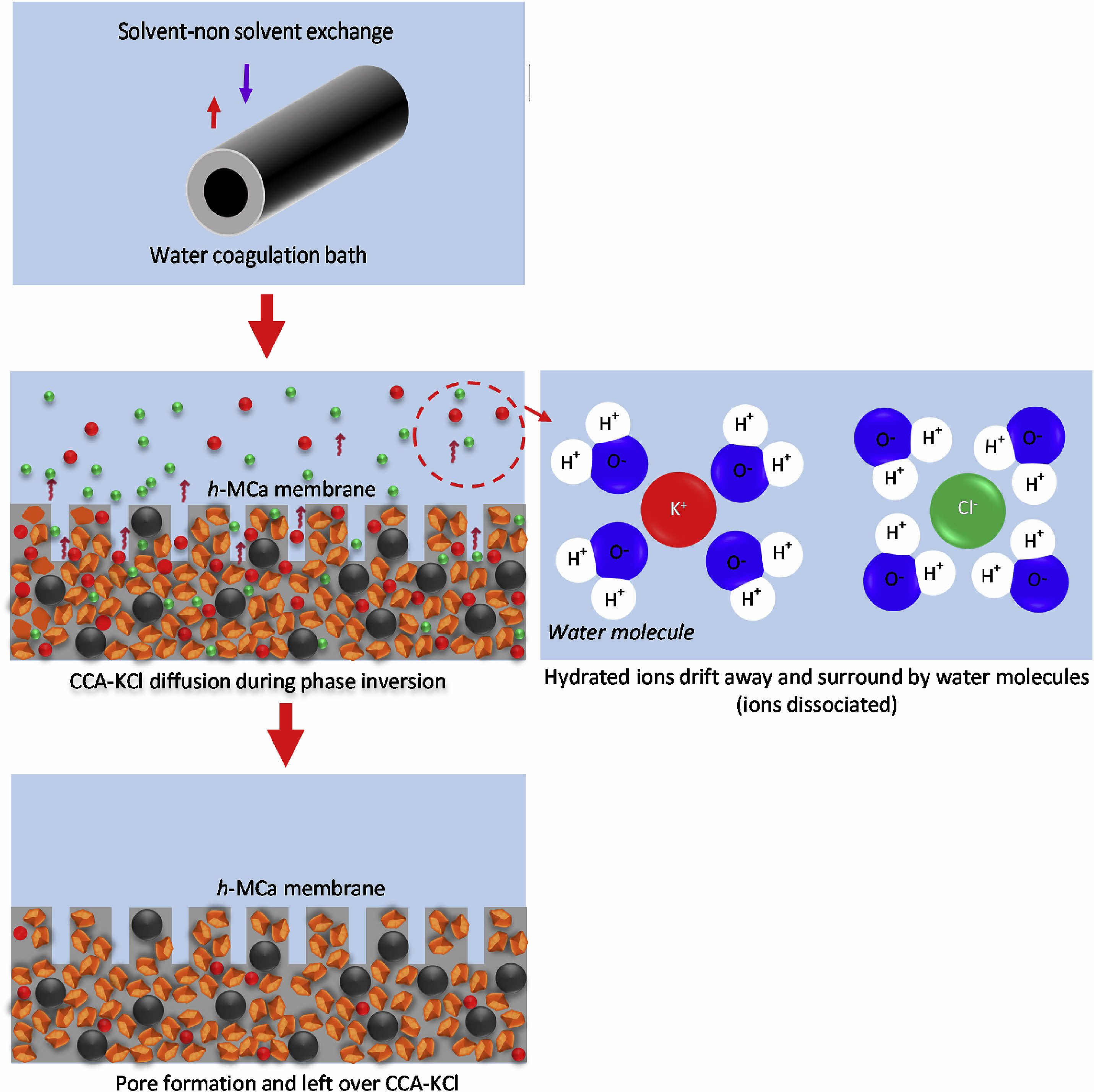
Straw ash is produced after crop combustion, which contains a lot of inorganic elements, such as K, Cl, Na, Ca, and P. In particular, the KCl, K2SO4, or KHCO3 contained in it can reduce the melting temperature of the ceramic, making it an ideal cosolvent [110]. Kamarudin et al. [111] used corn stalk ash and metakaolin as raw materials to prepare low-cost ceramic membrane supports by phase conversion sintering technology. At the sintering temperature of 1200 ℃, the permeability of the supports reached 1359.93 L/m2·h, and the compressive strength was 41.61 MPa. The dissolution mechanism of corn stalk ash in the preparation of membrane precursor is shown in Fig. 9.
Bagasse
As an agricultural waste, bagasse is a by-product of harvesting juice from sugarcane stalks. Studies have shown that the source of fiber, fiber age, harvesting process, and climatic conditions all affect the chemical composition and extracts of bagasse fiber, such as cellulose, hemicellulose, lignin, and ash, resulting in differences in the composition of bagasse [112]. According to the study of Bonassa et al. [113], bagasse has a porous structure composed of SiO2, Al2O3, CaO, Fe2O3, K2O, and silanol groups on the surface, which makes it easy to separate, catalyzed and adsorbed. Compared with rice husk ash, waste bagasse ash produces Al2O3 containing about 5 to 20 wt%, which can improve the strength of ceramic precursor [114]. Jamalludin et al. [115] prepared ceramic film support using waste bagasse ash. Firstly, bagasse was calcined at 800 °C, and then mixed with binder and dispersant and sintered. The permeability of the prepared support was about 466.2 L/m2·h.
|
Fig. 4 Raw materials for the preparation of low-costceramic membrane support. |
|
Fig. 5 SEM of bauxite-coal gangue based ceramic membrane support at different sintering temperatures [75]. |
|
Fig. 6 SEM images of ceramic film support at different sintering temperatures (a) 1100 °C, (b) 1200 °C, (c) 1300 °C, (d) 1400 °C [88]. |
|
Fig. 7 Effect of preparation conditions on mullite whisker ceramic membrane support (a) amount of sintering additives, (b) amount of whisker, and (c) molding pressure [96]. |
|
Fig. 8 Flow chart of preparation of ceramic film support by steel slag [105]. |
|
Fig. 9 Dissolution mechanism of corn stalk ash in preparation of membrane precursors [111]. |
|
Table 4 Properties of ceramic membrane supports prepared from fly ash with different particle sizes. |
In summary, this paper describes and analyzes the research progress of low-cost ceramic membrane supports in terms of preparation technology, material types, and performance differences. In the production of low-cost supports, factors such as the molding process, material composition, particle size distribution, additives, and types of pore-making agents need to be considered. The selection of the forming process can control the pore size and porosity of the prepared support. In terms of various low-cost alternative materials, natural materials, industrial solid waste, and agricultural solid waste are ideal raw materials. The use of the above raw materials can effectively reduce the sintering temperature and production cost, and bring better economic, social, and environmental benefits while absorbing solid waste. Although the use of low-cost raw materials instead of traditional materials to prepare ceramic membrane supports has made certain progress, the relevant direction is still worthy of in-depth exploration, which can be considered from the following aspects:
(1) The preparation process of low-cost ceramic film supports has been improved, but some key technical problems in the preparation process still need to be solved to ensure the density and stability of the support body, as well as improve its service life. At the same time, there is a lack of systematic research on the influence of different molding processes on the performance of the prepared support, and suitable molding processes should be adopted according to the characteristics of different raw materials to ensure the maximum performance and economic cost of the support.
(2) The low-cost ceramic membrane supports developed at present have good thermal stability, chemical stability, and high mechanical strength, but their performance is still far from that of traditional alumina ceramic membrane supports. It is necessary to further study the influence of different pore-forming agents, binders, surfactants, flocculants, coagulants, lubricants, and plasticizers on the improvement of supporting performance.
(3) Raw materials from different sources have different compositions and contain impurity elements, especially industrial solid waste and agricultural solid waste. Therefore, it is necessary to further study the pretreatment methods of each raw material to improve the purity, reduce the negative impact, ensure the uniformity and stability of the performance of the support body, and avoid the emission of toxic and harmful gases during the firing process.
(4) At present, low-cost ceramic membrane supports are only applied in some specific fields such as high-temperature filtration and flue gas filtration, and their applications in other fields still need to be further expanded.
The authors sincerely acknowledge the National Natural Science Foundation of China (51904212) for financial support.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
- 1. P. Wu, Y.Z. Xu, Z.X. Huang, and J.C. Zhang, J. Ceram. Process. Res. 16[1] (2015) 102-106.
-

- 2. Y.P. Bao, W.J. Lee, T. Lim, R. Wang, and X. Hu, Appl. Catal. B-Enviro. 254 (2019) 37.
-

- 3. K. Shima, Y. Funato, N. Sato, Y. Fukushima, T. Momose, and Y. Shimogaki, ACS Appl. Mater. Interfaces. 12[45] (2020) 51016-51025.
-

- 4. Y.W. Sun, M.L. Xie, Q.L. Liu, D.G. Ma, N. Ji, and C.F. Song, Chem. Ind. Eng. Prog. 36[5] (2017) 1880-1889.
-

- 5. Z. Tong, Y. Li, Y. Xiao, Q. Dong, Y. Zou, and H.J. Ji, Mater. Rep. 36[24] (2022) 53-58.
-

- 6. Q. Jiang, J. Zhou, Y. Miao, S.R. Yang, M. Zhou, Z.X. Zhong, and W.H. Xing, J. Membrane Sci. 610 (2020) 118238.
-

- 7. M. Wiener, B. Valdez, A. Eliezer, R. Salinas, and C. Lora, Corros. Rev. 37[2] (2018) 103-113.
-

- 8. N.H. Ismail, W.N.W. Salleh, A.F. Ismail, H. Hasbullah, N. Yusof, F. Aziz, and J. Jaafar, Sep. Purif. Technol. 233 (2020) 116007.
-

- 9. W.A. Meulenberg, F. Schulze-Küppers, W. Deibert, T.V. Gestel, and S. Baumann, ChemBioEng Rev. 6[6] (2019) 198-208.
-

- 10. A.K. Fard, G. McKay, A. Buekenhoudt, H.A. Sulaiti, F. Motmans, M. Khraisheh, and M. Atieh, Materials 11[1] (2018) 74.
-

- 11. T. Barbora, T. Jan, S. Kamil, M.Ondřej, and G. Lucie, J. Ceram. Process. Res. 21[6] (2020) 712-724.
-

- 12. S.M. Samaei, S. Gato-Trinidad, and A. Altaee, Sep. Purif. Technol. 200 (2018) 198-220.
-

- 13. L. Sawunyama, O.C. Olatunde, O.A. Oyewo, M.F. Bopape, and D.C. Onwudiwe, Heliyon 10 (2024) e24344.
-

- 14. Z. Tong, M.Y. Li, D.C. Li, K. Wu, and X.Y. Yang, J. Ceram. Process. Res. 24[2] (2023) 308-320.
-

- 15. S. Kim and Y. Kim, J. Ceram. Process. Res. 19[3] (2018) 236-242.
-

- 16. K.S. Kumaar, N. Muralimohan, P. Kulanthaivelc, and S. Sathiskumar, J. Ceram. Process. Res. 23[6] (2022) 892-901.
-

- 17. X. Da, X. Chen, B. Sun, J.J. Wen, M.H. Qiu, and Y.Q. Fan, J. Membrane Sci. 504 (2016) 29-39.
-

- 18. H.R. Mahdavi, M. Arzani, and T. Mohammadi, Ceram. Int. 44[9] (2018) 10281.
-

- 19. D. Wang, K. Li, and W.K. Teo, J. Membrane Sci. 138[2] (1998) 193-201.
-

- 20. W.B. Yang, Z. Tong, Y. Li, Z.G. Wei, J.X. Zhang, and L. Liu, J. Mater. Sci. Eng. 41[5] (2023) 806-811.
-

- 21. S.K. Hubadillah, M.H.D. Othman, T. Matsuura, A.F. Ismail, M.A. Rahman, Z. Harun, J. Jaafar, and M. Nomura, Ceram. Int. 44[5] (2018) 4538-4560.
-

- 22. J.H. Kim, G.J. Choi, J.K. Lee, S.J. Sim, Y.D. Kim, and Y.S. Cho, J. Mater. Sci. 33[5] (1998) 1253-1262.
-

- 23. S.F. Anis, R. Hashaikeh, and N. Hilal, Desalination 452 (2019) 159-195.
-

- 24. Y. Wang, B.W. Ma, M. Ulbricht, Y.C. Dong, and X. Zhao, Water Res. 226 (2022) 119173.
-

- 25. S.A. Aani, T.N. Mustafa, and N. Hilal, J. Water Pro. Eng. 35 (2020) 101241.
-

- 26. I.H. Song, B.S. Bae, J.H. Ha, and J. Lee, Ceram. Int. 43[13] (2017) 10502-10507.
-

- 27. W.Y. Zhu, Y. Liu, K. Guan, C. Peng, and J.Q. Wu, J. Eur. Ceram. Soc. 39[4] (2019) 1712-1716.
-

- 28. S. Jana, A. Saikia, M.K. Purkait, and K. Mohanty, Chem. Eng. J. 170[1] (2011) 209-219.
-

- 29. Y.Z. Tang, G.M. Peng, J.S. Tian, X.P. Wu, H.F. Chen, and W.Y. Zhang, Electroplating Finishing. 37[16] (2018) 732-737.
-

- 30. Y. Wang, W. Li, Y. Xia, X.L. Jiao, and D.R. Chen, J. Mater. Chem. A 2[36] (2014) 15124-15131.
-

- 31. Z. Zhu, J. Xiao, W. He, T. Wang, Z.L. Wei, and Y.C. Dong, J. Eur. Ceram. Soc. 35[11] (2015) 3187-3194.
-

- 32. B.F.K. Kingsbury and K. Li, J. Membrane Sci. 328[1] (2009) 134-140.
-

- 33. M.M. Lorente-Ayza, S. Mestre, M. Menéndez, and E. Sánchez, J. Eur. Ceram. Soc. 35[13] (2015) 3681-3691.
-

- 34. K.J. Krakowiak, P.B. Lourençoa, and F.J. Ulm, J. Am. Ceram. Soc. 94[9] (2011) 3012-3022.
-

- 35. S.L.S. Rain and R.V. Kumar, Case Stud. Chem. Environ. Eng. 4 (2021) 100149.
-

- 36. Y.F. Cheng, Y.G. Yu, C. Peng, and J.Q. Wu, Ceram. Int. 46[8] (2020) 11297-11303.
-

- 37. G.D. Li, B.L. Wu, and H. Zhang, J. Chin. Ceram. Soc. 28[6] (2000) 550-552,556.
-

- 38. T. Isobe, M. Shimizu, S. Matsushita, and A. Nakajima, J. Asian Ceram. Soc. 1[1] (2013) 65-70.
-

- 39. B.K. Nandi, R. Uppaluri, and M.K. Purkait, Appl. Clay Sci. 42[1-2] (2008) 102-110.
-

- 40. S.K. Hubadillah, M.R. Jamalludin, M.H.D. Othman, and Y. Iwamoto, Ceram. Int. 48[17] (2022) 24157-24191.
-

- 41. J. Luyten, A. Buekenhoudt, W. Adriansens, J. Cooymans, H. Weyten, F. Servaes, and R. Leysen, Solid State Ionics 135[1-4] (2000) 637-642.
-

- 42. R. Sarbatly, J. Appl. Sci. 11[13] (2011) 2306-2312.
-

- 43. X. Yan, Z. Tong, J.Y. Wang, S. Zhang, T. Liu, and G.R. Zhou, J. Synthetic Cryst. 48[7] (2019) 1208-1213.
- 44. J. Ma, C.M. Cheng, Q. Liu, and Y.F. Niu, Bull. Chin. Ceramic Soc. 41[10] (2022) 3634-3636.
- 45. M. Rafya, W. Misrar, L. Saadi, M. Mansori, M. Waqif, A. Hafidi, N. Zehhar, and F. Benkhalti, Mater. Chem. Phys. 295 (2023) 127030.
-

- 46. N. Ediza, I. Tatarb, and A. Aydnc, J. Ceram. Process. Res. 16[1] (2015) 129-136.
-

- 47. S.C. Huang, C.T. Huang, S.Y. Lu, and K.S. Chou, J Porous Mat. 6[2] (1999) 153-159.
-

- 48. M. Bellotto, A. Gualtieri, G. Artioli, and S.M. Clark, Phys. Chem. Miner. 22 (1995) 207-217.
-

- 49. M. Abbasi and D. Mowla, Desalin. Water Treat. 52[13-15] (2014) 2481-2493.
-

- 50. I. Hedfi, N. Hamdi, E. Srasra, and M.A. Rodríguez, Appl. Clay Sci. 101 (2014) 574-578.
-

- 51. N.H. Mohtor, M.H.D. Othman, A.F. Ismail, M.A. Rahman, J. Jaafar, and N.A. Hashim, Environ. Sci. Pollut. R. 24[19] (2017) 15905-15917.
-

- 52. Z. Zhu, Z. Wei, W. Sun, Jie Hou d, B.H. He, and Y.C. Dong, Appl. Clay Sci. 120 (2016) 135-141.
-

- 53. A. Harabi, F. Zenikheri, B. Boudaira, F. Bouzerara, A. Guechi, and L. Foughali, J. Eur. Ceram. Soc. 34[5] (2014) 1329-1340.
-

- 54. D. Vasanth, G. Pugazhenthi, and R. Uppaluri, J. Membrane Sci. 379[1-2] (2011) 154-163.
-

- 55. J. Zhou, X. Zhang, Y. Wang, A. Larbot, and X.B. Hu, J. Porous Mat. 17[1] (2010) 1-9.
-

- 56. A.M. Ben, N. Hamdi, M.A. Rodriguez, K. Mahmoudi, and E. Srasra, Ceram. Int. 44[2] (2018) 2328-2335.
-

- 57. S.K. Hubadillah, M.H.D. Othman, A.F. Ismail, M.A. Rahman, and J. Jaafar, Sep. Purif. Technol. 214 (2019) 31-39.
-

- 58. H.S. Khadijah, P. Kumar, M.H.D. Othman, A.F. Ismail, M.A. Rahman, and J. Jaafar, RSC Adv. 8[6] (2018) 2986-2995.
-

- 59. A. Harabi, A. Guechi, and S. Condom, Procedia Eng. 33 (2012) 220-224.
-

- 60. A. Guechi, A. Harabi, S. Condoum, F. Zenikheri, B. Boudaira, F. Bouzerara, and L. Foughali, Desalin. Water Treat. 57[12] (2016) 5246-2552.
-

- 61. N. Kouras, A. Harabi, F. Bouzerara, L. Foughali, A. Policicchio, S. Stelitano, F. Galiano, and A. Figoli, J. Eur. Ceram. Soc. 37[9] (2017) 3159-3165.
-

- 62. B. Boudaira, A. Harabia, F. Bouzerara, and S. Condom, Desalin. Water Treat. 9[1-3] (2009) 142-148.
-

- 63. P. Monash and G. Pugazhenthi, Int. J. Appl. Ceram. Tec. 8[1] (2011) 227-238.
-

- 64. G. Chen, X. Ge, Y. Wang, W.H. Xing, and Y.Z. Guo, Ceram. Int. 41[7] (2015) 8282-8287.
-

- 65. A. Dhivya and A. Keshav, J. Indian Chem. Soc. 99[7] (2022) 100557.
-

- 66. Y. Zhu and D.J. Chen, Mater. Design 113 (2017) 60-67.
-

- 67. Z. Tong, K.P. Huang, B.W. Yang, and J.X. Zhang, Mater. Rep. 35[6] (2021) 6054-6059.
-

- 68. N. Saffaj, M. Persin, S.A. Younsi, A. Albizane, M. Creti, and A. Larbot, Appl. Clay Sci. 31[1] (2006) 110-119.
-

- 69. Y. Anbri, N. Tijani, J. Coronas, E. Mateo, M. Menéndez, and J. Bentama, Desalination 221[1-3] (2008) 419-424.
-

- 70. M. Khedidja, M. Bechir, S. Feyda, and T.M. Ali, Solid State Sci. 148 (2024) 107430.
-

- 71. H. Elomari, B. Achiou, M. Ouammou, A. Albizane, J. Bennazha, and S.A. Younssi, Desalin. Water Treat. 57[43] (2016) 20298-20306.
-

- 72. R.J. Galán-Arboledas, T. Cotes, C. Martínez, and S. Bueno, Desalin. Water Treat. 57[6] (2016) 2633-2639.
-

- 73. A. Abdullayev, M. Bekheet, D. Hanaor, and A. Gurlo, Membranes 9[9] (2019) 105.
-

- 74. W. Fan, D. Zou, J. Xu, X.F. Chen, M.H. Qiu, and Y.Q. Fan, Membranes 11[9] (2021) 711.
-

- 75. Q. Lu, X.F. Dong, Z.W. Zhu, and Y.C. Dong, J. Hazard. Mater. 273 (2014) 136-145.
-

- 76. А.I. Ivanets, А.I. Rat׳ko, Т.А. Azarova, S.М. Azarov, S.H. Al-Khowaiter, О. Al-Harbi, S.V. Shemchonok, V.А. Dobysh, V.А. Tarasevich, V.Е. Agabekov, and А.А. Rat׳ko, Ceram. Int. 40[8] (2014) 12343-12351.
-

- 77. A. Ivanets and V. Agabekov, Chem. J. Mold. 12[1] (2017) 67-73.
-

- 78. H. Aloulou, H. Bouhamed, B. Raja, and R.B. Amar, Desalin. Water Treat. 78 (2017) 41-48.
-

- 79. S.J. Zhu, Y. Chen, X.Y. Deng, J.B. Li, W.D. Qiu, and W. Zhang, Mater. Rep. 24[14] (2010) 70-73,88.
- 80. P.D.R. Roque-Malherbe, W.D. Valle, F. Marquez, J. Duconge, and M.F.A. Goosen, Sep. Sci. Technol. 41[1] (2006) 73-96.
-

- 81. Y.C. Dong, S.F. Chen, X.B. Zhang, J.K. Yang, X.Q. Liu, and G.Y. Meng, J. Membrane Sci. 281[1-2] (2006) 592-599.
-

- 82. P. Hristov, A. Yoleva, S. Djambazov, I. Chukovska, and D. Dimitrov, J. Univ. Chem. Technol. Metall. 47[4] (2012) 476-480.
-

- 83. Y. Zhou, C.L. Yun, X.G. Luo, and Y. Wang, J. Southwest Univ. Sci. Technol. 37[2] (2022) 1-8.
-

- 84. X.B. Zhang, X.C. Hu, X.L. Chen, and X.J. Liu, Acta Mineral. Sin. 27[1] (2007) 6-10.
-

- 85. A. Karim, B. Achiou, A. Bouazizi, A. Aaddane, M. Ouammou, M. Bouziane, J. Bennazha, and S.A. Younssi, J. Environ. Chem. Eng. 6[1] (2018) 1475-1485.
-

- 86. B. Achiou, H. Elomari, M. Ouammou, and A.A. Albizane, J. Mater. Environ. Sci. 9 (2018) 1013-1021.
-

- 87. L. Zhu, Y. Dong, L. Li, J. Liu, and S.J. You, RSC Adv. 5[15] (2015) 11163-11174.
-

- 88. J. Liu, Y. Dong, X. Dong, S. Hampshire, L. Zhu, Z.W. Zhu, and L.L. Li, J. Eur. Ceram. Soc. 36[4] (2016) 1059-11074.
-

- 89. Y. Dong, J. Zhou, B. Lin, Y.Q. Wang, S.L. Wang, L.F. Miao, Y. Lang, X.Q. Liu, and G.Y. Meng, J. Hazard. Mater. 172[1] (2009) 180-186.
-

- 90. L. Zhu, Y.C. Dong, S. Hampshire, S. Cerneaux, and L.Winnubst, J. Eur. Ceram. Soc. 35[2] (2015) 711-721.
-

- 91. Z. Wei, J. Hou, and Z. Zhu. J. Alloy. Compd. 683 (2016) 474-480.
-

- 92. J. Cao, X. Dong, L. Li, Y.C. Dong, and S. Hampshire, J. Eur. Ceram. Soc. 34[13] (2014) 3181-3194.
-

- 93. M.Y. Li, Y.N. Guo, Z. Tong, H. Zhan, S.K. Cui, L. Liu, Membrane Sci. Technol. 43[3] (2023) 94-103.
-

- 94. I. Jedidi, S. Khemakhem, A. Larbot, and R.B. Amar, Ceram. Int. 35[7] (2009) 2747-2753.
-

- 95. Y.C. Dong, S. Hampshire, J.E. Zhou, B. Lin, Z.L. Ji, X.Z. Zhang, and G.Y. Meng, J. Hazard. Mater. 180[1-3] (2010) 173-180.
-

- 96. D. Zou, M. Qiu, X. Chen, E. Drioli, and Y.Q. Fan, Sep. Purifi. Technol. 210 (2019) 511-520.
-

- 97. Y. Wang, Y. Wang, P. Nian, W.T. Wang, D. Wei, N. Xu, Z.X. Wu, T.T. Zhu, and Y.B. Wei, Ceram. Int. 49[12] (2023) 19798-19805.
-

- 98. H. Ji, M. Fang, Z. Huang, K. Chen, Y.G. Xu, Y.G. Liu, and J.T. Huang, Ceram. Int. 39[6] (2013) 6841-6846.
-

- 99. T. Yang, X.M. Hou, and G.Z. Zhou. Refractories 48[3] (2014) 197-200.
- 100. M.Y. Li, Y.N. Guo, Z. Tong, and K. Wu, J. Ceram. 44[2] (2023) 328-336.
-

- 101. L. Fan, M.K. Tian, J. Zhang, and Y.F. Zhang, Bull. Chin. Ceramic Soc. 37[5] (2018) 1781-1787.
-

- 102. K. Man, Q. Zhu, L. Li, and Z.P. Xing, Ceram. Int. 43[10] (2017) 7565-7572.
-

- 103. W. Wang, W. Chen, and H. Liu, Ceram. Int. 45[8] (2019) 9852-9857.
-

- 104. A.L. Xavier, T.V. Oliveira, W. Klitzke, A.B. Mariano, D. Eiras, and R.B. Vieira, Appl. Clay Sci. 168 (2019) 260-268.
-

- 105. A.L. Xavier, D.E.L. Fetzer, T.V. Oliveira, D. Eiras, F.A.P. Voll, and R.B. Vieira, Ceram. Int. 48[16] (2022) 23273-23283.
-

- 106. O. Manfredi, W. Wuth, and I. Bohlinger, JOM 49[11] (1997) 48-51.
-

- 107. M.H.A. Aziz, M.H.D. Othman, N.A. Hashim, M.A. Rahman, J. Jaafar, S.K. Hubadillah, and Z.S. Tai, Ceram. Int. 45[2] (2019) 2069-2078.
-

- 108. M.F. Serra, M.S. Conconi, M.R. Gauna, G. Suárez, E.F. Aglietti, and N.M. Rendtorff, J. Asian Ceram. Soc. 4[1] (2016) 61-67.
-

- 109. S.K. Hubadillah, M.H.D. Othman, A.F. Ismail, M.A. Rahman, J. Jaafar, Y. Iwamoto, S. Honda, M.I.H.M. Dzahir, and M.Z.M. Yusop, Ceram. Int. 44[9] (2018) 10498-10509.
-

- 110. R. Xiao, X. Chen, F. Wang, and G.S. Yu, Renew. Energ. 36[1] (2011) 244-249.
-

- 111. N.H. Kamarudin, Z. Harun, M.H.D. Othman, T. Abdullahi, S.S. Bahri, N.H. Kamarudin, M.Z. Yunos, and W.N.W. Salleh, Ceram. Int. 46[2] (2020) 1512-1525.
-

- 112. A. Bahurudeen, D. Kanraj, V.G. Dev, and M. Santhanam, Cement Concrete Comp. 59 (2015) 77-88.
-

- 113. G. Bonassa, L.T. Schneider, H.J. Alves, T.R.W. Meier, E.P. Frigo, and J.G. Teleken, J. Environ. Chem. Eng. 4[4] (2016) 4091-4099.
-

- 114. K.C.P. Faria, R.F. Gurgel, and J.N.F. Holanda, J. Environ. Manage. 101 (2012) 7-12.
-

- 115. M.R. Jamalludin, Z. Harun, M.H.D. Othman, S.K. Hubadillah, M.Z. Yunos, and A.F. Ismail, Ceram. Int. 44[15] (2018) 18450-18461.
-

 This Article
This Article
-
2024; 25(3): 389-403
Published on Jun 30, 2024
- 10.36410/jcpr.2024.25.3.389
- Received on Mar 9, 2024
- Revised on Apr 12, 2024
- Accepted on Apr 23, 2024
 Services
Services
- Abstract
introduction
ceramic membrane
low-cost raw materials
conclusion and prospect
- Acknowledgements
- Disclosure Statement
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Xiang Zhang
-
Joint International Research Laboratory of Refractories and Metallurgy, Ministry of Education, Wuhan University of Science and Technology, Wuhan 430081, Hubei, China
Tel : +86-158-2718-1426 Fax: +86-027-68862529 E-mail: zx91@wust.edu.cn - E-mail: zx91@wust.edu.cn






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.