- Use of geopolymer-derived leucite as a reinforcement in dental bioceramic composites
Cengiz Bagci*
Department of Metallurgical and Materials Engineering, Faculty of Engineering, Hitit University, Corum 19030, Turkey
This article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Leucite (K2O·Al2O3·4SiO2) converted from geopolymers as a sustainable approach, was used as an alternative to feldspar, one of the three components of dental ceramics. Leucite crystals were obtained from a dried potassium geopolymer of the composition K2O·Al2O3·4SiO2·11H2O by heating at 1200 oC for 3 h in an open-air furnace. Produced leucite was crushed into small parts, powdered in a planetary mill and then sieved to sub-63 micron size. Leucite crystals were then replaced by feldspars at a range from 0-100 (wt.%) in all three parts of dental ceramic slurries. The slurries were molded to 1 cm3 and subsequently heat-treated at 1300 oC-1450 ºC/4.5 h with a heating rate of 10 ºC/min. Final products were microstructurally characterized with XRD, SEM-EDS and mechanically based on Weibull analysis of compressive tests. Due to the phase transformation occurring in leucite, it was determined that the amount of leucite had a significant effect on the structural integrity and therefore the mechanical properties of the final dental material. Results of the statistical analysis showed that the replacement of 50% leucite exhibited the highest compressive strength of (49.3±10.5 MPa) compared to the other samples consistent with microstructural analysis
Keywords: Geopolymer, Leucite, Dental Ceramics
Feldspathic dental ceramics with a typical composition of 75 wt.% feldspar, 20 wt.% quartz and 5 wt.% kaolin in the traditional clay (kaolin), quartz and feldspar ternary material systems have been used for many years in dentistry [1, 2]. They mainly include leucite (K2O·Al2O3·4SiO2) crystals dispersed in a glass matrix [3]. Leucite itself is a glass ceramic with a high melting point (Tm ~1693 oC) and a wide coefficient of thermal expansion (~17 × 10−6 K−1) [4]. Thanks to these properties, leucite is being favored in many applications such as cermets, thermal barrier coatings or ceramic matrix composites, in particular, metal-ceramic replacements in dental ceramics as a reinforcement phase [5]. Because leucite base dental prostheses not only have a high aesthetic quality, enabling visual characteristics that mimic natural teeth [6] but also have improved mechanical properties due to the suppression of stresses caused by the thermal expansion difference of the components during the production of the dental ceramics. To feldspathic replacement of the leucite is effective at increasing the mechanical properties of dental ceramics over that of conventional feldspathic ones [7]. Leucite can be in-situ synthesized from potassium feldspar by solid-state reaction [8], sol-gel method [9], hydrothermal and decomposition of zeolites [10]. A compatible matrix-reinforcement interface is achieved; however, long processing time and inhomogeneous nucleation of the leucite crystals in the glass matrix are the main problems in these processes [11]. So direct incorporation of synthetic leucite crystals into the dental matrix could be enhanced the mechanical properties by a homogeneous distribution of the crystals [12]. Geopolymers (GPs) are ambient temperature XRD-amorphous, ceramic-like structural materials and there is a recent trend related to converting them to high-temperature crystal phases such as nepheline [13], leucite, pollucite [14], SiC [15], and Si3N4 [16, 17]. Leucite crystals can be synthesized from kaolin but GPs already include its high-temperature [18] analogue, metakaolin as an aluminosilicate solid [15]. Metakaolin has been usually preferred in GP formulation over other aluminosilicate counterparts due to its higher purity and more reactivity in alkaline silicate solution which is the liquid side of preparation of a GP [19, 20]. So, geopolymerization includes room temperature alkali treatment of any aluminosilicate [21] to produce nanoparticulate and nonporous solids. This may be helping to synthesize more high-purity leucite crystals. Of course, determining the mixing ratios and conventional heat treatment conditions after the synthesis of leucite to be used as a replacement is essential, as these will affect the final properties of the dental ceramics to be obtained. As mentioned above, due to the high thermal expansion of leucite, it can tolerate the thermal incompatibility of the constituents of the composite to be formed by heat treatment in the structure. Thus, it can both contribute to the mechanical properties and prevent the aesthetic reduction caused by the internal stresses that will occur due to these thermal incompatibilities. However, attention has been drawn to the tetragonal-cubic phase transformation of leucite in dental ceramics, which results in an increase in volume at temperatures above about 625 °C [22]. Although the content of additions in mullite-modified dental ceramics is reported up to 50% [23] or waste glass up to 75% in the literature [24] there are limited studies on the amount of leucite [25, 26]. To avoid the negative effects of the phase transformation, the amount of leucite replacement could require to be optimized by using a wide range. 1350 oC-1450 oC temperature range with the varying heating rate was used for 3Y-stabilized zirconia for dental applications [27] 55 oC/min and 10 oC/min was used as the heating rate for leucite-based dental ceramics [28]. Both studies reported that the heating rate was not significant effect on the density and mechanical properties of resultant ceramics. So, 10 oC/min could be used as a typical heating rate like all other traditional ceramics.
As a strategy with a sustainable approach, this study related to directly using the geopolymer-derived leucite crystals as a feldspathic replacement with varying ratios (0-100 wt.%) in all three parts of dental ceramics and microstructural investigation by XRD and SEM-EDS and mechanical characterization of resultant composites by subsequent Weibull-based statistical evaluation.
Production of leucite crystals and dental ceramics
In the synthesis of leucite crystals, potassium geopolymer (KGP) monoliths (K2O·Al2O3·4SiO2·11H2O) produced by using the classical geopolymer route, briefly described in the introduction above and the details of which were given in our previous study, were used [29]. Quartz, feldspar and kaolin, which are the three components of dental ceramics, were obtained from Eti Maden, Turkey. To increase crystallization, KGP monoliths were cut into small pieces and then heat-treated in an open-air furnace at 1200 ºC for 3 hours to synthesize leucite. The synthesized leucite was then additionally crushed into approximately 5 mm size and planetary milled at 500 rpm for 10 minutes. Powdered leucite crystals and the starting materials, quartz, feldspar and kaolin were separately sieved to a size of less than 63 microns, ready to be prepared for dental ceramics. The compositions of the dental porcelain parts were (85 wt.% feldspars, 10 wt.% quartz and 5 wt.% kaolin), (75 wt.% feldspar, 20 wt.% quartz and 5 wt.% kaolin) and (65 wt.% feldspar, 25 wt.% quartz and 10 wt.% kaolin) for opaque, dentine and transparent parts, respectively. Leucite was replaced with feldspar contents in all three parts of dental ceramic in weight ratios of 0%, 25%, 50%, 75%, and 100%. Prepared leucite and feldspar mixtures were homogenized in the planetary ball mill at 250 rpm/5 min. The homogenous compositions for opaque parts were first mixed with deionized water to obtain ceramic slurries and then cast into a 1 cm3 delrin mold. After drying at 60 ºC for 30 min, the green compacts were de-molded and then dentine and transparent slurries were prepared by the same route and hand applied over the opaque samples by drying between the layers at the same condition, respectively. Subsequently, the dental ceramics were carried out heat treatment between 1300 ºC-1450 ºC/4.5 h with a heating rate of 10 ºC/min by gradually increasing the temperature for each increasing amount of leucite.
Microstructural and mechanical characterization
The crystallinity of the leucite was confirmed by X-ray diffraction (Bruker D8 Advance) using Cu Kα radiation (1.5418 Å wavelengths). Scans ran from 10° to 60° 2θ at a 0.01° step and 0.1 s/step. Surfaces micro topographies of resultant dental ceramics were examined by scanning electron microscope (SEM) equipped with energy dispersive spectroscopy (EDS) analysis (Quanta, 450). The SEM examinations were based on secondary electron imaging (SEI) at an accelerating voltage of 20 kV. The compressive strengths of the samples were made by Shimadzu mechanical testing machine using a 100 kN load cell at 0.5 mm/min. The Weibull distribution function was also utilized to confirm the data from compressive tests. The hardness of the dental ceramics was measured using a Vickers hardness tester (Metkon DUROLINE-M) with a load of 5 N and a dwell time of 10 s.
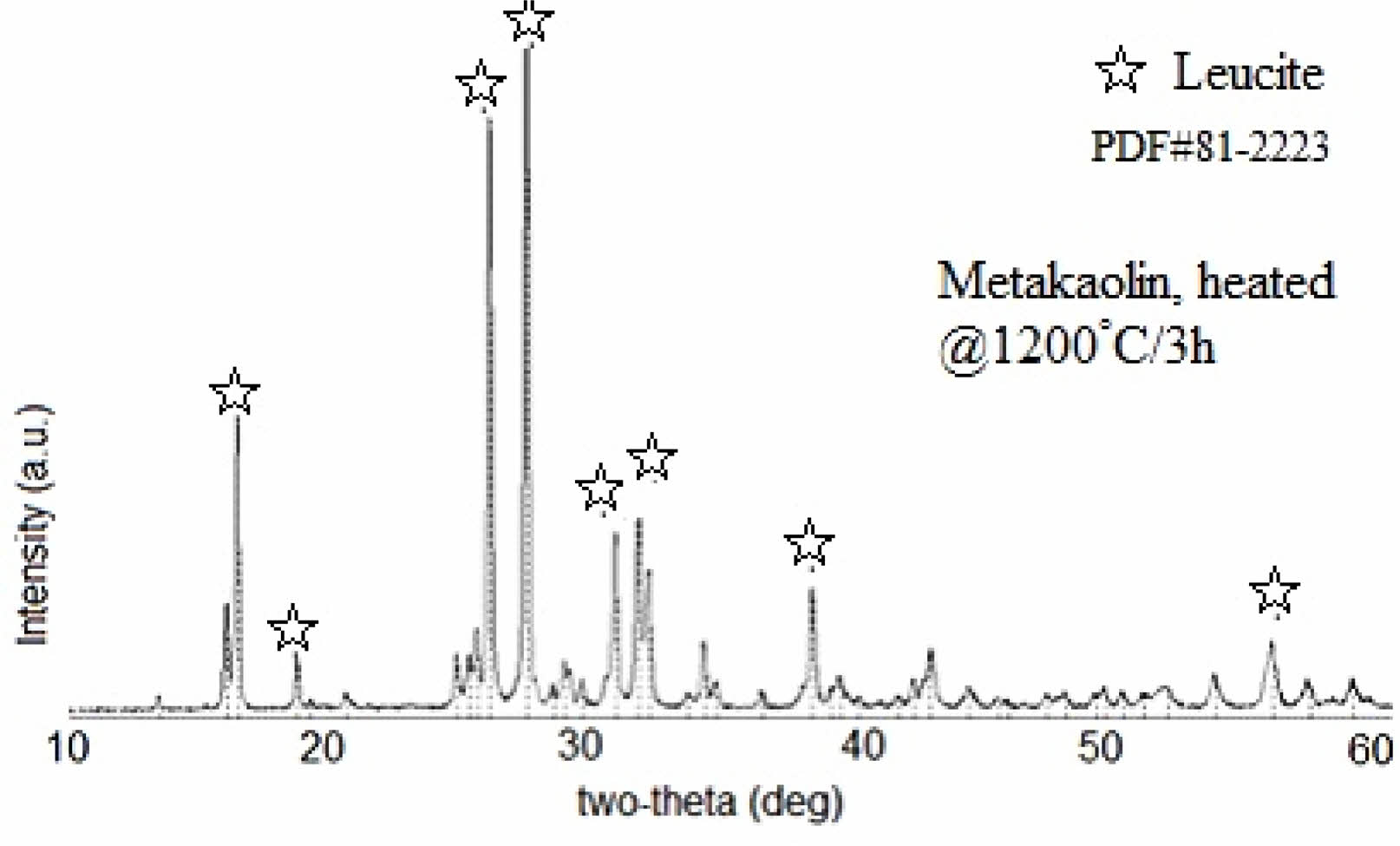
Figure 1 shows X-ray diffraction patterns of KGP precursor after being heated at 1200 oC/3 h in the open-air furnace. Main XRD peaks which were observed at 2θ = 16.37°, 25.81°, 27.25°, 31.58°, 33.92°, 35.10°, and 43.9° were ascribed to the leucite characteristic patterns [8] and a trace amount of kalsilite [30-32]. This means that KGP was well converted into high-purity leucite crystals with the help of alkali treated and nanoparticulate nature of GP precursor [4].
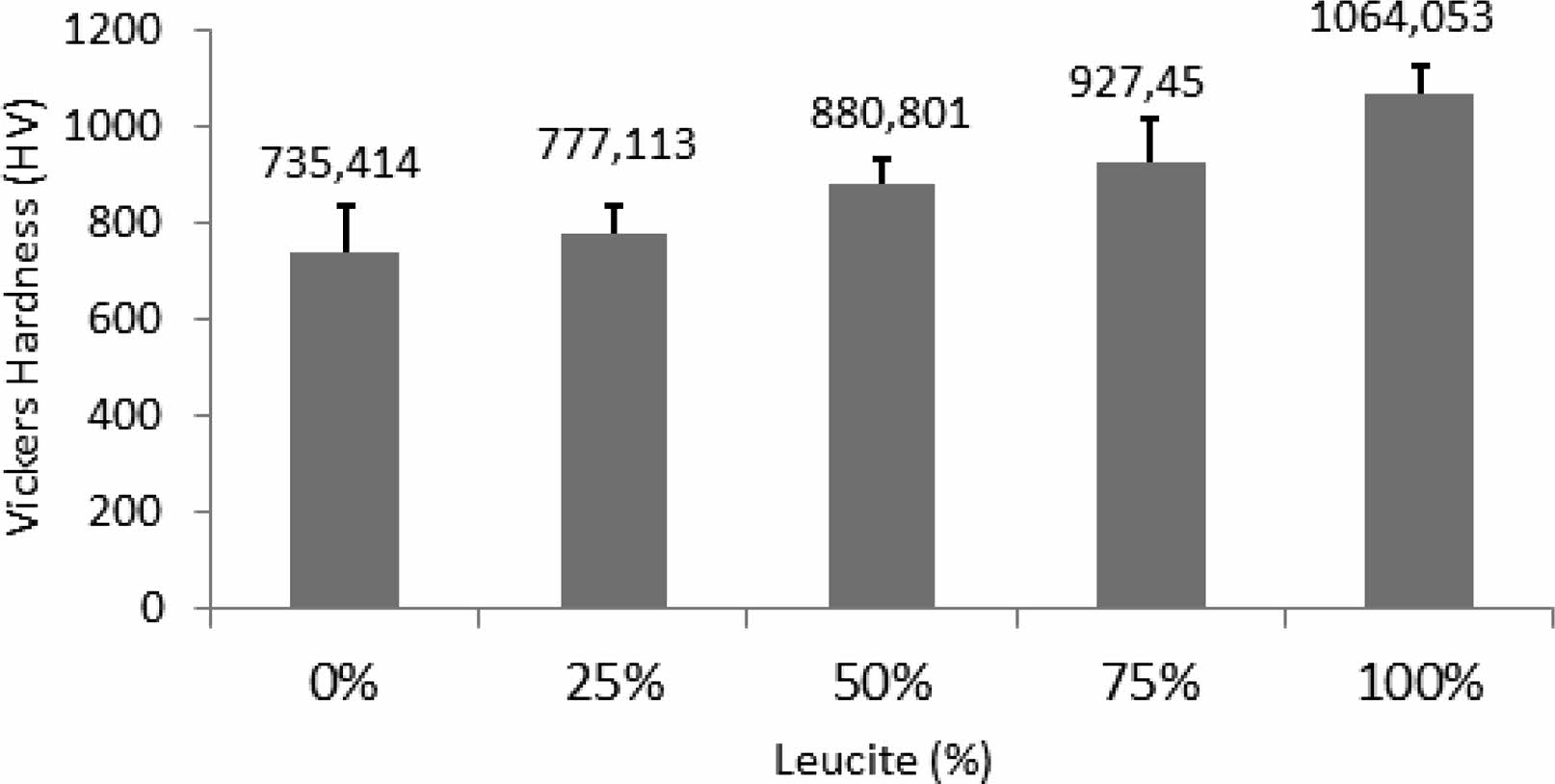
Figure 2 depicts Vickers hardness results of dental ceramics versus leucite content (wt.%). With increasing the leucite amount in dental ceramic, it is seen a proportional increment of the Vickers hardness of the composites. This was because leucite crystals introduced into the dental matrix served to improve the hardness value of the composites [11]. It should also be noted that the 50% sample had the smallest standard deviation and subsequently the smallest error bar among the other composite samples. That means this sample had finer structural integrity than the other samples. Structural integrity is a very crucial parameter in traditional ceramic materials for mechanical properties [33]. So, cracks that originated during the dehydration of glass matrix like the ceramic structure are seen as a problem that cannot be overcome and limits its industrial use because it weakens the mechanical properties [34, 35]. In addition, it can be predicted that the expansion in the structure due to the phase transformation together with the increase in leucite will cause cracks and deterioration of the samples [22, 36].
SEM micrographs of the dental ceramics with varying amounts of leucite content were represented in Fig. 3. Relatively enough sinterability of all samples sintered at 1300 ºC-1450 ºC/4.5 h can be confirmed from the micrographs. It was possible to see that all samples included pores due to dehydration during drying as traditional ceramics are. The pores were in different sizes from micro to macro [37]. Samples containing lower amounts of leucite (Fig. 3a and b) generally contained fewer pores of larger sizes than other samples. Increasing the amount of leucite provoke not only reduces the pore’s size but also resulted in the narrower size distribution of pores (Fig. 3c) [1]. Samples including 50% leucite replacement sintered at 1400 ºC exhibited better structural integrity and uniform structure than other samples. The homogeneous distribution of the pores was supposed to prevent crack propagation by crack reflection mechanism and this could be reflected in further mechanical properties [38]. In the samples with 0%, 25%, and 50% leucite addition, the pores were successful in preventing cracks created by the stresses caused by the tetragonal to cubic phase transformation in leucite. It has been reported that the cubic phase transformation, which starts at 625 °C, increases with increasing temperature and the volume of the cubic phase is approximately 1% more than the tetragonal one [39]. In this study, the temperature was increased for sinterability due to the high melting point of leucite in direct proportion to the increased leucite content. Accordingly, over 50% of leucite replacement destroyed structural integrities by the suggested leucite phase-transition mechanism, which predominantly occurred at higher amounts of leucite contents. A connected pores region is seen as evidence of deterioration of structural integrity in Fig. 3d. The connected pores gave rise to growth of the pores in Fig. 3e [36]. Although some stabilizing oxide agents such as Cs2O that limit or reduce the effects of this phase transformation are used [40, 41], the current study was limited to the optimization of the amount of leucite to control this effect.
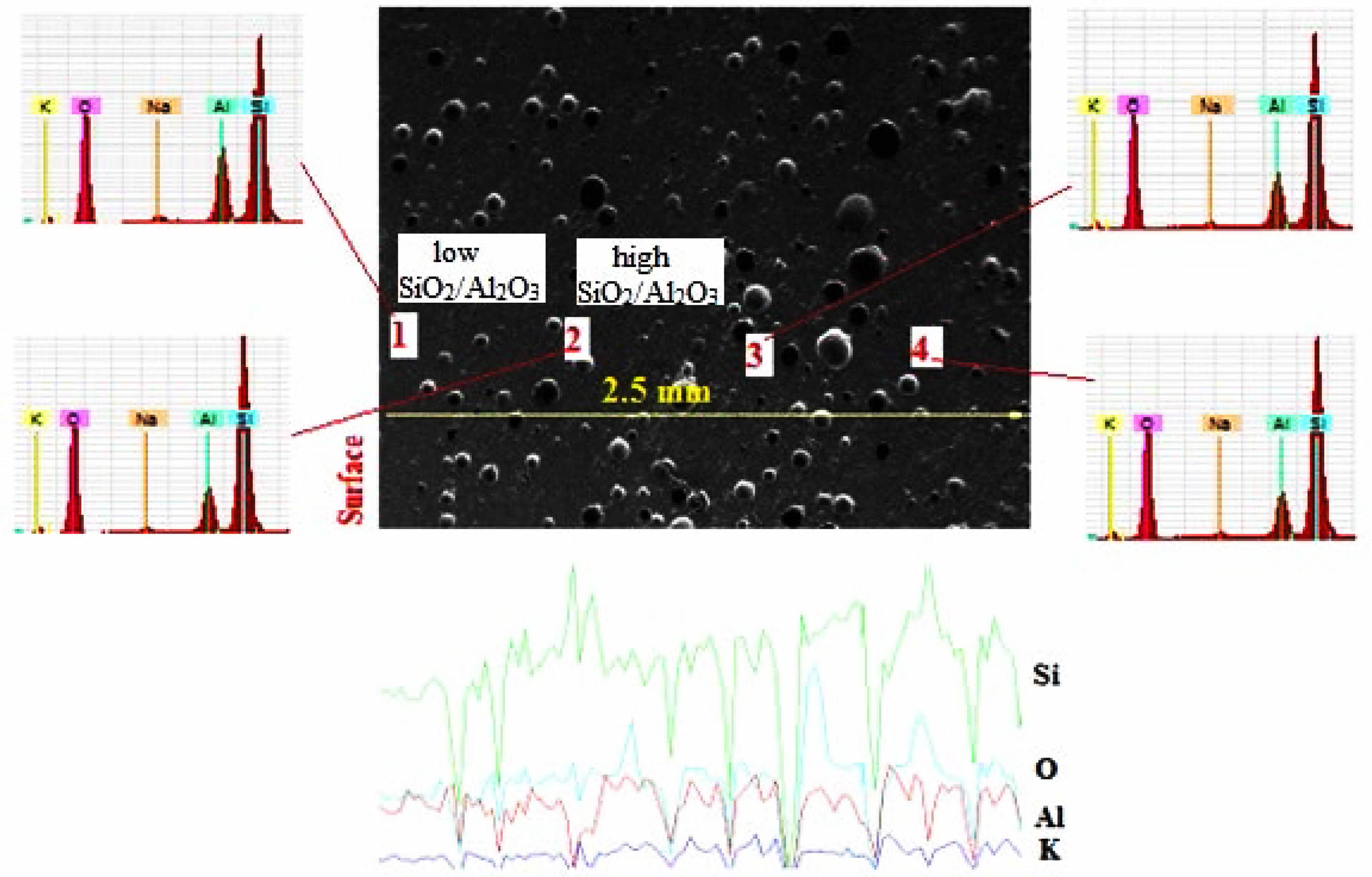
Figure 4 shows SEM-EDS analysis of the 50% dental ceramic which was optimized from the microstructural assessment of all samples in Fig. 3. Considering the SiO2/Al2O3 ratio, point analyses taken from four different parts of the microstructure are examined, while points 1 and 3 with low SiO2/Al2O3 ratio are attributed to leucite (K2O·Al2O3·4SiO2) [4], points 2 and 4 with high SiO2/Al2O3 ratio were defined potassium aluminum silicate as the feldspar (K2O·Al2O3·6SiO2) [42]. From these point analyses, it was seen that leucite exhibited a homogeneous distribution within the structure, as expected from a composite material. Subsequently, when the line scan analysis from the surface to the center of 2.5 mm is examined, it confirmed the phases determined from point analyses and supported the structural homogeneity by the point analysis.
Weibull parameters of all the samples calculated from the compressive strength of eight different measurements for each sample are shown in Table 1. It is seen that 50% dental ceramic showed the highest Weibull strength and narrower confidence interval among the other samples. This was consistent with the microstructural evaluation above as the dental ceramic kept its structural integrity until the addition of 50% leucite. In samples containing leucite below this ratio, although the average Weibull strength gives values close to 50% [43], these were relatively low due to insufficient reinforcement. Consistent with the microstructure results, over 50% of leucite served for more tetragonal to cubic phase transformation [44]. Due to this phase transformation, the expansion in the leucite lattice and the resulting stresses in the structure disrupted the structural integrity. It is seen that this deterioration in the structural integrity dramatically reduced the compressive strength and gave rise to the larger confidence intervals of the samples above 50% leucite content.
|
Fig. 1 X-ray diffraction patterns of KGP precursor after being heated at 1200 oC/3 h in the open-air furnace. |
|
Fig. 2 Vickers hardness results of dental ceramics versus leucite content (wt.%). |

|
Fig. 3 SEM micrographs of the dental ceramics which has leucite content of a) 0%, b) 25%, c) 50%, d) 75%, e) 100% wt. and were heat-treated at 1300 ºC-1450 ºC/4.5 h. |
|
Fig. 4 SEM-EDS analysis of the dental ceramic which has a 50% leucite content and heat-treated at 1400ºC. |
This study introduces using of geopolymer-derived leucite as a tunable replacement in the production of dental bioceramic composites. Increasing the amount of leucite replacement increased the hardness of dental ceramics. SEM micrographs showed that over 50% of leucite replacement destroyed structural integrity. Consistent with 50% leucite replacement showed the highest compressive strength of (49.3±10.5 MPa) and its probability plot of compressive strength showed a smaller confidence interval. This means that the negative effects of cubic phase transformation in leucite in the composite can be compensated by optimizing the leucite amount by 50% and the heat treatment temperature (1400 ºC) without using any stabilizer. Leucite, which is synthesized from geopolymer with a sustainable approach, has capable of being proposed as an alternative reinforcement in metal-free bioceramic composites.
The author wishes to thank Res. Assists. B. Alkan, BSc. S. Yildirim and BSc. K. Sevinc of Hitit University for their help with XRD analysis and preliminary studies.
- 1. B. Onwona-Agyeman, N. Lyczko, D.P. Minh, A. Nzihou, and A. Yaya, J. Ceram. Process. Res. 21[1] (2020) 35-41.
-

- 2. H. Celik, J. Ceram. Process. Res. 11[5] (2010) 622-626.
-

- 3. P. He, Z. Yang, J. Yang, X. Duan, D. Jia, S. Wang, Y. Zhou, Y. Wang, and P. Zhang, Compos. Sci. Technol. 107 (2015) 44-53.
-

- 4. N. Xie, J.L. Bell, and W.M. Kriven, J. Am. Ceram. Soc. 93 (2010) 2644-2649.
-

- 5. K. Alexandra, M. Mrazova, M. Kohoutkova, and J. Klouzek, Chemicke Listy 107[11] (2013) 856-861.
- 6. F.A. Da Silva, C.N. Barbato, S.C. Franca, A.L.N. Silva, De Andrade, M.C.J. Mater. Eng. Perform. 26 (2017) 5027-5031.
-

- 7. M.H Atala, E.B.G. Aygun, and A. Doğan, J. Ceram. Process. Res. 21 (2020) 407-415.
-

- 8. H. Salimkhani, E. Asghari Fesaghandis, S. Salimkhani, B. Abdolalipour, A. Motei Dizaji, T. Joodi, and A. Bordbar‐Khiabani, Int. J. App. Ceram. Tech. 16 (2019) 552-561.
-

- 9. C. Liu, Sri. Komarneni, and R. Roy, J. Am. Ceram. Soc. 77 (1994) 3105-3112.
-

- 10. R.I. Bedard and E.M. Flaningen, US Patent 5,071,801 (1991).
- 11. V. Šatava, A. Kloužkova, D. Ležal, and M. Novotna, Leucite porcelain. Ceram. Silik. 46 (2002) 37-40.
- 12. M. Novotna, V. Šatava, D. Ležal, A. Kloužkova, and P. Kostka, Int. Solid State Phenom. 90 (2003) 377-382.
-

- 13. C. Kuenzel, L.M. Grover, L. Vandeperre, A.R. Boccaccini, and C.R. Cheeseman, J. Eur. Ceram. Soc. 33[2] (2013) 251-258.
-

- 14. A.J. Steveson and W.M. Kriven, J. Am. Ceram. Soc. 104[7] (2021) 3397-3410.
-

- 15. C. Bagci, G.P. Kutyla, K.C. Seymour, and W.M. Kriven, J. Am. Ceram. Soc. 99[7] (2016) 2521-2530.
-

- 16. C. Bagci, G.P. Kutyla, and W.M. Kriven, Ceram. Eng. Sci. Proc. 38[10] (2014) 15-28.
-

- 17. C. Bagci, Q. Yang, and W.M. Kriven, J. Am. Ceram. Soc. 102[11] (2019) 6542-6551.
-

- 18. S. Hashimoto, A. Yamaguchi, K. Fukuda, and S. Zhang, Mater. Res. Bull. 40 (2005) 1577-1583.
-

- 19. N. Ediz, I. Tatar, and A. Aydin, J. Ceram. Process. Res. 16[1] (2015) 129-136.
-

- 20. T.H. Ahn and J.S. Ryou, J. Ceram. Process. Res. 15[4] (2014) 216-220.
-

- 21. Y. Kim. S. Kim, and C. Jung, J. Ceram. Process. Res. 17[11] (2016) 1202-1207.
-

- 22. I.L. Denry, J.A. Holloway, and H.O. Colijn, J. Biomed. Mater. Res. 54[3] (2001) 351-359.
-

- 23. L.M. Gao, J. Li, Y. Li, and F.Q. Zhang, J. Ceram. Process. Res. 12[6] (2011) 640-645.
-

- 24. M.M. Salman and H.T. Nhabih, J. Ceram. Process. Res. 21[3] (2020) 371-377.
-

- 25. P.F. Cesar, H.N. Yoshimura, W.G.M. Junior, and C.Y. Okada, J. Dent. 33[9] (2005) 721-729.
-

- 26. J.R. Mackert Jr, and C.M. Russell, Int. J. Prosthodont. 9[3] (1996) 261-265.
- 27. Y. Wang, H. Huang, L. Gao, and F. Zhang, J. Ceram. Process. Res. 12[4] (2011) 473-476.
-

- 28. C. Fredericci, H.N. Yoshimura, A.L. Molisani, M.M. Pinto, and P.F. Cesar, Ceram. Int. 37 (2011) 1073-1078.
-

- 29. C. Bagci, G.P. Kutyla, and W.M. Kriven, Ceram. Int. 43 (2017) 14784-14790.
-

- 30. X. Chatzistavrou, D. Esteve, E. Hatzistavrou, E. Kontonasaki, K.M. Paraskevopoulos, and A.R. Boccaccini. Mater. Sci. Eng. C. 30[5] (2010) 730-739.
-

- 31. Y. Zhang, M. Lv, D. Chen, and J. Wu. Mater. Lett. 61[14-15] (2007) 2978-2981.
-

- 32. V.C. Farmer, Min. Soc. Mon. 4 (1974) 331-363.
- 33. S. Yasin and H. Ahlatci, J. Ceram. Process. Res. 16[6] (2015) 662-666.
-

- 34. P. He, D. Jia, and S. Wang, J. Eur. Ceram. Soc. 33 (2013) 689-698.
-

- 35. Z. Zhang, Y. Yi, X. Wang, J. Guoc, D. Li, L. He, and S. Zhang, J. Mech. Behav. Biomed. Mater. 74 (2017) 111- 117.
-

- 36. D.C. Palmer, E.K., Salje, and W.W. Schmahl, Phys. and Chem. of Minerals 16 (1989) 714-719.
-

- 37. A. Theocharopoulos, X. Chen, R.M. Wilson, R. Hill, and M.J. Cattell, Dent. Mater. 29 (2013) 1149-1157.
-

- 38. C.C. Gonzaga, P.F. Cesar, W.G. Miranda Jr, and H.N. Yoshimura, Dent. Mater. 27 (2011) 394-406.
-

- 39. A.M.T. Bell and C.M.B. Henderson, J. Solid State Chem. 284 (2020) 121142.
-

- 40. M.A. Rouf, L. Hermansson, and R. Carlsson, Trans. J. Brit. Ceram. Soc. 77 (1978) 36-39.
- 41. S.T. Rasmussen, C.L. Groh, and W.J. O’Brien, Dent. Mater. 14 (1998) 202-211.
-

- 42. P.F. Cesar, F.N. Soki, H.N. Yoshimura, C.C. Gonzaga, and V. Styopkin, Dent. Mater. 24 (2008) 1114-1122.
-

- 43. B. Zhang, F. Qian, X. Duan, and B. Wu, J. Wuhan Univ. Technol. Mater. Sci. Ed. 24 (2009) 72-74.
-

- 44. J.R. Mackert, Jr, M.B. Butts, and C.W. Fairhurst, Dent. Mater. 2 (1986) 32-36.
-

 This Article
This Article
-
2023; 24(4): 700-704
Published on Aug 31, 2023
- 10.36410/jcpr.2023.24.4.700
- Received on Apr 25, 2023
- Revised on May 22, 2023
- Accepted on May 25, 2023
 Services
Services
- Abstract
introduction
experimental details
results and discussions
conclusions
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Cengiz Bagci
-
Department of Metallurgical and Materials Engineering, Faculty of Engineering, Hitit University, Corum 19030, Turkey
Tel : +90[364]2191200 Fax: +90[364]2191399 - E-mail: cengizbagci@gmail.com







 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.