- Mechanical properties of CaO-MgO-Al2O3-SiO2-based glass–ceramic glaze for ceramic tiles fabricated by controlled heat-treatment
Jeong-U Eoma, Seunggu Kanga, Kangduk Kima,* and Jin-Ho Kimb,**
aDepartment of Advanced Materials Science and Engineering, Kyonggi University, Suwon 16227, Korea
bKorea Institute of Ceramic Engineering & Technology, Icheon 17303, KoreaThis article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Glass–ceramic glazes were prepared using a CaO-MgO-Al2O3-SiO2 system and were used to fabricate low-temperature firing, high-hardness glazes in ceramic tiles. Additives (B2O3) and a nucleating agent (TiO2) were added to the glaze, and crystalline glazes were obtained by sintering at 1000 ~ 1100 oC for 30 or 60 min. X-ray diffraction analysis of the glazes suggested that cordierite (Mg2Al4Si5O18) and anorthite (CaAl2Si2O8) crystal phases were present, and the peak intensity of the cordierite crystal phase increased with increasing sintering temperature. All samples showed high crystallinities of 70% or more, irrespective of sintering temperature or time. The glaze density increased with increasing sintering temperature and decreased at 1100 oC. The Vickers hardness test results of the glazes indicated high hardness values of 6.79 and 6.77 GPa after 30 min heat treatment at 1000 oC and 60 min at 1050 oC, respectively. Glass-ceramic glaze with high hardness at low temperature was fabricated through crystallization of glaze for tiles
Keywords: CaO-MgO-Al2O3-SiO2, Glass–Ceramic Glazes, Hardness, Thermal Expansion Coefficient, Sintering
A glaze is an amorphous phase film that is coated on the surface of a clay body, which is used in the ceramic industry to improve a product’s esthetic and mechanical properties, such as hardness and strength. However, amorphous glazes do not impart high mechanical properties, such as abrasion resistance and hardness, and are characterized by surface properties such as brightness and texture, which are degraded with time [1]. In recent years, ongoing research in the ceramic tile industry has targeted the application of a glass–ceramic glaze with a functional crystal phase, as an alternative to the conventional amorphous glaze, to improve the mechanical properties of tiles [2].
Glass–ceramic glazes form one or more crystal phases within an amorphous matrix through a crystallization mechanism of nucleation and growth upon heat treatment. These glazes are characterized by improved mechanical, chemical, and thermal properties compared to those of conventional amorphous glazes, and such properties are dependent on the type of crystal phase, crystallinity, and microstructure. [3]. The cordierite (2MgO–2Al2O3–5SiO2) crystal phase is known to have low coefficient of thermal expansion (10–22 × 10−7 oC−1; 25–800 oC) and dielectric constant, high chemical durability and heat resistance, and excellent mechanical properties. Currently, research on MgO-Al2O3-SiO2-based glass–ceramic glazes is actively being conducted. [4, 5]. Crystalline glazes of MgO-Al2O3-SiO2 generally exhibit the surface crystallization behavior; however, when a nucleating agent such as TiO2 or ZrO2 is added, glazes with the microscopic crystallization behavior can be manufactured [6]. In general, MgO-Al2O3-SiO2-based glass–ceramic glazes are known to have high melting temperatures, which can be lowered along with the sintering temperature through the addition of a flux (B2O3 or P2O5) [7]. Demirci et al. reported that the addition of a small amount of B2O3 to a MgO-Al2O3-SiO2 system affects the crystallization behavior and thermal expansion coefficient of the glaze [8]. Ferrari et al. reported that cordierite crystallization in MgO-Al2O3-SiO2 glass–ceramic glazes was possible through B2O3 addition followed by rapid sintering, resulting in sufficiently high glaze density and excellent mechanical and chemical properties [9]. ]. G. Sumana et. al. reported that the reduced the surface roughness to 3.73 µm and increased the Vickers hardness to 5.04 GPa through microwave sintering in a CaO-MgO-Al2O3-SiO2 system[10]. Kim et al. reported that glass-ceramic glaze with high hardness was fabricated by adding CaO and B2O3 as fluxes to MgO-Al2O3-SiO2 frit [11]. In addition, the melting temperature of MgO-Al2O3-SiO2-based glazes can be lowered, and the thermal expansion coefficient can be controlled upon the addition of CaO. The use of a glass–ceramic glaze with a thermal expansion coefficient similar to that of a clay body improves the adhesion at the interface between the glaze and clay body [12-15].
In this study, B2O3 was used as a flux for the low-temperature melting of CaO-MgO-Al2O3-SiO2-based glazes with high melting temperatures. After the addition of TiO2 as a nucleating agent for glaze crystallization, the crystallization behavior and hardness characteristics of the glazes were recorded under various heat-treatment conditions (varying temperature and time). In addition, the hardness, degree of crystallinity, and thermal expansion coefficient as well as the physical properties of density and water absorption of the prepared glass–ceramic CaO-MgO-Al2O3-SiO2 glazes were evaluated.
Commercially available materials were obtained from KOJUNDO Chemical Co., Japan, (CaCO3, 99%; MgO, 99.9%; SiO2, 99.9%; B2O3, 99.9%; TiO2, 99.9%) or SANCHUN PURE Chemical Co., Korea, (Al2O3, 99.0%) and used without further purification.
CaCO3, MgO, SiO2, and Al2O3 were used as raw materials for the preparation of the CaO-MgO-Al2O3-SiO2 ceramic-glass glaze, B2O3 was used as a flux to lower the sintering temperature, and TiO2 was added as a nucleating agent to generate cordierite and anorthite crystal phases. The raw materials were combined and ball-milled in the solid-state for 24 h using zirconia balls. The mixed raw material was subsequently placed in an alumina crucible and melted in an electric furnace at 1450 oC for 90 min, followed by cooling with distilled water at room temperature. The resulting suspended glaze was mixed by sonication, and dried at 100 oC for 24 h. The dried glaze was pulverized to a particle size of <45 μm, followed by heat treatment at the desired temperature with a heating rate 10 oC min−i rate to prepare a crystalline glaze. Table 1 summarizes the batch composition of the raw materials and heat-treatment conditions for the fabrication of the glass–ceramic glaze.
The crystal phase (PANalytical, Xpertpro, Netherlands) and crystallinity (D8 ADVANCE, Bruker Co., U.S.A.; EVA program) of the glass–ceramic glazes were characterized by X-ray diffraction (XRD) analysis. The glaze microstructure was analyzed by scanning electron microscopy (SEM; Nova NanoSEM 450, FEI Co., USA), and the surface and cut surface were polished and etched with 3 wt% hydrofluoric acid (HF) for 1 min prior to analysis. Energy dispersive qualitative analysis was performed through X-ray spectroscopy (SEM/EDS; EMAX 7593H, HORIBA, Ltd., Japan). Fourier transform infrared (FTIR) absorbance spectra of the glass–ceramic glazes were measured using an FTIR spectrometer (Nicolet iS50, Thermo Fisher Scientific, USA). The density and water absorption of the glazes were measured using the KS L 1001 ¡°Ceramic Tile” test method. The hardness of the glass–ceramic glaze surface was measured by applying a load of 1 kgf using a Vickers hardness tester (HM-124, MITUTOYO Co., Japan). To determine the thermal properties, the thermal expansion coefficient (dilatometry, DIL 402C, NETAZCH Co., Germany) was measured.
|
Table 1 Batch Compositions and Heat Treatment Conditions for the Preparation of Glass-ceramic Glazes. |
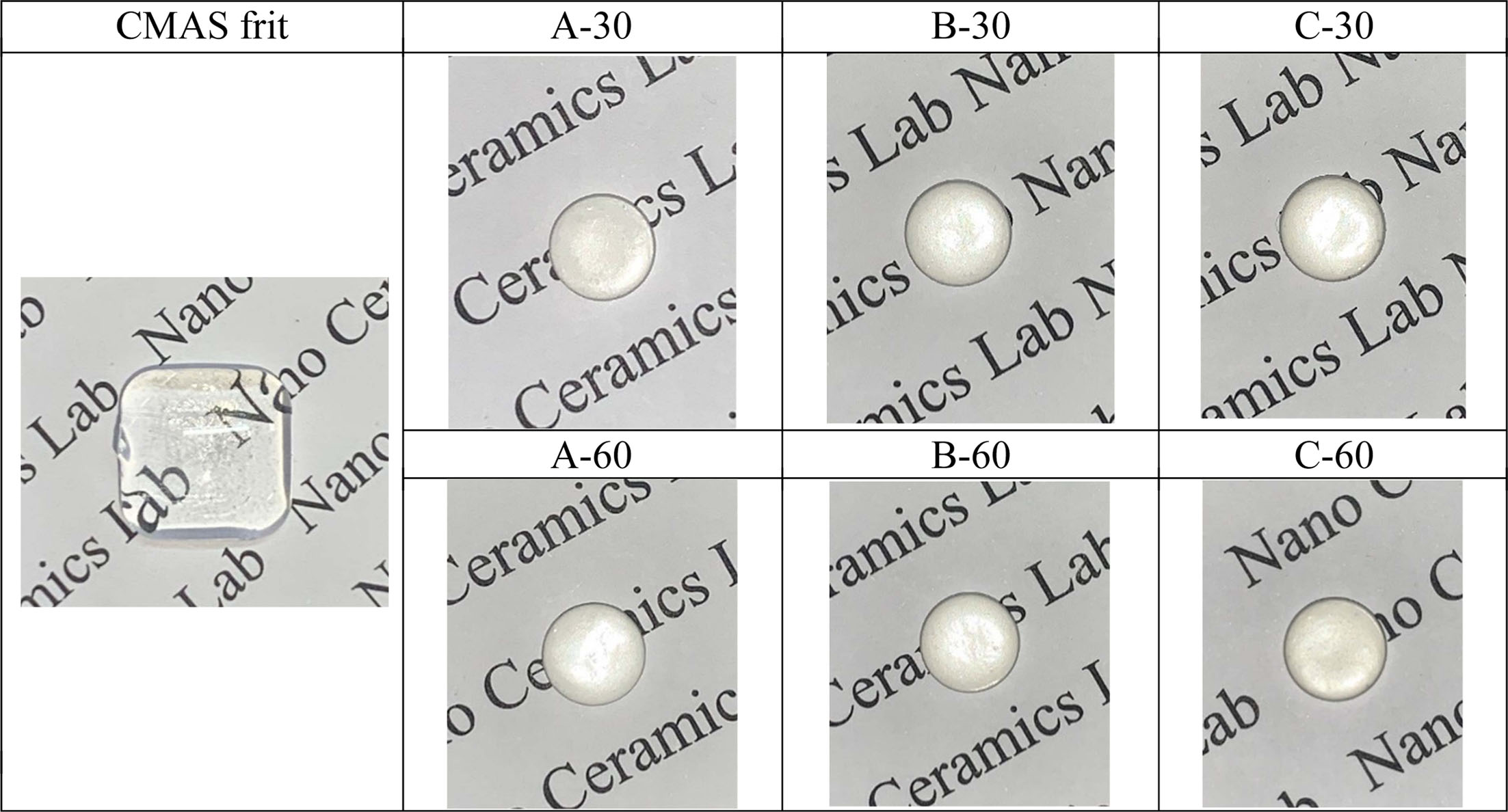
Fig. 1 shows an image of the melted frit and the glass–ceramic glaze, indicating that the melted frit (heat treatment at 1450 oC for 90 min) was optically colorless and transparent, while the glass–ceramic glaze frit (heat treatment at 1000–1100 oC for 30, 60 min) was colorless and opaque.
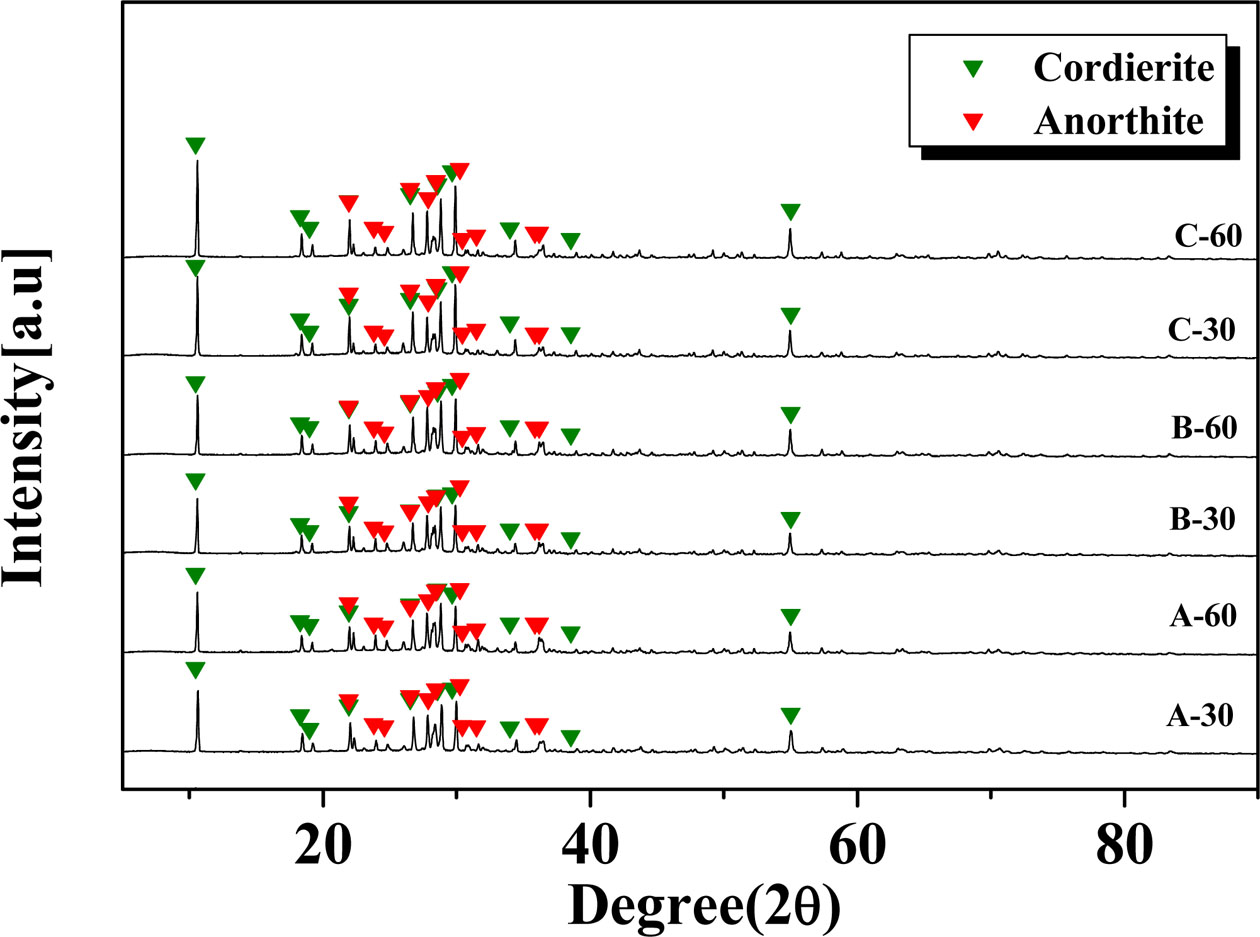
Fig. 2 shows the XRD patterns of the glazes obtained under various heat-treatment conditions (temperature range of 1000–1100 oC and a holding time of 30 or 60 min). Glass–ceramic glazes with cordierite (Mg2Al4Si5O18) and anorthite (CaAl2Si2O8) crystalline phases were observed under all heat-treatment conditions. In addition, the peak intensity of the cordierite crystalline phase increased with increasing heat-treatment temperature. According to Tabit et al., ceramics composed of cordierite and anorthite crystalline phases exhibit high mechanical strength, and mechanical strength was improved with larger amounts of cordierite in the crystalline phase [16].
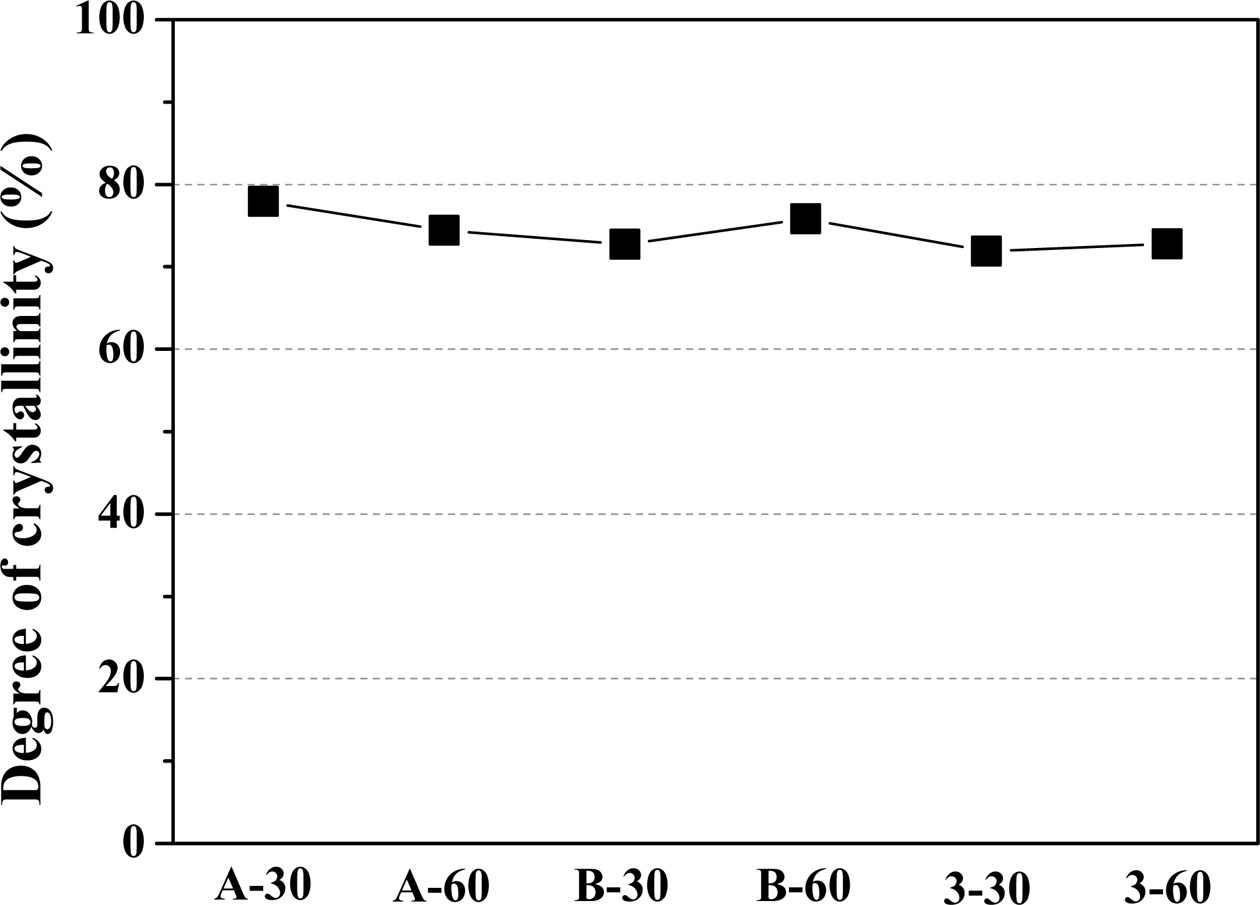
Fig. 3 shows the degree of crystallinity of the glass–ceramic glaze heat-treated for 30, 60 min at the temperature of 1000 oC~1100 oC. At a 30 min holding time, increasing the heat-treatment temperature from 1000 oC to 1100 oC resulted in a decrease in the degree of crystallinity of the glaze from 77.9% to 71.9%. At the longer holding time (60 min), the degree of crystallinity progressed from 74.4% to 75.8% and 72.8% with increasing heat-treatment temperature. The glazes showed a high degree of crystallinity (>70%), regardless of the heat-treatment conditions.
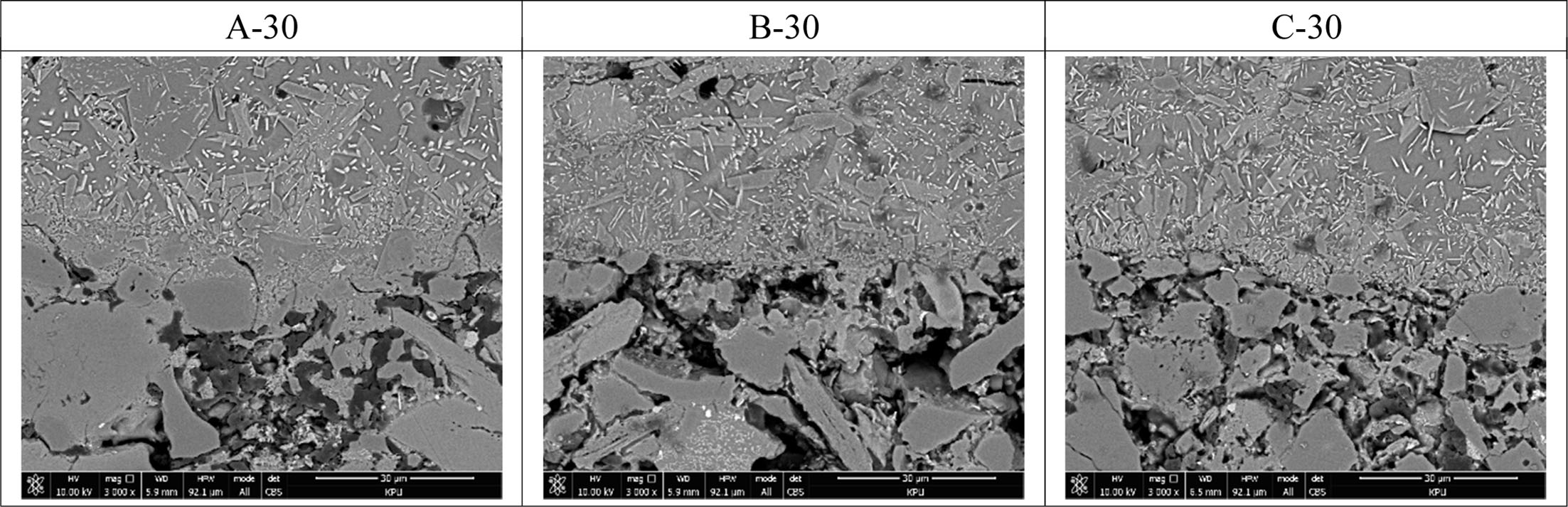
Fig. 4 shows the microstructure of the interface between the glaze layer and the clay body of tiles heat-treated for 30 min at the temperature of 1000 oC~ 1100 oC. In the upper glaze layer, the crystal phases of various sizes and habits (plates and needles), forming a dense structure with the lower clay body layer, were observed.
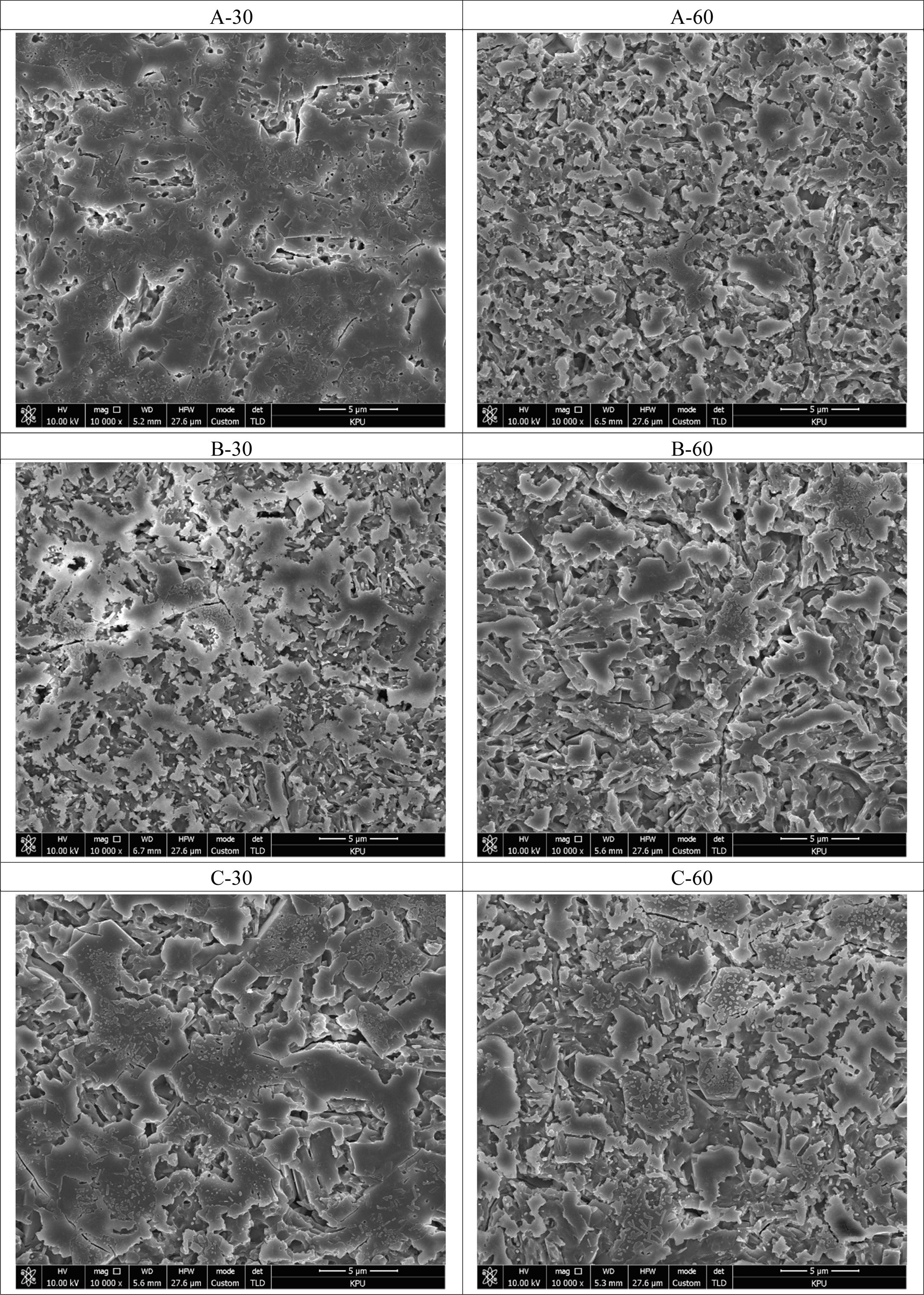
Fig. 5 shows the SEM images of the surface micro- structure of the glass–ceramic glaze that were heat-treated for 30, 60 min at the temperature of 1000 oC ~1100 oC. The surface of the glaze heat-treated for 30 min at 1000 oC had a denser structure than those of other glazes, and minute surface cracks were observed at the heat-treatment temperatures of 1100 oC and above.
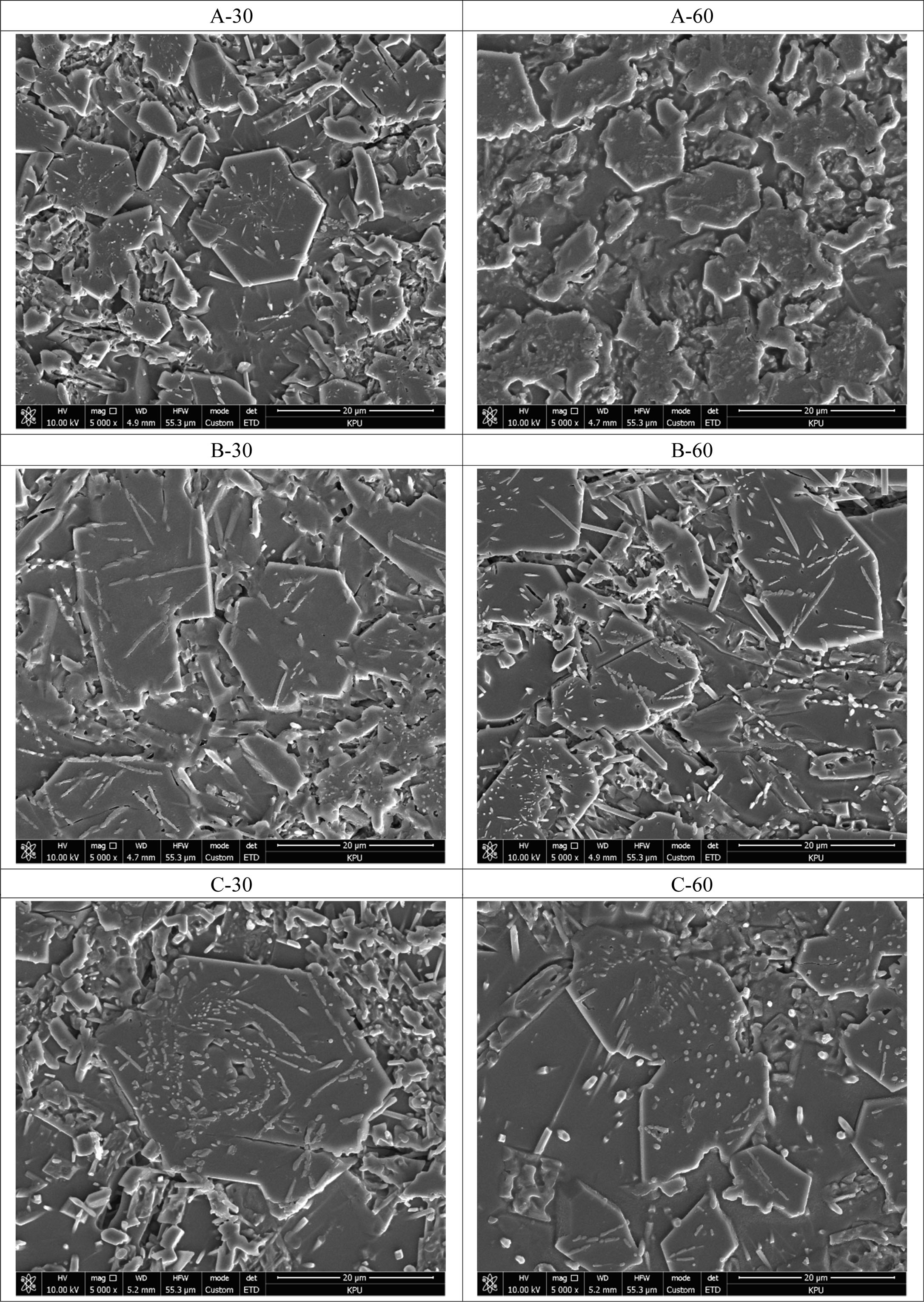
Fig. 6 shows the SEM images of the fracture surface of the glass–ceramic glazes heat-treated for 30, 60 min at the temperature of 1000 oC~1100 oC. Under all heat-treatment conditions investigated, hexagonal and rod-like crystalline phases were observed, with sizes ranging from a few to several tens of micrometers. The size of the hexagonal crystalline phase increased with increasing heat-treatment temperature. Under the maximum tem- perature studied (1100 oC), the glaze microstructure exhibited cracks that traversed the crystalline phase; these cracks were observed irrespective of holding time. As reported by Apel et al., crack propagation in glass–ceramic glazes occurs through the glass matrix and the crystalline phase with the crack terminating in the region where the density of the crystalline increases [17]. Cracks are considered to be caused by the abnormal contraction and expansion of the glaze because the heat-treatment temperature exceeds that at which the glaze exhibits plastic behavior [18].
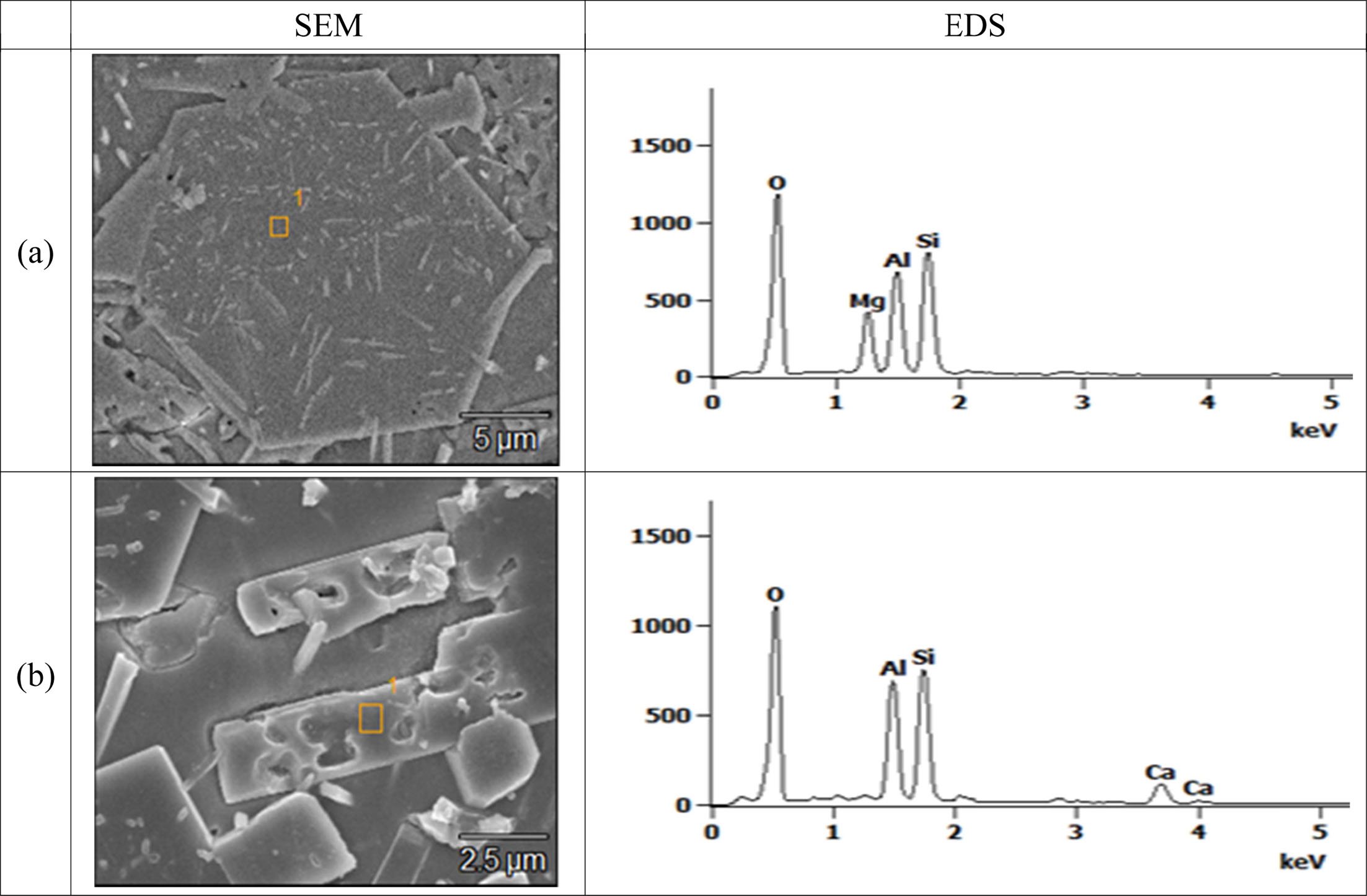
Fig. 7 shows the quantitative analysis results of the hexagonal and rod-shaped crystal phases observed by SEM/EDS. The hexagonal crystal phase (a) was identified as the cordierite (Mg2Al4Si5O18) crystal phase, as determined by the following elemental contents: Mg, 8.6 wt%; Al, 16.5 wt%; Si, 28.2 wt%; and O, 46.7 wt%. The rod-shaped crystal phase (b) was identified as an anorthite (CaAl2Si2O8) crystal phase, according to the following components: Ca, 11.6 wt%; Al, 11.9 wt%; Si, 17.9 wt%; and O 64.1 wt%.
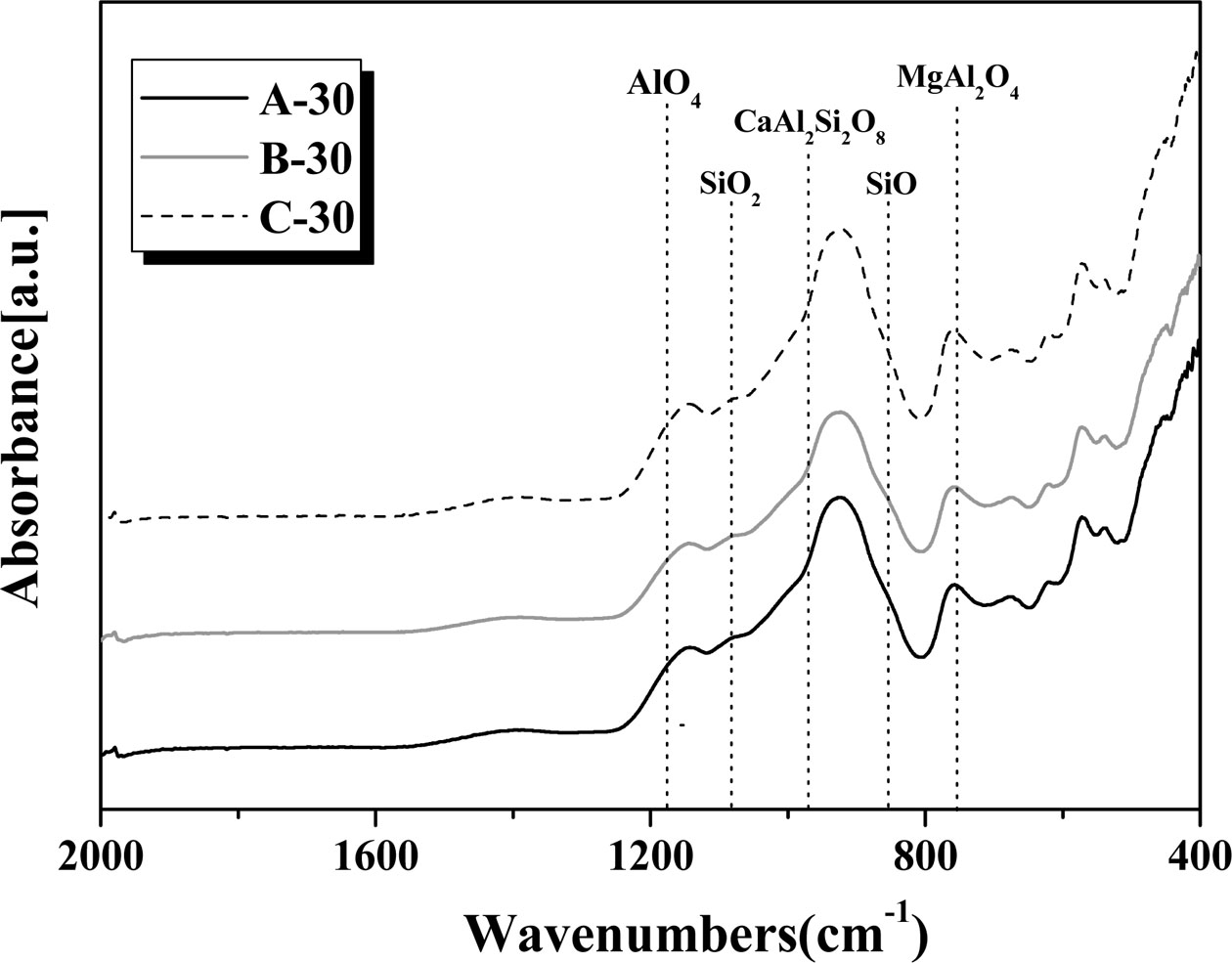
Fig. 8 shows the FTIR spectra of the glass–ceramic glazes heat-treated for 30 min at the temperature of 1000~1100 oC with a holding time of 30 or 60 min. The absorption peaks near 853 and 1179 cm−1 correspond to the vibrations of Al-O4 tetrahedra and Si-O bonds, respectively [19, 20]. The absorption peaks at 1080–1088 cm−1 arise due to the presence of amorphous SiO2, and the Si-O bands can be attributed to the α-coordination of the nanocrystals. The nanocrystalline α-cordierite structure contains both Si-O-Al and Si-O-Mg bonds [19, 21, 22]. The absorption peak at approximately 975 cm−1 is attributed to the anorthite crystal phase [23]. Several sharp and narrow absorption peaks arise in the 1300–800 and 800–500 cm−1 regions, which can be ascribed to the crystallization of SiO2 within the amorphous glass structure.
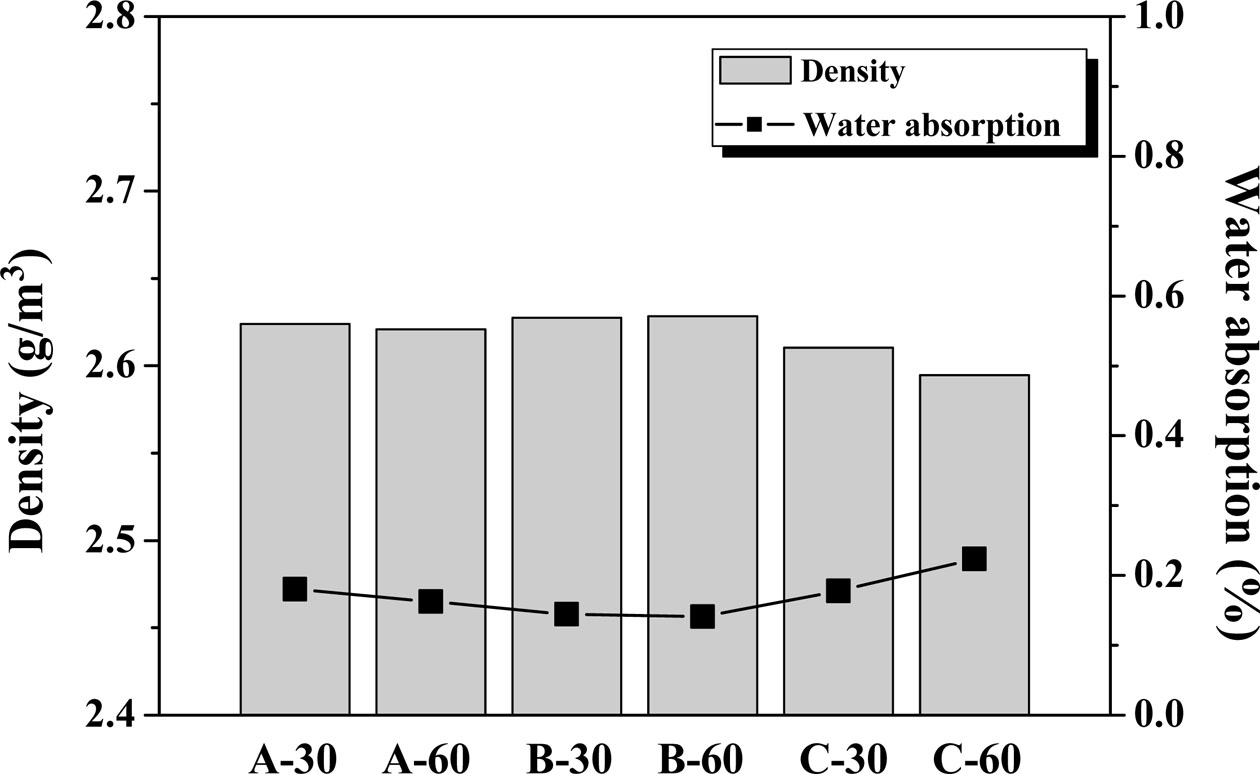
Fig. 9 shows the density and water absorption of the glass–ceramic glaze heat-treated for 30, 60 min at the temperature of 1000 oC~1100 oC. The density of the glass–ceramic glaze was 2.6–2.65 g/cm3, and holding time had no impact on this range at the maximum heat-treatment temperature (1100 oC). The overall density of the cordierite crystal phase was measured to be 2.65 g/cm3, and the anorthite crystal phase density was 2.73 g/cm3. The studied glass–ceramic glazes exhibit a high degree of crystallinity (~75%) and are considered to have lower densities than their individual crystalline phases. The water absorption of the glass–ceramic glazes was found to be low (≤0.2%), with the highest water absorption observed at 1100 oC. The water absorption of the studied crystalline glazes satisfies the water absorption criterion (<3.0%) of KS L 1001 “Ceramic tiles”. In addition, it also satisfies the water absorption criterion (<0.5%) of porcelain tiles in ISO 10545-3 “Ceramic tiles-Part 3: Determination of water absorption, apparent porosity, apparent relative density and bulk density” [24, 25]. The decrease in density and increase in water absorption with increasing heat-treatment tem- perature is thought to be the result of cracking during the heat treatment for glaze crystallization, which was confirmed by the microstructure of the glaze shown in Fig. 6.
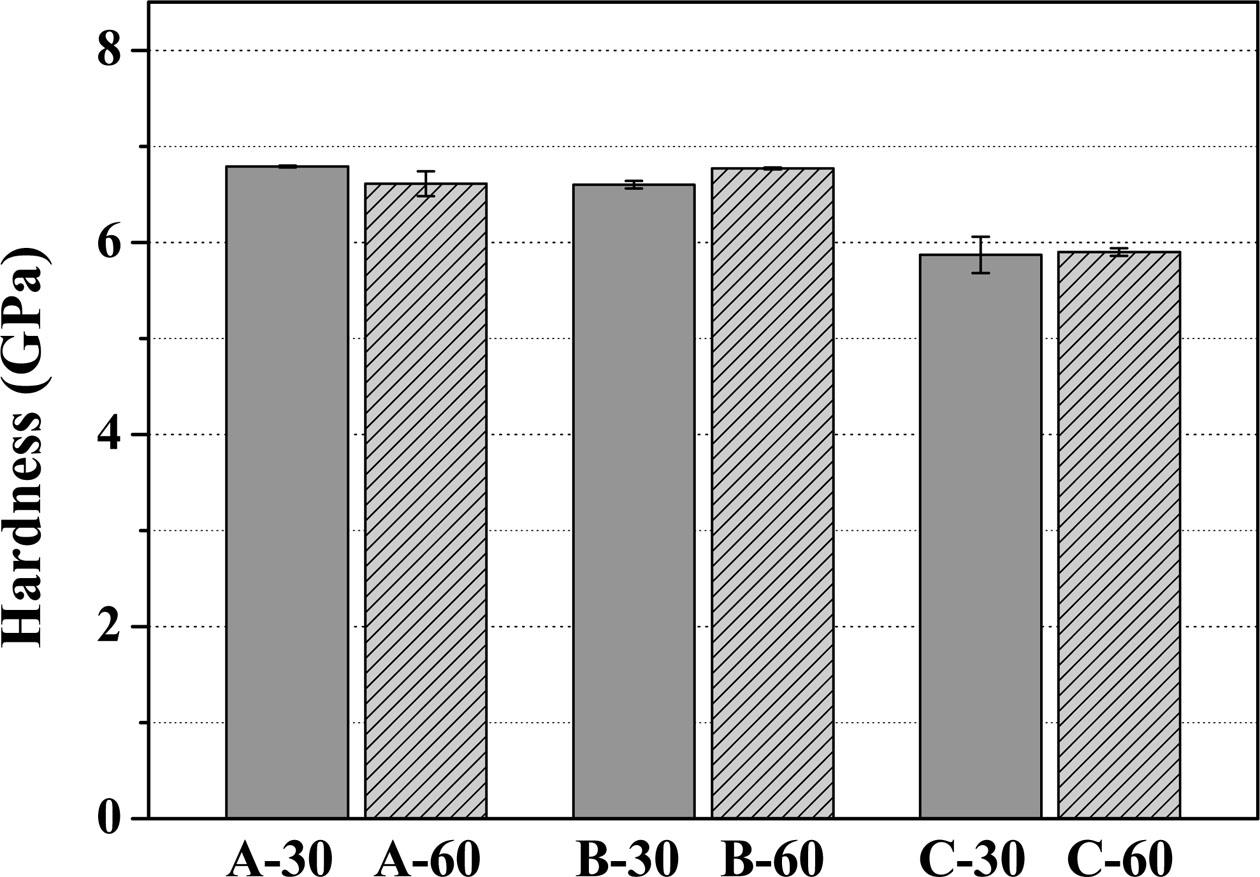
Fig. 10 shows the Vickers hardness of the glass–ceramic glazes heat-treated for 30, 60 min at the tem- perature of 1000 oC~1100 oC. The glazes heat-treated at 1000 oC for 30 or 60 min showed hardness values of 6.79 and 6.61 GPa, respectively. Moreover, the glazes heat-treated at 1050 oC for 30 or 60 min gave hardness values of 6.60 and 6.77 GPa, respectively. However, heating the glaze to 1100 oC resulted in a decrease in hardness, with measurements below 6.0 GPa. A report from Sultana et al. describes that the hardness of glass–ceramic glazes depends on the type of crystalline phase, the quantity of individual crystalline components, the composition of the residual glass phase, and the porosity of the glaze surface [26]. Furthermore, Wang et al. reported that density and porosity are also important factors that affect the hardness of ceramics [27]. Therefore, the decrease in the hardness of the glaze heat-treated at 1100 oC can be ascribed to crack formation and decrease in glaze density.
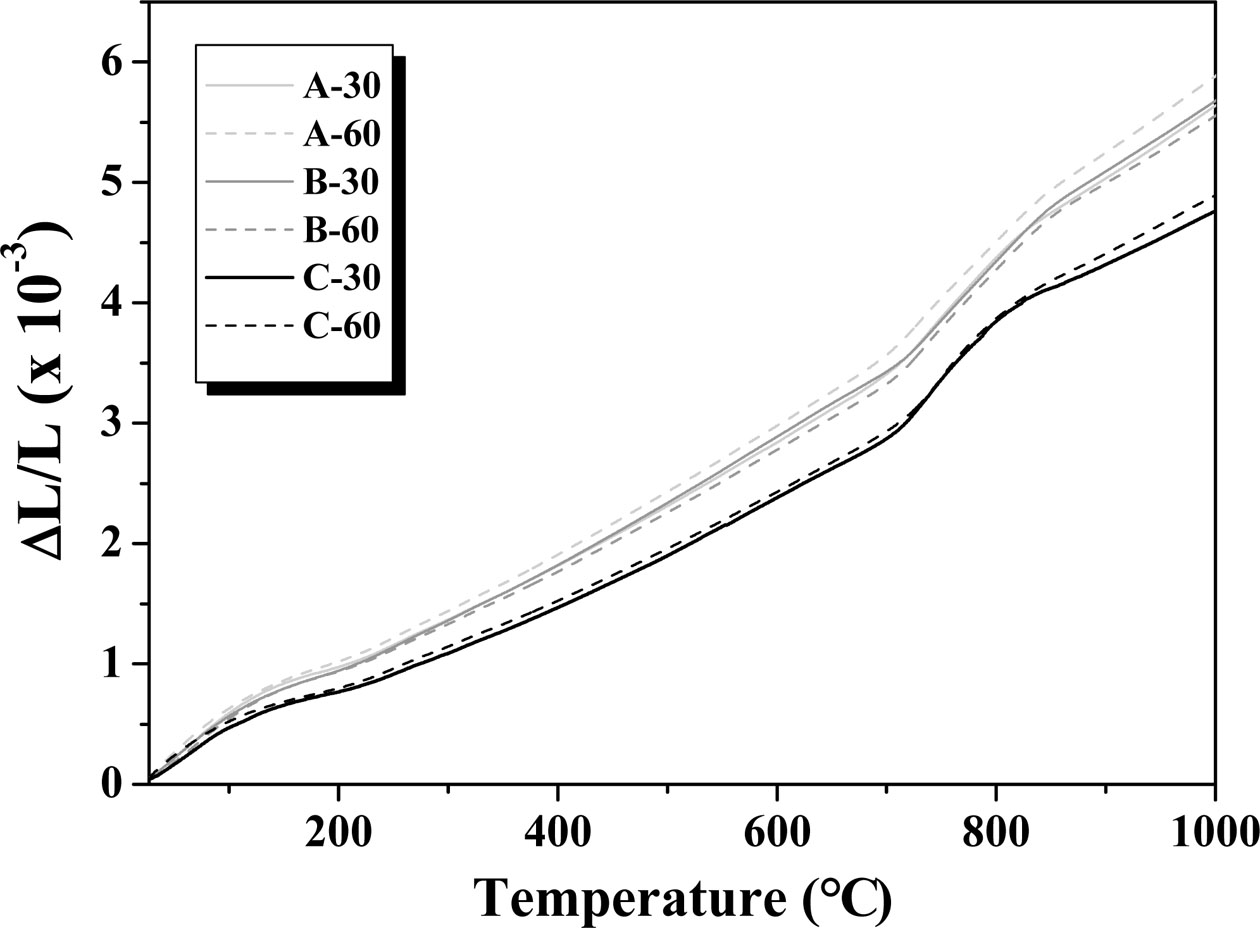
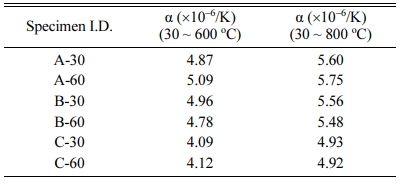
Table 2 and Fig. 11 show the thermal expansion coefficient (α) of the glass–ceramic glazes heat-treated for 30, 60 min at the temperature of 1000 oC~1100 oC. The heat treatment at 1000 oC resulted in a thermal expansion coefficient of 5.75×10-6 oC−1, while a lower coefficient of 4.93×10-6 oC−1 was obtained when the heat treatment was carried out at 1100 oC. When the thermal coefficient of the glaze and the clay body are poorly matched, the glaze can be subjected to compressive force (thermal expansion coefficient of the glaze < thermal expansion coefficient of the clay body) or traction force (thermal expansion coefficient of the glaze > thermal expansion coefficient of the clay body) to allow the formation of stress-induced defects in the glaze [28]. Fig. 12 13 14 15 16
|
Fig. 1 The Optical image of the melts frit (heat treatment at 1450 oC for 90 min) and glass-ceramic glaze (heat treatment at 1000 oC ~ 1100 oC for 30, 60 min). |
|
Fig. 2 The XRD of the glass-ceramic glaze heat-treated for 30, 60 min at the temperature of 1000 oC~1100 oC. |
|
Fig. 3 The degree of crystallinity of glass-ceramic heat-treated for 30, 60 min at the temperature of 1000 oC ~ 1100 oC |
|
Fig. 4 SEM image of fracture surface of glaze/body in tile heat-treated for 30 min at the temperature of 1000 oC ~ 1100 oC. |
|
Fig. 5 The surface of the glass-ceramic glaze heat-treated for 30, 60 min at the temperature of 1000 oC ~ 1100 oC. |
|
Fig. 6 . The fracture surface of the glass-ceramic glaze heat-treated for 30, 60 min at the temperature of 1000 oC ~ 1100 oC. |
|
Fig. 7 The EDS of the (a) hexagonal and (b) rod-shaped crystal phases in the glass-ceramic glaze A-60. |
|
Fig. 8 FTIR Absorbance spectra of glass–ceramics glaze heattreated for 30 min at the temperature of 1000 oC ~ 1100 oC. |
|
Fig. 9 The density and water absorption of the glass-ceramic glaze heat-treated for 30, 60 min at the temperature of 1000 oC ~ 1100 oC. |
|
Fig. 10 A Vickers hardness of the glass-ceramic glaze heat-treated for 30, 60 min at the temperature of 1000 oC ~ 1100 oC. |
|
Fig. 11 CTE of the glass-ceramic glaze heat-treated for 30, 60 min at the temperature of 1000 oC ~ 1100 oC. |
|
Table 2 CTE of glass-ceramic glazes heat-treated for 30, 60 min at the temperature of 1000 oC ~ 1100 oC |
In this study, the crystallization of the CaO-MgO-Al2O3-SiO2-based glaze was performed in order to fabricate a glaze with high hardness at a temperature lower than that utilized in the conventional tile manu- facturing process. B2O3 and TiO2 were added as a flux to lower the heat-treatment temperature and as a nucleating agent to improve glaze crystallization, respectively. A temperature range of 1000–1100 oC and a holding time of 30 or 60 min were employed as the experimental heat-treatment conditions. According to XRD analysis, cordierite (Mg2Al4Si5O18) and anorthite (CaAl2Si2O8) crystal phases were observed under all heat-treatment conditions, and the peak intensity of the cordierite crystalline phase increased as the heat-treatment temperature increased. High crystallinities over 70% were observed under all heat-treatment con- ditions. SEM analysis of the microstructure revealed that the cordierite crystal phase size increased with increasing heat-treatment temperature, leading to the formation of cracks within the crystalline phase of the glaze at the maximum heat-treatment temperature (1100 oC). But increasing the heat-treatment temperature caused a decrease in the glass–ceramic glaze density and an increase in the water absorption. The Vickers hardness of the glass–ceramic glaze prepared at 1000 oC with 30 min holding time was measured to be 6.79 GPa, which decreased to 5.87 GPa when the heat-treatment temperature was increased to 1100 oC due to internal cracking. The thermal expansion coefficient of the glass–ceramic glaze heat-treated at 1000 oC was 5.75×10-6 oC−1. In this study, it was possible to manu- facture a glass-ceramic glaze with high hardness at a low temperature through crystallization of the glaze. In particular, it is expected that the working temperature range during ceramic tiles manufacturing process can be broadened due to the stable microstructure of the glass-ceramic glaze in the low temperature.
This work was supported by the Technology Innova- tion Program (20010483, Key technology development of porcelain ceramic with high energy efficiency) funded by the Ministry of Trade, Industry & Energy (MOTIE, Korea).
- 1. J.R. Taylor and A.C. Bull, in “Ceramics Glaze Technology” (Oxford: Pergamon press, 1986) p.1-10.
- 2. R. Casasola, J.M. Rincon, R. Romero, J. Mater. Sci. 47 (2012) 553-582.
-

- 3. S. Ghosh, K.S. Pal, N. Dandapat, J. Ghosh, and S. Datta, J. Eur. Ceram. Soc. 33[5] (2013) 935-942.
-

- 4. O.A. Al-Harbi and E.M.A. Hamzawy, Ceram. Int. 40[4] (2014) 5283-5288.
-

- 5. S.A.M. Abdel-Hameed, and I.M. Bakr, J. Eur. Ceram. Soc. 27[2-3] (2007) 1893-1897.
-

- 6. A. Hunger, G. Carl, and C. Russel, Solid State Sci. 12[9] (2010) 1570-1574.
-

- 7. H. Wang, S. Xu, S. Zhai, D. Deng, and H. Ju, J. Mater. Sci. Tech. 26[4] (2010) 351-354.
-

- 8. Y. Demirci and E. EGunay, J. Ceram. Process. Res. 12[3] (2011) 352-356.
- 9. A.M. Ferrari, L. Barbieri, C. Leonelli, T. Manfredini, C. Siligardi, and A.B. Corradi, J. Am. Ceram. Soc. 80[7] (1997) 1757-1766.
-

- 10. S. Ghosh, K.S. Pal, A.K. Mandal, N. Biswas, M. Bhattacharya and P. Bandyopadhyay, Mater. Charact. 95 (2014) 192-200.
-

- 11. Y.R. Rho, K.D. Kim, and J.H. Kim, J. Ceram. Process. Res. 21[S1] (2020) 9-15.
-

- 12. R.C. De Vekey, A.J. Majumdar, Glass Technol. 14 (1973) 125-134.
- 13. R.C. De Vekey, A.J. Majumdar, Glass Technol. 15 (1974) 71-80.
- 14. G.H. Chen, J. Alloys Compd. 455[1-2] (2008) 298-302
-

- 15. R. Xie, L. Huang, X. Fu, and Y. Chen, J. Eur. Ceram. Soc. 18[7] (1998) 901-905.
-

- 16. K. Tabit, M. Waqif, and L. Saadi, Mater. Chem. Phys. 254 (2020) 123472.
-

- 17. E. Apel, J. Deubener, A. Bernard, M. Holand, R. Muller, H. Kappert, V. Rheinberger, and W. Holand, J. Mech. Behavior. Biomed. Mater. 1[4] (2018) 313-325.
-

- 18. H.G. No, S.M. Kim, U.S. Kim, and W.S. Cho, J. Kor. Ceram. Soc. 53[5] 2019 535-540.
-

- 19. R. Petrovic, D. Janackovic, S. Zec, S. Drmanic, and L. Kostic-Gvozdenovic, J. Sol-Gel Sci. Technol. 28[1] (2003) 111-118.
-

- 20. M.K. Naskar, and M. Chatterjee, J. Eur. Ceram. Soc. 24[13] (2004) 3499-3508.
-

- 21. A.S. Majumdar, and G. Mathew, J. Geo. Soc. India. 86[1] (2015) 80-92.
-

- 22. J. Castvan, S. Lazarevic, T.A. Orlovic, R. Petrovic, and D. Janackovic, Ceram. Int. 33[7] (2007) 1263-1268.
-

- 23. L. Torrisi, V. Venuti, V. Crupi, L. Silipigni, M. Cutroneo, G. Paladini, A. Torrisi, V. Havránek, A. Macková, M. Russa, G. Birarda, L. Vaccari, A. Macchia, F. Khalilli, M. R, and D. Majolino, Heritage 2[3] (2019) 1852-1873.
-

- 24. Korean Standard, No. KS L 1001 (2019) p.1-34.
- 25. European Standard, No. ISO 10545-3 (2018) p.1-8.
- 26. M.S. Sultana, A.N. Ahmed, M.N. Zaman, and M.A. Rahman, IOP Conf. Ser.: Mater. Sci. Eng. 438 (2018) 012018.
-

- 27. Z. Wang, Z. Shi, W. Wang, S. Wang, and C. Han, Ceram. Int. 45[11] (2019) 13865-13873.
-

- 28. M. Kavanova, A. Klouzkova, and J. Klouzek, Ceramics-Silikaty. 61[3] (2017) 267-275.
-

 This Article
This Article
-
2021; 22(5): 568-575
Published on Oct 31, 2021
- 10.36410/jcpr.2021.22.5.568
- Received on Mar 31, 2021
- Revised on Apr 17, 2021
- Accepted on Apr 28, 2021
 Services
Services
- Abstract
introduction
experimental materials and methods
results and discussions
conclusions
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Kangduk Kim a, and Jin-Ho Kim b
-
aDepartment of Advanced Materials Science and Engineering, Kyonggi University, Suwon 16227, Korea
Tel: +82–10-6206-6290
bKorea Institute of Ceramic Engineering & Technology, Icheon 17303, Korea
Tel: +82–10-8914-8707 - E-mail: solidwaste@kyonggi.ac.kr, jino.kim@kicet.re.kr






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.