- Preparation of nano-TiO2/ purified diatomite coating and its photocatalytic properties of formaldehyde degradation
Lijian Wanga, Junwei Zhaoa, Yujiang Wanga and Shuilin Zhengb,*
aMaterials Science and Engineering School & Henan Key Laboratory of Special Protective Materials, Luoyang Institute of Science and Technology, Luoyang 471023, P. R. China
bSchool of Chemical and Environmental Engineering, China University of Mining and Technology, Beijing 100083, P. R. China
The nano-TiO2 / purified
diatomite composite materials with core-shell structures were prepared by
hydrolysis precipitation using purified diatomite as carrier and titanium
sulfate as precursor. The composite materials were characterized by
transmission electron microscope (TEM), X-ray diffraction (XRD), low
temperature nitrogen adsorption and mercury porosimetry. The photocatalytic
composite coating based on the nano-TiO2 / prified diatomite
composite materials was prepared and its properties of formaldehyde degradation
were investigated. Meanwhile, the degradation mechanism of formaldehyde gas was
discussed. The results showed that the nano-TiO2 / prified diatomite
composite materials were formed core-shell structures, and the TiO2
nanoparticles were anatase with the average size of about 10 nm.The test results show that the
formaldehyde purification performance of the coatings has met the technical
requirements of Class I materials in the Chinese national standard JC/T 1074-2008.
Keywords: Nano-TiO2 / prified diatomite, Photocatalytic coating, Degradation, Formaldehyde gas
With the improvement of people's living standard and
health consciousness, the materials of green environmental protection for interior
decoration become more and more popular. The conventional
decorative materials will gradually release hazardous substances such as
formaldehyde after decoration [1, 2]. Therefore, the indoor gas pollution
has become an urgent problem to be solved. Study on the
environmentally friendly interior decoration materials is significant
in academia as well as application. Formaldehyde, as a common indoor air
pollution chemical, poses a serious threat to people's health [3, 4]. At
present, the methods of removing indoor formaldehyde include physical
adsorption, plant absorption and purification, windowing ventilation, air
purification and catalytic degradation. In recent years, the photocatalytic
degradation method has become available to purify indoor formaldehyde due to
its low energy consumption, simple operation, mild reaction conditions and the
ability to reduce secondary pollution [5, 6]. However, the traditional
methods of adsorption or chemical degradation are not effective in the degradation of
formaldehyde or cause secondary pollution. Therefore,
it is urgent to develop economical and efficient
formaldehyde reduction strategy. Semiconductor
photocatalysis belongs to a new technology for environmental pollution control. The
electronic structures of various semiconductor metal oxides and sulfides as
catalysts make them exhibit excellent photocatalytic activity. Nanoscale
titanium dioxide (Nano-TiO2) is one of the most studied
photocatalytic materials [7-14]. The nano-TiO2 advantages
of photocatalytic degradation of formaldehyde include strong
oxidation ability, complete degradation and no secondary pollution [5].
However, the poor dispersion of nano-TiO2 limits its application in
decorative materials. In order to improve the photocatalytic ability, the
nano-TiO2 coated large porous materials with high specific surface
area have been studied [15]. The obtained composites can not only
prevent the aggregation of fine nano-TiO2 particles,
but also improve the photodecomposition performance [16]. In recent years,
various porous materials have been developed as carriers of
nano-TiO2 [17-20]. Because of the larger specific surface
area, these carriers actually increase the local
concentration of organic compounds and avoid volatilization or dissociation of intermediate
products. Correspondingly, the photocatalyst reaction
rate was accelerated [21, 22].
Diatomite is a kind of siliceous and biogenic siliceous
sedimentary rock, which is mainly composed of the remains of ancient diatoms.
The main chemical composition of diatomite is SiO2. The relative
density of pure and dry diatomite is 0.4-0.9 g/cm3, and the pore
size distribution is 50-800 nm. The multifunctional composites can be
constructed by using the natural pores of diatomite [6, 23-27].
In this study, the nano-TiO2 / purified
diatomite composite materials
were firstly synthesized by typical hydrolytic precipitation method using
titanic sulphate as precursor. And then, the novel photocatalytic coating was
innovatively developed based on the prepared nano-TiO2 / purified
diatomite composite materials, which can effectively degrade
indoor formaldehyde gas. The photocatalytic activity of the
nano-TiO2 / purified diatomite coating
was studied through the photocatalytic degradation of
formaldehyde with a white light source.
Materials
and reagents
Diatomite powder was purchased from Meiston Powder
Material Co., Ltd. (Linjiang City, Jilin Province). Titanic
sulphate (Ti(SO4)2) was purchased from Jingxiang Chemical
Plant (Changping, Beijing). All the chemicals used in the
experiment were analytical pure. The deionized water was prepared in
laboratory, which has a resistivity not less than 18.2 MΩ.
Purification
of diatomite
In order to eliminate the influence of impurities on the
properties of synthetic materials, diatomite was firstly purified. Diatomite
(10.0 g) was calcined at 550 ºC for one hour, and then immersed in 50%
sulphuric acid solution (40.0 g) for 2 h. After centrifuging and washing twice
with water, the purified diatomite was obtained.
Preparation
of nano-TiO2 / purified diatomite composite materials
In an ice water bath, the purified diatomite and deionized
water with a mass ratio of 1:24 were placed in a beaker under vigorous
stirring. And then the diluted sulfuric acid was added to adjust the pH of the
solution to 3. After that, the Ti (SO4)2 solution (3.0 mol/L,
10 mL) was slowly dripped by a constant current pump. Ti (SO4)2
was used as titanium source. Then, the ammonium sulphate solution (1.5 mol/L,
20 mL) was pumped into the beaker. After continuous mixing for half an hour,
the above mixed solution was heated to 40 ºC and remained at this
temperature for half an hour. The pH value of the mixture was adjusted to 5.5
with diluted ammonia solution. After one hour of continuous reaction, the white precipitate
was collected by centrifuging, filtration, washing, drying and calcination at
650 ºC for 2 h. The nano-TiO2 / purified diatomite composite
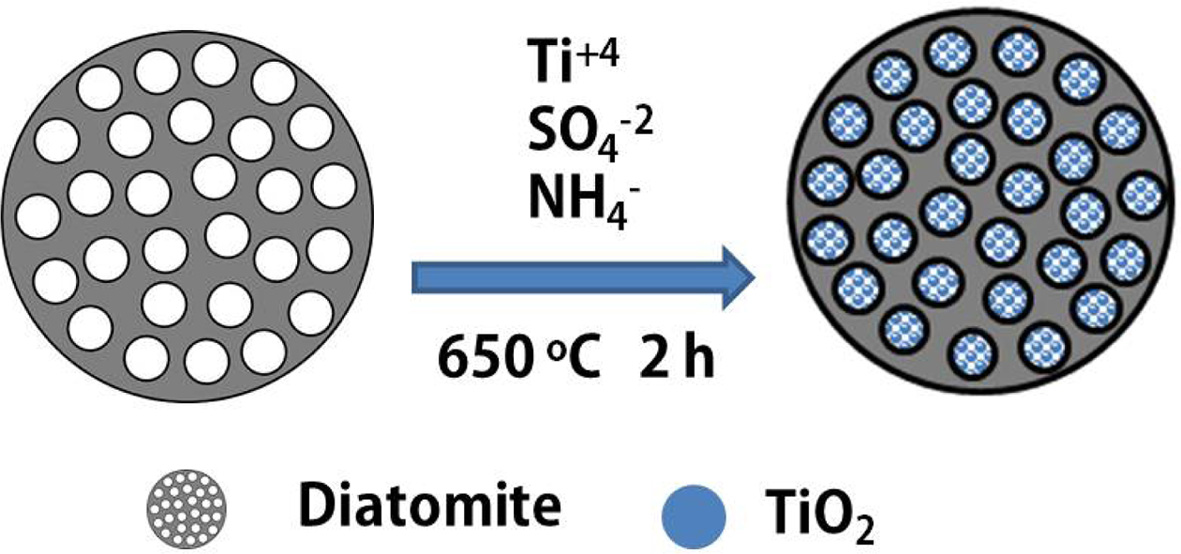
materials were prepared. The preparation process was shown in Fig. 1.
Preparation
of photocatalytic coating
Deionized water (302.0 g), sodium acrylate salt water
dispersant (5.0 g), defoamer (F111, 2.0 g) and film-forming additives (5.0 g)
were added to a beaker in turn. After being uniform stirred for 5 min, some
nano-TiO2 / purified diatomite composite materials (48.0 g) were
slowly added. At a speed of 2000 r/min, the water-borne
colloid was obtained by stirring and dispersing for half an hour. The pigments
and fillers of calcined kaolin (100.0 g), light calcium carbonate (100.0 g) and
talcum powder (100.0 g) were added into the water-borne colloid by vigorous
stirring for half an hour and then styrene acrylate emulsion (110.0 g) was
added by mild stirring. The pH value of the mixed system was adjusted to 8 with
neutralizer. Finally, thickener (ASE-60, 5.0 g) and levelling agent (RM-2020,
10.0 g) were added with stirring evenly and aging for one hour. The prepared
nano-TiO2/ diatomite photocatalytic coating was obtained.
Characterization
The samples for TEM observation were fabricated by RTO
metal-embedded chip micron-nano characterization method.
First, the powder particles were embedded in copper by ion deposition without
disturbance, and then the samples were thinned to nanometer thickness by
mechanical grinding, polishing and ion shearing. The samples were analyzed by
Hitachi H-800 Microscope for image analysis to observe the profile of nano-TiO2
coated on the surface of diatomite particles. The crystal structure of
nano-TiO2 was analyzed by Brooke X-ray diffractometer (XRD). The
pore size and pore volume were measured by NOVA4000 high-speed automatic
specific surface and porosity meter produced by Conta Company and Autopore IV
9500 mercury intrusion meter produced by Mike Company in the
United States, and the specific surface area of samples was
measured by ST-2000 of Beijing Beibu Instrument Technology Company.
Photocatalytic coating
for degradation of formaldehyde gas
The formaldehyde gas
purification performance of the photocatalytic coatings was tested by
entrusting National Building Material
Industry Technology Monitoring and Research Center of China based on JC/1074-2008
“Cleaning Performance of Indoor Air Purification Functional Coatings”. The
detection process was as follows: First, the indoor environment was simulated
by the environment test chamber under the visible white light source. The
temperature was set at 23 ± 0.5 oC and the humidity was
set at 45 ± 3%. Second, the formaldehyde solution with a
concentration of 37% - 40% at 2.5 μL was dripped onto a petri dish and put into
the environmental test chamber to make it fully volatilized in the
environmental test chamber. Third, the photocatalytic coating was coated on 0.1
m2 glass (250 g/m2, twice), and irradiated by white
fluorescent light source (30 W) to produce photocatalytic effect. The distance
between the light source and the sample was 70 cm. Fourth, acetylacetone
spectrophotometry (GB/T 15516-1995) was used to
detect the concentration of formaldehyde. The gas in the 10 L cabin was
sampled every 6 h to detect the
change of formaldehyde concentration in the environmental test cabin. Fifth, the control group was
conducted in another environmental chamber. According to the above experimental
steps, the glass plate is not coated with photocatalytic coating in step 3.
|
Fig. 1 Preparation schematic diagram of the nano-TiO2/purified
diatomite composite materials. |
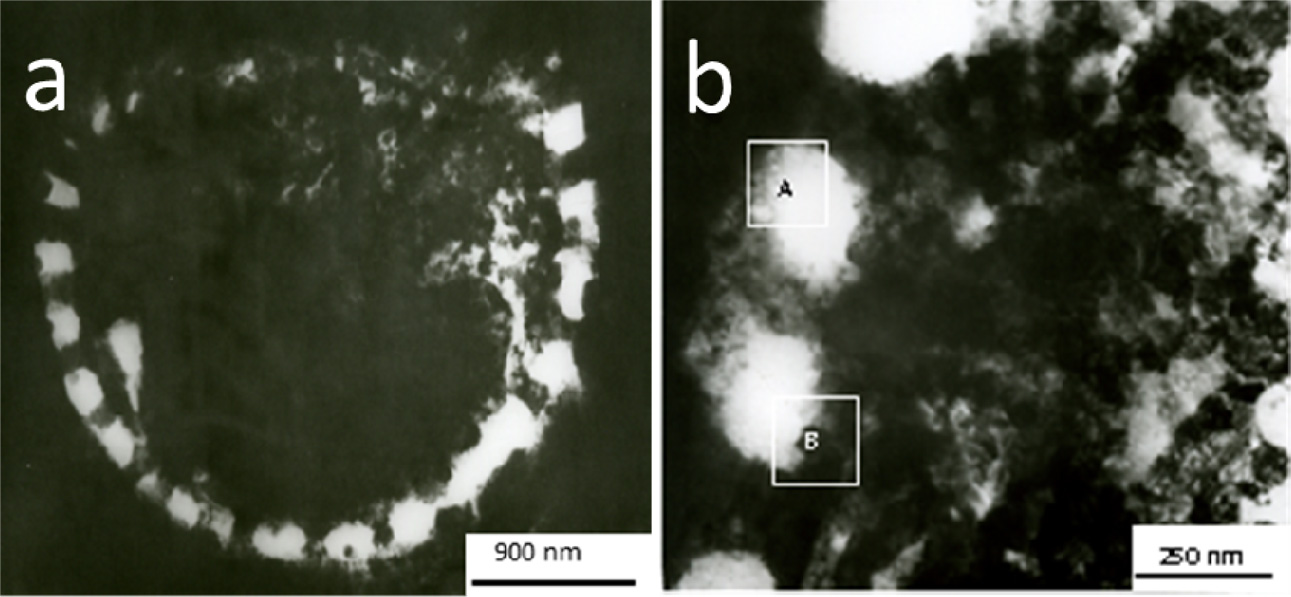
Fig. 2 shows the TEM of cross-section of the nano-TiO2 / purified
diatomite composite materials. Fig. 2(a) is a TEM image of the RTO profile of
nano-TiO2 loaded on the surface of a circular sieve diatomite. Fig.
2(b) is a locally magnified image of the interface between nano-TiO2
and diatomite. It can be seen from Fig. 2(b) that the prepared
nano-TiO2 / purified diatomite composites
show obvious core-shell structure with diatomite as core and nano-TiO2
as shell. The core-shell structure is closely bonded with each other. The thickness
of the coated shell is about 230 nm. The micropores on the surface of diatomite
have been covered by nano-TiO2, and the macropores on the surface
are clearly observed.
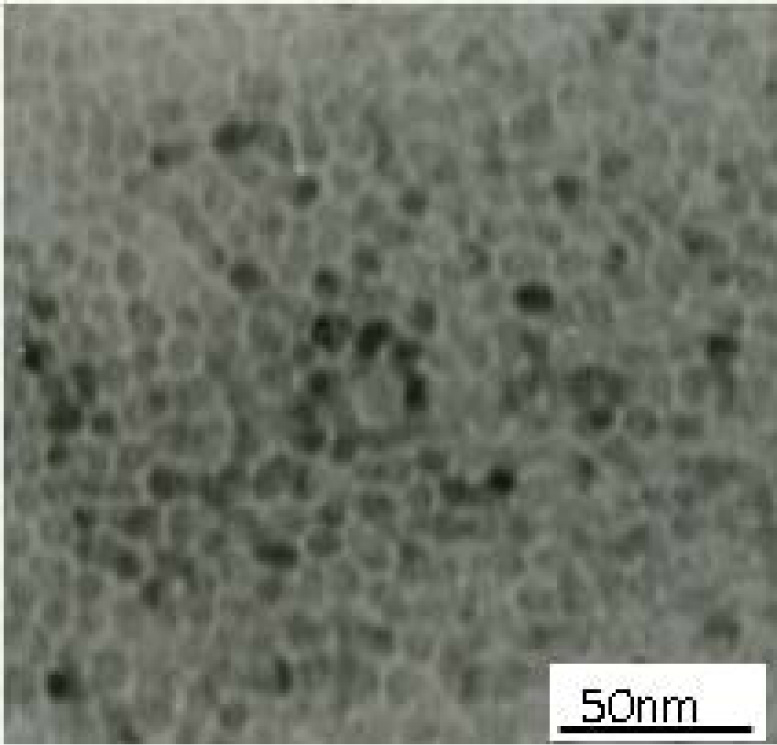
Fig. 3 is a magnified TEM image of point A in Fig. 2(b).
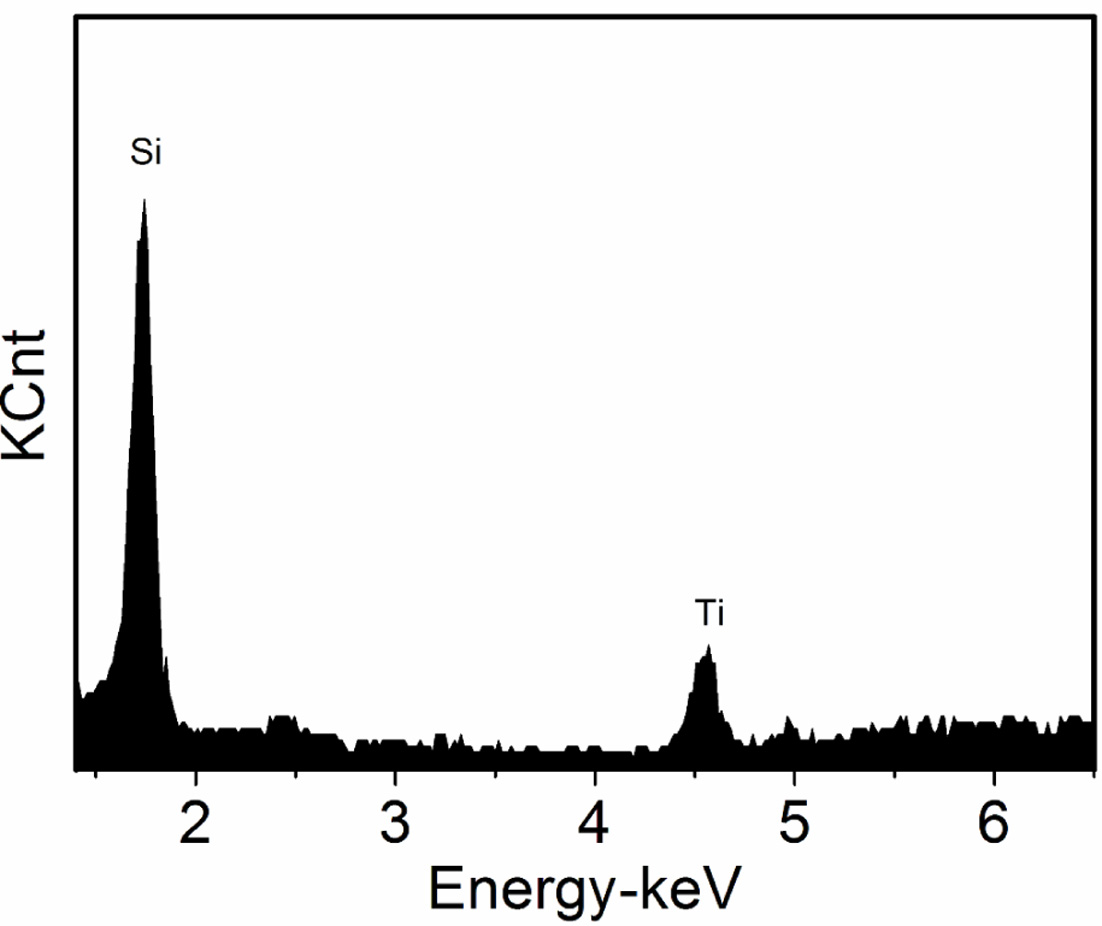
The average size of titanium dioxide at point A is about 10 nm. Fig. 4 is the
result of energy spectrum analysis at point B in Fig. 1(b), where is the
interface between titanium dioxide and diatomite. The peaks of Si and Ti
elements can be observed in the spectrum. It is further proved that the
nano-TiO2 / purified diatomite composite materials is formed.
The synthesized nano-TiO2 / purified diatomite
composite materials were analyzed by XRD. In
the previous work, the temperature of crystalline phase
transformation of TiO2 in the composite powder is about 800 oC
[25]. Therefore, in this work, the synthesized nano-TiO2 / purified
diatomite composite materials were calcined at 650 oC for 2 h.
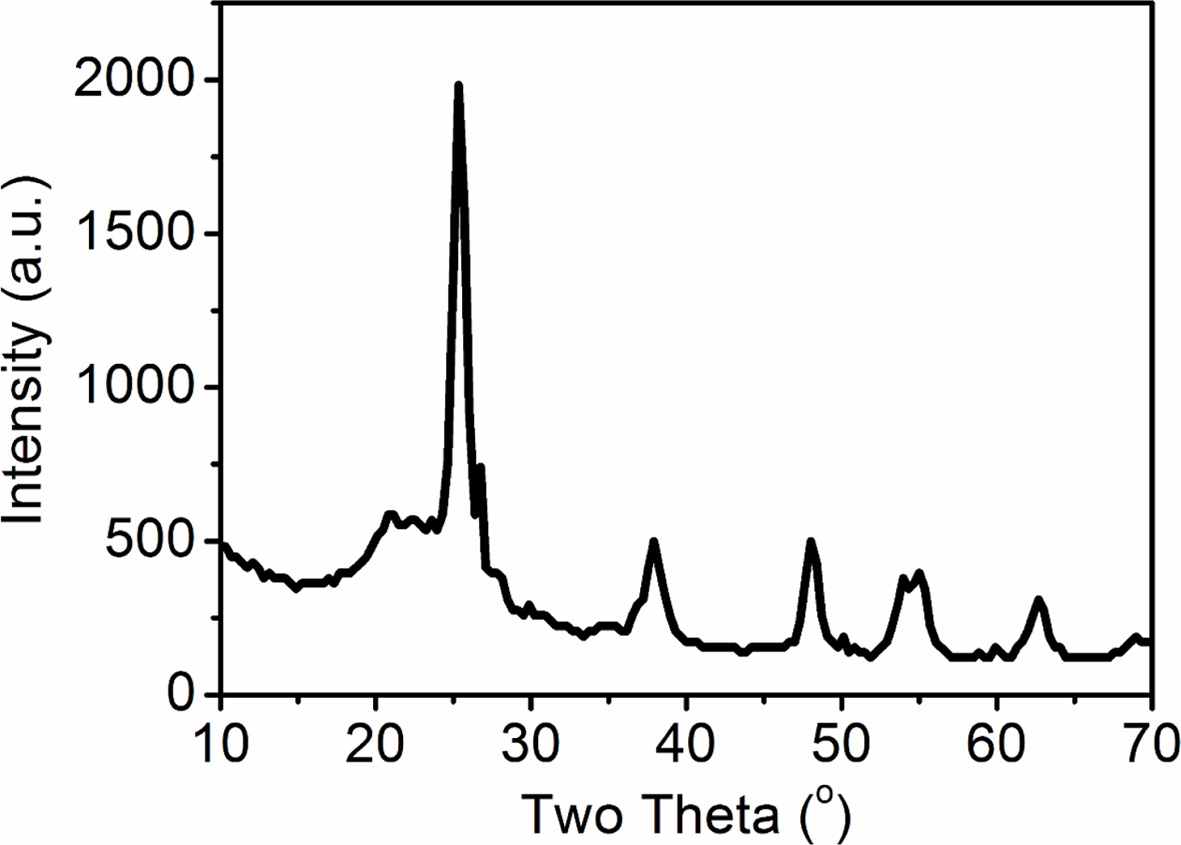
The results are shown in Fig. 5. The diffraction peaks of the prepared samples
are 25.2, 37.7 and 48.0 degrees, which are matched well with the standard
spectra of anatase-type titanium dioxide (JCPDS71-167). This indicates that the
crystal structure of titanium dioxide loaded on the surface of diatomite is
anatase. The average single crystal size of the sample can be calculated by
Scherrer formula: D = Kλ/Bcosθ, where K is Scherrer constant,
K = 0.89, λ = 0.15406 nm and B is the half-width of the
crystal plane with the strongest diffraction peak (101). The average size of
the loaded titanium dioxide is about 11 nm, which is consistent with that
observed by TEM.
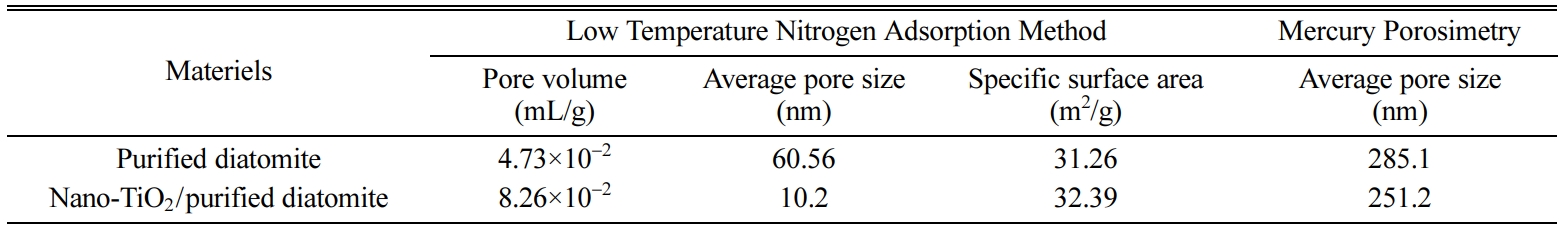
The pore size and specific surface area of the purified diatomite
and the nano-TiO2 / purified diatomite composite
materials were determined by low temperature nitrogen adsorption and mercury
porosimetry, respectively, which are
summarized in Table 1. The results of nitrogen adsorption at low temperature
show that the mesoporous volume and
specific surface area of the nano-TiO2 / purified diatomite
composite materials are significantly higher than that of the purified
diatomite, while the average pore size is significantly lower than that of the
purified diatomite. These results indicate that the nano-TiO2 / purified
diatomite composite materials own mesoporous structure. The results of mercury
intrusion test show that the average pore size of the nano-TiO2 / purified
diatomite composite materials become lower, which further indicate that the
inner surface of the larger pore of purified diatomite was loaded with nano-
TiO2.
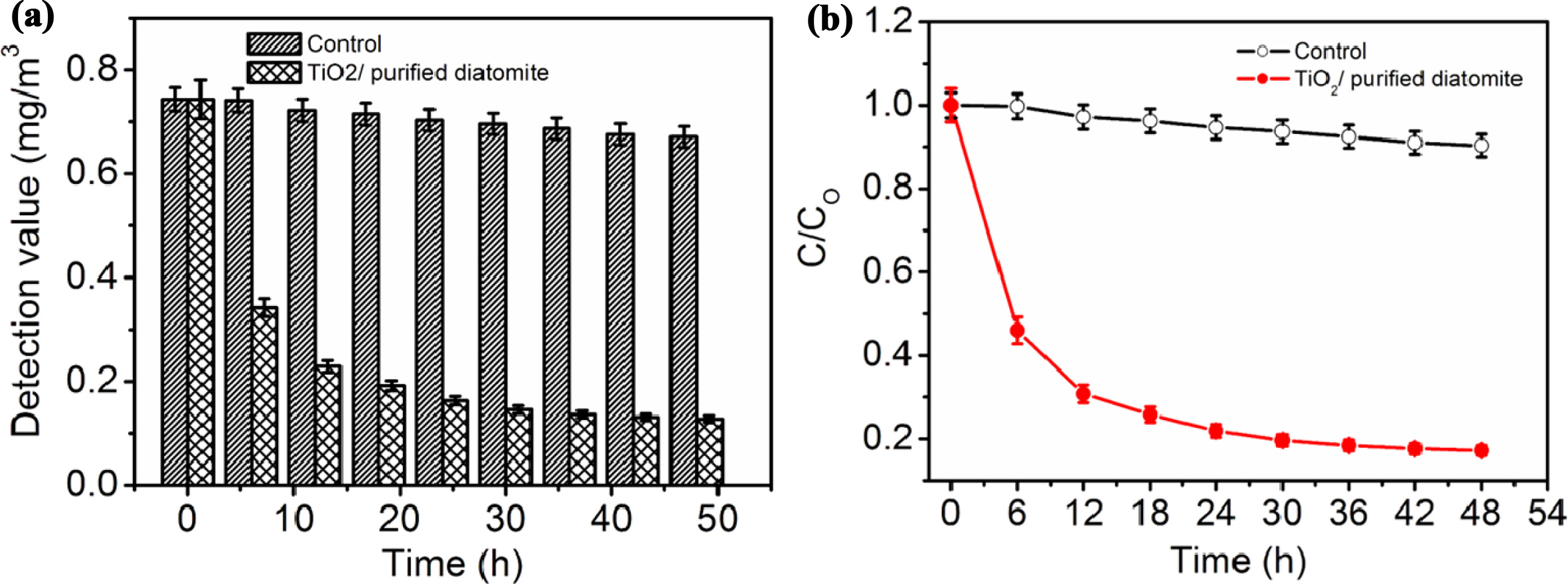
The formaldehyde gas purification performance of the
photocatalytic coating was tested by entrusting National Building Material
Industry Technology Monitoring and Research Center of China. The test results of
formaldehyde gas degradation
are shown in Fig. 6. As shown in Fig. 6, the degradation rate of the
photocatalytic coating increases gradually with the prolongation of irradiation
time. After 48 h of illumination, the degradation rate of the photocatalytic
coating reached 82.8%, which meets the technical requirements of Class I
materials in the standard JC/T 1074-2008 of China. Correspondingly, the control
group without the photocatalytic
coating had lower photocatalytic degradation rate.
It is well known that the degradation rate of substrates in photocatalytic reactions was
achieved by adsorbing on the surface of catalysts or reaching the surface of
catalysts of free molecules [28]. In our case, the main mechanism of formaldehyde
gas degradation in photocatalytic coating is that the molecules of formaldehyde
gas were adsorbed on the surface of the coatings firstly, and then were
oxidized and degraded by the catalyst in the coating under irradiation of
fluorescent light source. The prepared photocatalytic coating consists of the
nano-TiO2 / purified diatomite composite materials, which increase
the adsorption of the coating. The adsorption effect enriches
formaldehyde and other pollutants in the air onto the surface of
the photocatalytic coating, thus accelerating the degradation
of pollutants by accepting electrons and holes directly on the surface of the
nano-TiO2 / purified diatomite.
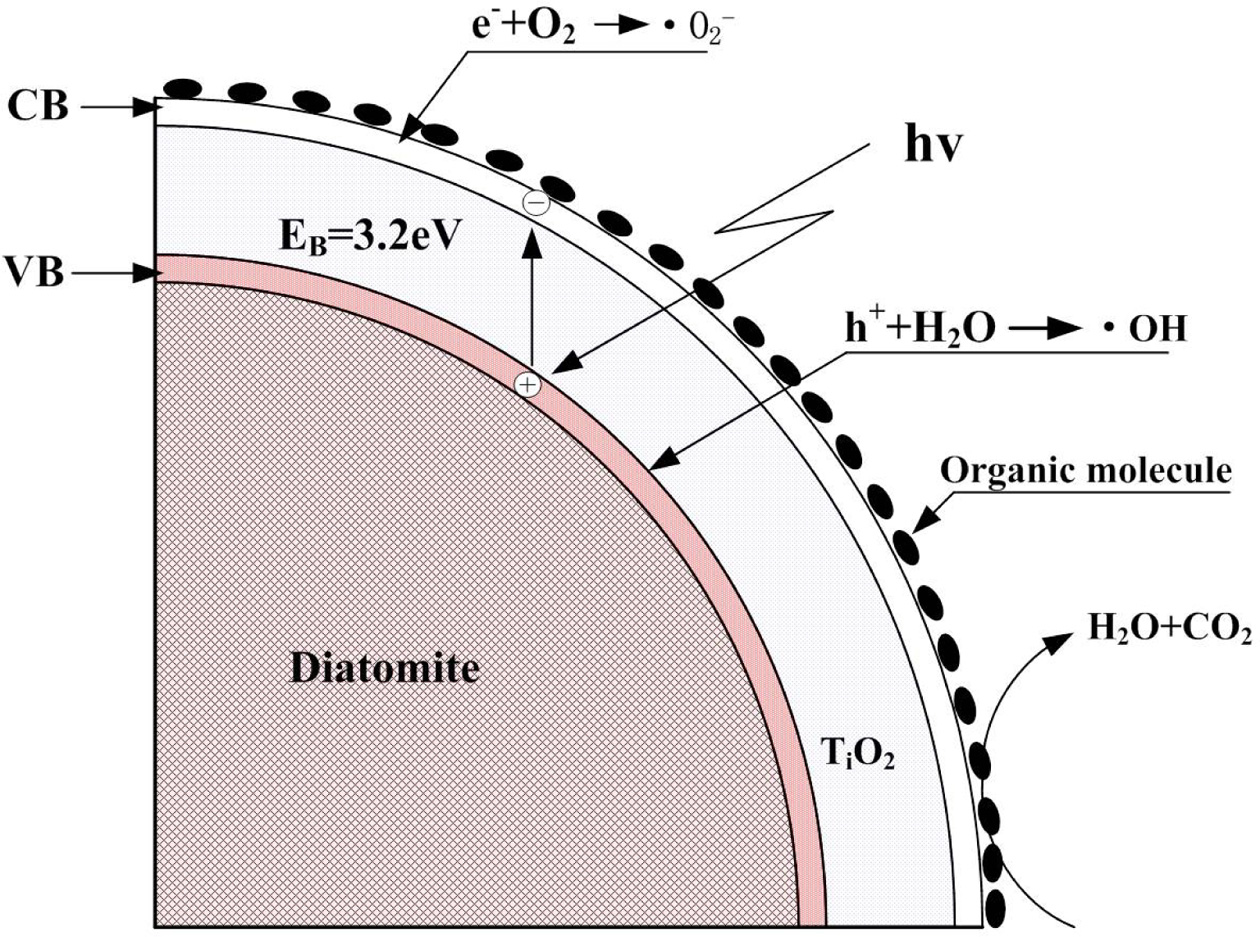
Fig. 7 is a schematic diagram of the photocatalytic
degradation mechanism of formaldehyde by the nano-TiO2 / purified
diatomite composites with core-shell structures. In Fig. 7, the photon energy
which is greater than the band gap of titanium dioxide (3.2 eV) directly excite
electrons transition from the top of valence band to the bottom of conduction
band, resulting in the formation of electron and hole pairs of nano-TiO2 in
photocatalytic coating:
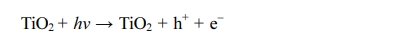
Photogenerated holes can react directly with pollutants
already adsorbed on the surface of coating and oxidize them, or react with
hydroxyl groups and water to produce hydroxyl radicals ·OH.
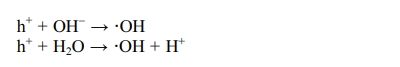
Photogenerated electrons react with oxygen molecules
adsorbed on the coating surface to produce superoxide radicals. Molecular
oxygen not only participates in the reduction reaction, but also is another
source of hydroxyl radicals on the surface. The specific reaction formulas are
as follows:
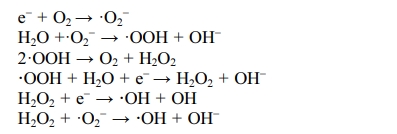
In the above reaction, hydroxyl radicals (·OH) and
superoxide radicals (·O2-) have been produced. They are
very active free radicals with strong oxidation ability, which can directly
oxidize various organic substances to small inorganic molecules such as CO2
and H2O. Moreover, the oxidation reaction generally does not stop in
the intermediate step and does not produce intermediate products because of
their strong oxidation ability. The above analysis shows that the degradation
of formaldehyde gas is determined by the adsorption and photocatalytic
degradation properties of the nano-TiO2 / purified diatomite
coating, and the degradation effect is the result of the synergistic effect of
the two.
|
Fig. 2 TEM of cross-section of the nano-TiO2/purified diatomite
composite materials. |
|
Fig. 3 The magnified TEM of point A in Fig. 2(b). |
|
Fig. 4 EDS spectrum of the nano-TiO2/purified diatomite
composites. |
|
Fig. 5 XRD patterns of the nano-TiO2/purified diatomite
composite materials. |
|
Fig. 6 The test results of formaldehyde gas degradation with and without the photocatalytic coating, respectively. |
|
Fig. 7 Schematic diagram about the photocatalytic degradation
mechanism of the nano-TiO2/purified diatomite coating. |
|
Table 1 The pore size and specific surface area of the purified diatomite and the nano-TiO2/purified diatomite composite materials |
The nano-TiO2 / purified diatomite composites
were prepared by hydrolytic precipitation method using purified diatomite as
carrier and titanium sulfate as precursor. The nano-TiO2 / purified
diatomite composite particles show obvious core-shell structure with
diatomite as core and nano-TiO2 as shell. The nano-TiO2 / purified
diatomite photocatalytic coating was prepared by using the prepared nano-TiO2 / purified
diatomite composite as filler. The test results of the formaldehyde
degradation performance of the coating show that the formaldehyde
degradation performance of the coating has met the technical
requirements of Class I materials in the national
standard JC/T 1074-2008 of China. The degrading effect of photocatalytic
coating on formaldehyde gas is the result of the synergistic effect of
adsorption and photocatalytic degradation.
This work was supported by 135 National Key R&D
Program Projects of the Ministry of Science and Technology
(2016YFC0700901) and the Opening Foun- dation
of Henan Key Laboratory of Special Protective Materials (Grant No.
SZKFJJ201903).
The authors declare no competing financial interests.
- 1. K.-S. Liu, F.-Y. Huang, S.B. Hayward, J. Wesolowski, and K. Sexton, Environ. Health Perspect. 94 (1991) 91-94.
-

- 2. C.H. Ao, S.C. Lee, J.Z. Yu, and J.H. Xu, Appl. Catal., B 54 (2004) 41-50.
-

- 3. O. Merk and G. Speit, Environ. Mol. Mutagen. 32[3] (1998) 260-268.
-

- 4. C.W. Kim, J.S. Song, Y.S. Ahn, S.H. Park, J.W. Park, J.H. Noh, and C.S. Hong, Yonsei Med. J. 42 (2001) 440-445.
-

- 5. A. Fujishima, T.N. Rao, and D.A. Tryk, J. Photochem. Photobiol., C 1 (2000) 1-21.
-

- 6. Q. Sun, H. Li, S. Zheng, and Z. Sun, Appl. Surf. Sci. 311 (2014) 369-376.
-

- 7. N. Uekawa, M. Suzuki, T. Ohmiya, F. Mori, Y.J. Wu, and K. Kakegawa, Int. J. Mater. Res. 18[4] (2003) 797-803.
-

- 8. H. Choi, E. Stathatos, and D.D. Dionysiou, Thin Solid Films 510 (2006) 107-114.
-

- 9. A. López, D. Acosta, A. I. Martínez, and J. Santiago, Powder Technol. 202 (2010) 111-117.
-

- 10. X.Z. Li, H. Liu, L.F. Cheng, and H. J. Tong, Environ. Sci. Technol. 37[17] (2003) 3989-399
-

- 11. S. Narakaew, J. Ceram. Process. Res. 18[1] (2017) 36-40.
- 12. A. Maddu, I. Deni, and I. Sofiana, J. Ceram. Process. Res. 19[1] (2018) 25-31.
- 13. Y.-S. Song, M.-H. Lee, B.-Y. Kim, and D.Y. Lee, J. Ceram. Process. Res. 20[2] (2019) 182-186.
-

- 14. Y.-S. Song, S. Son, D.Y. Lee, M.-H. Lee, and B.-Y. Kim, J. Ceram. Process. Res. 17[11] (2016) 1197-1201.
- 15. R.-q. Gao, Q. Sun, Z. Fang, G.-t. Li, M.-z. Jia, and X.-m. Hou, Int. J. Miner. Metall. Mater. 25[1] (2018) 73-79.
-

- 16. R.-q. Gao and X.-m. Hou, Int. J. Miner. Metall. Mater. 20[6] (2013) 593-597.
-

- 17. S. Qiu, S. Xu, F. Ma, and J. Yang, Powder Technol. 210 (2011) 83-86.
-

- 18. J. Wang, B. He, and X.Z. Kong, Appl. Surf. Sci. 327 (2015) 406-412.
-

- 19. T. Georgakopoulos, N. Todorova, K. Pomoni, and C. Trapalis, J. Non-Cryst. Solids 410 (2015) 135-141.
-

- 20. E.P. Reddy, L. Davydov, and P. Smirniotis, Appl. Catal., B 42 (2003) 1-11.
-

- 21. S.-y. Lu, Q.-l. Wang, A.G. Buekens, J.-h. Yan, X.-d. Li, and K.-f. Cen, Chem. Eng. J. 195-196 (2012) 233-240.
-

- 22. B. Wang, G. Zhang, X. Leng, Z. Sun, and S. Zheng, J. Hazard. Mater. 285 (2015) 212-220.
-

- 23. Y. Chen and K. Liu, J. Hazard. Mater. 324 (2017) 139-150.
-

- 24. K.-J. Hsien, W.-T. Tsai, and T.-Y. Su, J. Sol-Gel Sci. Technol. 51 (2009) 63-69.
-

- 25. Z. Sun, Z. Hu, Y. Yan, and S. Zheng, Appl. Surf. Sci. 314 (2014) 251-259.
-

- 26. Y. Xia, F. Li, Y. Jiang, M. Xia, B. Xue, and Y. Li, Appl. Surf. Sci. 303 (2014) 290-296.
-

- 27. G. Zhang, Z. Sun, Y. Duan, R. Ma, and S. Zheng, Appl. Surf. Sci. 412 (2017) 105-112.
-

- 28. C.S. Turchi and D.F. Ollis, J. Catal. 122 (1990) 178-192.
-

 This Article
This Article
-
2020; 21(6): 751-756
Published on Dec 31, 2020
- 10.36410/jcpr.2020.21.6.751
- Received on Nov 25, 2019
- Revised on Mar 13, 2020
- Accepted on Mar 16, 2020
 Services
Services
- Abstract
introduction
material and methods
results and discussion
conclusion
- Acknowledgements
- Conflict of Interest
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Shuilin Zheng
-
School of Chemical and Environmental Engineering, China University of Mining and Technology, Beijing 100083, P. R. China
Tel : +8637965928196 Fax: +8637965928196 - E-mail: shuilinzheng8@gmail.com






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.