- Nitrilotriacetic acid functionalization on Fe3O4 nanoparticle via amino and carboxylic functionalization
Jin Soon Hana,**, MiRae Youma,**, Hyun-Hee Choib, Yeon-Gil Jungb, Sung-Churl Choia and Gye Seok Ana,*
aHanyang university 04763 Division of Materials Science and Engineering, Hanyang University, 222 Wangsimni-ro, Seongdong-gu, Republic of Korea
bSchool of Materials Science and Engineering, Changwon National University, 20 Changwondaehak-ro, Changwon, Gyeongnam 51140, Republic of Korea
Since chelating chemical had
focused for enhancing bio-susceptibility and ability for the binding with
biomaterials, in this study, Nitrilotiriacetic acid (NTA) was utilized for the
developing chelate ligand attached superparamagnetic nanoparticle. In order to
conjugate NTA, carboxylic group was needed on the surface of substrate particle
for building peptide bond with amine group which was branched from the NTA
structure. Therefore Fe3O4 nanoparticle was
functionalized with various of amine precursor (Polyethyleneimine (PEI),
(3-Aminopropyl)triethoxysilane (APTES)), and carboxyl precursor (Polyacrylic
acid (PAA)) before the conjugation of NTA. Then NTA conjugation behavior was
estimated which is varied with the type of amine precursor which affects to the
surface properties.
Keywords: Magnetic nanoparticle, Chelate ligand, Nitilotriacetic acid, Functionalization
Chelate-based sensors play an important role in environmental
and biological fields as metal and chemical detectors, humidity sensors, and
biosensors, among others [1-5]. Metal ions act as stimulators by attaching
solely to the chelate ligand on the surface of the sensing particle; however,
the sensing particles can be stimulated by the secondly attached
chemical on the particular pair of chelate and metal ion. These
mechanisms were usually determined by the types of chelates, which were
represented by nitrilotriacetic acid (NTA), ethylenediamine
diacetate (EDDA), and ethylene-
diaminetetraacetic acid (EDTA) with different functional
groups and affinities for other materials [6]. Owing to these mechanisms,
chelates have been applied for sensing metal ions, chemical
reagents, and biomaterials. Especially in the case of
biosensors, they have been utilized for sensing protein or DNA, which is
directly connected to protein and genetic engineering [5, 9, 10].
To reinforcing sensing and recollecting, the
introduction of
magnetic properties was assumed as an effective way
for mass controlling particles by inducing a magnetic field. Fe3O4,
in particular, has paramagnetic properties that ensure reversible mobility in a
sensor particle. Therefore, nanoparticles having higher efficiency than the first
attached substrate have been utilized [10-14]. The surface plasmon resonance
(SPR) bio-sensor chip was based on the high refractive index of Fe3O4,
which was directly connected to the efficiency [15, 16]. In the case
of NTA, as an enzyme-free biosensor, the efficiency of the
sensor and controllability of nanoparticles have been the areas of focus [14,
17].
However,
nanoparticles were reported to have unexpected
toxicity that could cause DNA damage. There are many factors that determine the
toxicity of nanoparticles, including its concentration, particle size,
existence of coating layer, and total surface area [18].
To
further functionalize Fe3O4, various types of materials
were applied on its surface, such as noble metals,
carbon materials, and other chemical reagents [10-13]. For example, Fe3O4-Au
composite particle coated with horseradish peroxidase was utilized with
graphene sheets–Nafion film for detecting hydrogen peroxide based on its high surface activity [11].
Carbon-coated Fe3O4
with TiO2 layer was used as a photoelectrochemical biosensor for uric acid
[12]. In the case of NTA, NTA had
been built on intermediate polyacrylamide via the polymerization
process for separating his-tagged protein. Since γ-methacryloxypropyl
trimethoxysilane was polymerized to prepare the substrate layer of
NTA, the magnetic property of NTA attached Fe3O4
nanoparticle was reinforced;
however, this requires certain conditions, including prolonged reflux and noble
gas injection for protection [14].
In order to utilize this NTA conjugating method into mass
production, building these conditions with large scale were not preferred. And
the magnetic properties about Fe3O4 nanoparticle had been
decreased after the NTA conjugation with reflux condition. With these aspect,
utilizing the NTA attached Fe3O4 nanoparticle into
genetic or protein technology had been carried out only in a small scale, and
another method had to be established in order to get over this situation.
In this study, we methodologically reviewed the effects of
intermediate functionalization on the NTA attachment process on the surface of
Fe3O4 and the properties of NTA-attached Fe3O4
nanoparticles. In the first method, (3-aminopropyl)triethoxysilane (APTES) and
polyethylenimine (PEI) were utilized to attach the amine group on the Fe3O4
nanoparticle. Then, NTA was conjugated with the cross-linker before the amino-functionalized
Fe3O4 nanoparticle and NTA. The second
method involved direct conjugation via carboxylic functionalization with
polyacrylic acid (PAA), which did not require a cross-linker between the
particle and NTA. By comparing these samples, a more adequate method
for fabricating NTA attached Fe3O4 nanoparticle
was established.
Materials
The Fe3O4 nanoparticles were
prepared as starting materials following a previously reported polyol-based
method. [19, 20] APTES (Sigma Aldrich, USA) and PEI (Mw:
~ 25,000, branched, Sigma Aldrich, USA) were used for amino
functionalization, and PAA (Mw ~ 450,000,
Sigma Aldrich, USA) was used for carboxylic
functionalization. Then, Nα,Nα-Bis(carboxymethyl)-L-lysine hydrate (ABNTA, C10H18N2O6 · xH2O,
Sigma Aldrich, USA) was prepared as a chelate chemical. Glutaraldehyde solution
(GLH; 25%, Sigma Aldrich, USA) was also prepared for the cross-linker.
Amino
functionalization using APTES with the core-shell Fe3O4@SiO2
nanoparticle
Fe3O4 nanoparticles (2 g) were
prepared with a mixed solvent containing 450 mL ethyl alcohol and 50 mL
distilled water after the previously reported in-situ SiO2
coating process [19, 20, 27]. The dispersed solution
was set for stabilization and adjusted to basic pH. Then, 4 wt% of APTES was
injected dropwise as an ethyl alcohol-based solution for the amine attached
silicate precursor. After 8 h of vigorous stirring, the amino-functionalized
nanoparticle was collected by the magnetic field and washed with ethyl alcohol
and distilled water for several times.
Amino
functionalization using PEI with Fe3O4 nanoparticle
The prepared Fe3O4 nanoparticles (2
g) were dissolved in 100 mL of distilled water and 4 wt% of PEI
solution. Next, this solution was mixed for 30 min at room temperature before
heating to 80 oC. It was then reacted for 10 h with vigorous
stirring and cooled down until it reached the room temperature. The reacted
particle nanoparticle was collected by inducing magnetic field and stored as a
solution with distilled water after washing it several times with distilled
water.
Formation
of NTA chelating ligand on the surface of amino-functionalized Fe3O4
A total of 0.1 g of both distinctly amino-functionalized
Fe3O4 nanoparticles with APTES and PEI were prepared as a
solution with 100 mL of distilled water and mechanically stirred before the
reaction. Then, the glutaraldehyde solution was injected and mechanically
stirred for 8 h [21]. The particles were collected via the induced magnetic
field and washed with distilled water for removing the unreacted reagent and
modifying their acidity to the neutral state. Then, they were
re-dispersed at 100 mL of distilled water and mixed with the ABNTA
solution. Reaction occurred under the vigorous stirring
condition at room temperature for 24 h. Finally, the chelating ligand-attached
Fe3O4 nanoparticles were collected via magnets. It was
then washed and stored with distilled water.
Chelate
functionalization of Fe3O4 nanoparticle via carboxylic
functionalization with PAA
A dispersed solution (300 mL) containing 10 g Fe3O4
nanoparticles was prepared after washing several times with distilled water.
Then, PAA was prepared as a 100 mL solution with 4 wt% of
concentration. These solutions were mixed in a round bottom flask
and heated to 75 oC for 4 h. The mixed solution was mechanically
stirred at 300 rpm until the solution cooled down to room temperature. Next,
0.25 g of PAA-attached Fe3O4 was re-dispersed in 100 mL
distilled water and mixed with 100 mL ABNTA to obtain a concentration of 0.1 wt%.
These solutions were reacted for 8 h via mechanical stirring.
Then, the reacted Fe3O4 nanoparticle was washed
and stored in distilled water.
Characterization
The surface functional group of as-synthesized Fe3O4,
amino-functionalized Fe3O4, and NTA-attached Fe3O4
was analyzed via Fourier-transform infrared spectroscopy (FTIR, IRAffinity-1S,
Shimadzu, Japan) at 500 ~ 4,000 cm-1.
The FTIR spectra was used to judge the success of functionalization of Fe3O4
with the existing peaks of amine or carboxyl group of APTES, PEI, and NTA
and the differences in micro structure between different amine reagents.
Microscopic analysis was also conducted using a transmittance electron
microscope (HRTEM, Tecnai G2 F30 S-Twin, FEI, Netherlands) for the visual
identification of the coating layer on Fe3O4 particles.
Then, using the Zeta potential (Zetasizer, Malvern, USA), the changes on the
surface charges of Fe3O4 particles were observed after
amino functionalization and NTA attaching. Finally, the magnetic
properties of the as-synthesized and NTA-attached Fe3O4
particles were identified using a vibrating sample
magnetometer (VSM, Lake shore 7400, USA).
Morphological
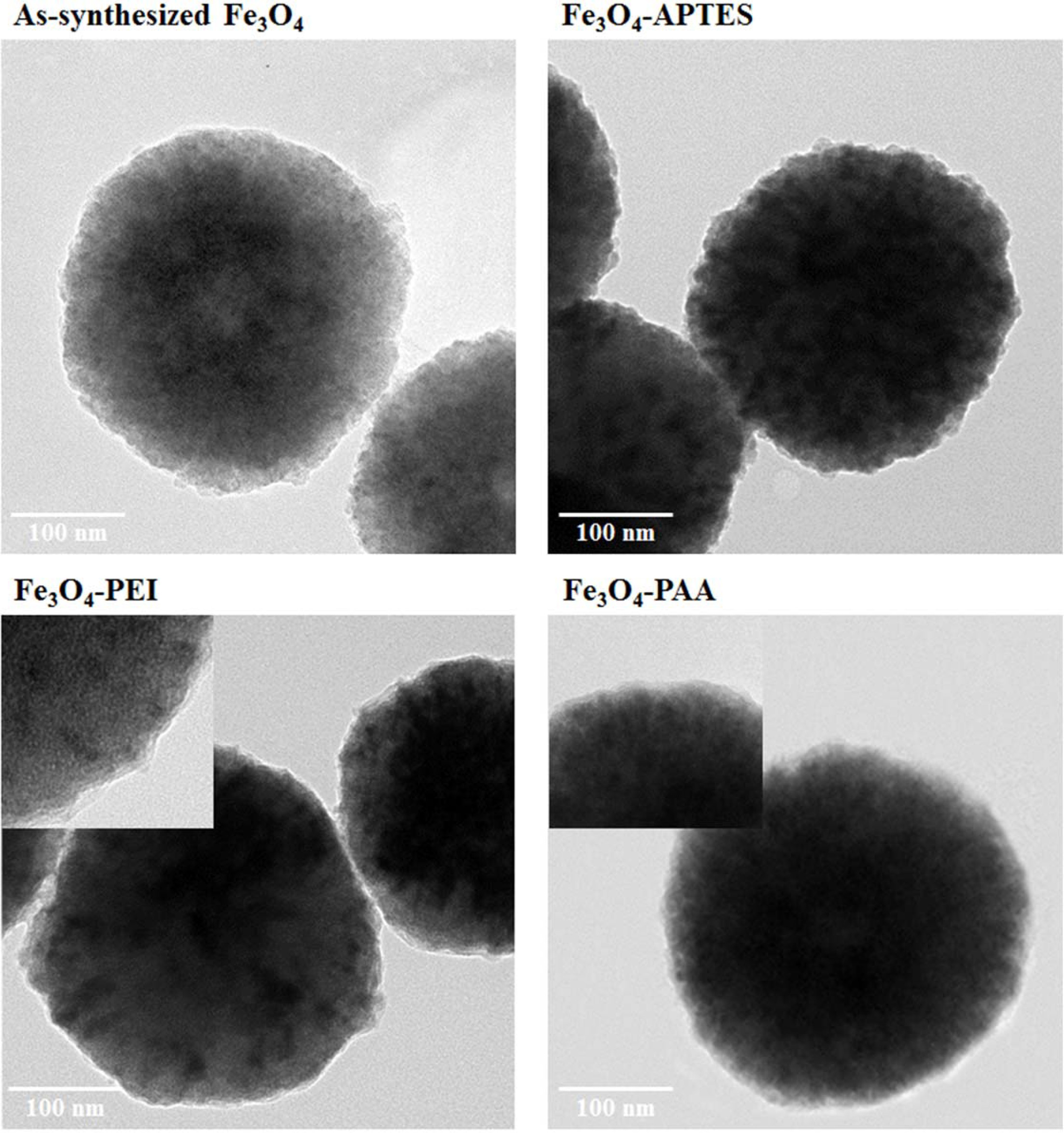
changes of Fe3O4 and intermediate functionalized Fe3O4
A transmittance electron microscopy (TEM) analysis was
performed to examine the surface and micro-
structure of the as-synthesized Fe3O4
nanoparticles, amino-functionalized Fe3O4 with APTES and
PEI, and carboxyl-functionalized Fe3O4 with PAA (Fig. 1).
The as-synthesized Fe3O4 nanoparticles
were observed about 300 nm in size. In the case of APTES-modified
particles, the observed particle size and the morphology were almost identical
to those of the as-synthesized particle. However, the Fe3O4
particles functionalized with PEI were found to be semi-spherical in shape,
with a size of approximately 300 nm. A coated layer with a thickness of
3 ~ 4 nm was formed on the surface of the PEI-treated Fe3O4
particles. In contrast, to be functionalized into carboxylic acid, the
PAA-treated Fe3O4 nanoparticles had a smooth and opaque
layer on their surface, with a thickness of 5–6 nm. The core size of
PAA-treated Fe3O4 was approximately 320 nm, which
was slightly greater than the as-synthesized Fe3O4, and it
had an almost perfectly spherical shape, in comparison with the
PEI-treated Fe3O4 particles.
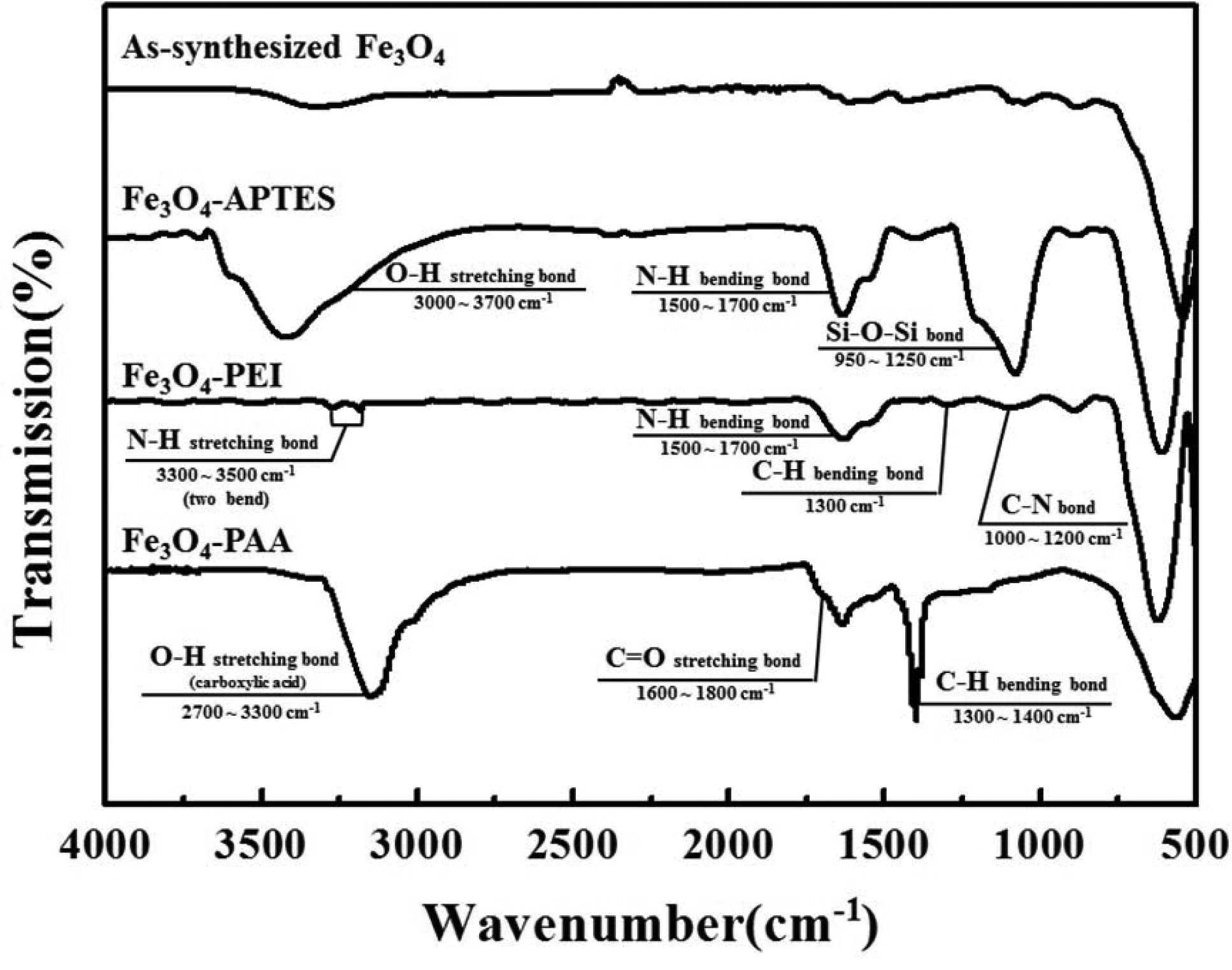
Functional
group on the Fe3O4 and intermediate functionalized Fe3O4
The FT-IR spectra of as-synthesized Fe3O4,
amino-functionalized Fe3O4 with APTES and PEI, and
carboxyl-functionalized Fe3O4 with PAA are illustrated in
Fig. 2. The Fe–O peak was observed at around 600 cm-1
for each particle [22]. After surface modification, a distinctive amine peak
was observed at 1,500 ~ 1,700 cm-1
owing to the bending of the N–H bending bond. Because the condensation of SiO2
was accompanied by the functionalization of APTES, an O–H stretching peak and
Si–O–Si peak were observed at around 3,000 and 950 ~ 1,250 cm-1, respectively. In the
case of another amino functionalization with
PEI, the polymeric carbon chains were
stuck on the surface of the particle; thus, the C–H bending peaks were observed
at 1,300 cm-1.
Additionally, in the spectrum of PEI used particle, C–N bending
and N–H stretching bonds were observed at 1,000 ~ 1,200 and
3,300 ~ 3,500 cm-1,
respectively, and these ranges were where the intensive peaks from O–H
stretching bond and Si–O–Si bond were found in the spectrum of the APTES used
particle [22, 23, 25]. Although some differences were observed, it was
confirmed that both surface-treated particles were successfully functionalized
with the amine group. In contrast, in the case of PAA treatment, the C–H
bending bond derived from the polymeric carbon chain was observed at
1,300 ~ 1,400 cm-1,
which was similar to the PEI-treated particles. Significant carboxylic bonds
were also found at 1,600 ~ 1,800 and 3,000 cm-1 for
the C=O and O–H stretching bonds, respectively. The O–H stretching bonds were
observed at 2,700 ~ 3,300 cm-1,
which was slightly lower than the range for the APTES-treated particles
(3,000 ~ 3,700 cm-1)
[25]. Considering the difference in the O–H stretching bond, we
determined that the carboxyl group was successfully attached on
the surface of Fe3O4 and that most of the O–H bonds
originated from the carboxylic acid instead of the hydroxyl group.
Discussion
about intermediate functionalization process of Fe3O4
As mentioned above, differences were observed in the
amino-functionalized particles with APTES and PEI, from the aforementioned
analysis. The functionalization mechanism with APTES is based on
hydrolysis and condensation, similar to the Stöber method [23].
During APTES treatment, the existing SiO2 layer was
etched with an ammonia solution, and the unreacted hydroxyl groups were exposed
via a hydrolysis reaction. These hydroxyl groups were used for the condensation
site with APTES, and this functional group was observed in the FT-IR spectrum
of Fe3O4 with APTES, at 3,000 ~ 3,700 cm-1.
However, as evident in the functionalized Fe3O4 with PEI
and PAA, the functionalization process did not include an intermediate layer;
thus, these precursors were directly attached to the surface of the Fe3O4
nanoparticles. However, owing to the molecular structure of APTES, Si–O–Si bond
could not be formed continuously; thus, the coated layer was not observed on
the surface of APTES-treated particles. In the case of PEI- and PAA-treated
particles, directly bound carbon chains created an opaque layer on the surface
of the Fe3O4 nanoparticle.
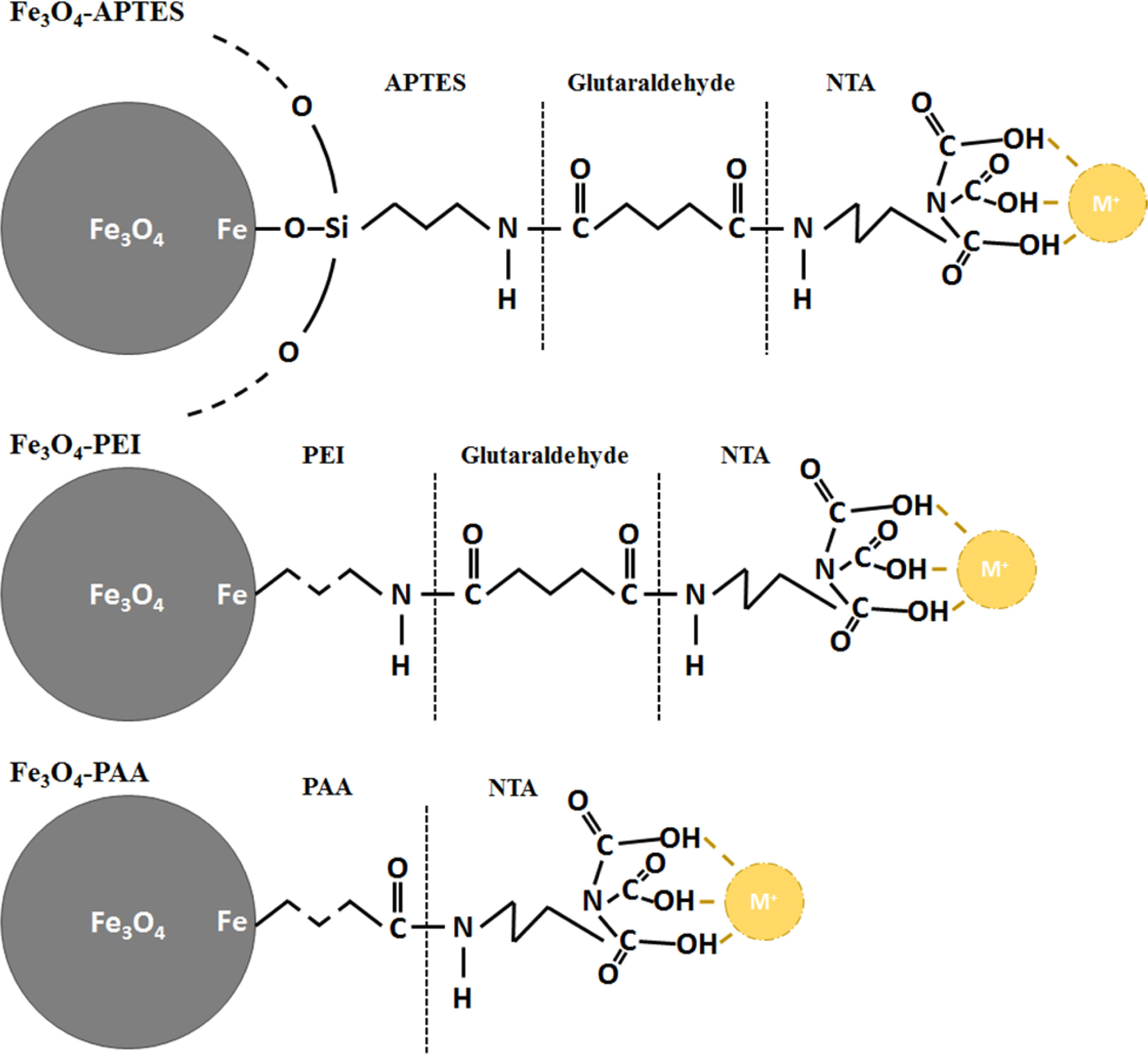
Scheme
about NTA conjugation and intermediate functionalization process of Fe3O4
Fig. 3 shows the chemical structures of Fe3O4
functionalized using various methods, along with a brief scheme of the
NTA-attaching mechanism on the surface of Fe3O4
nanoparticles. Because a condensation reaction involving electrostatic
attraction was used in the NTA-attaching mechanism, in the case of amine, the
Fe3O4 particles were attached with glutaraldehyde before
the conjugation with NTA. As evident from the FT-IR spectra, the intermediate
SiO2 layer or carbon chain exhibited the most significant difference
between APTES- and PEI-treated Fe3O4. This difference
could derive different conjugation properties with glutaral- dehyde owing
to the different concentrations of activation sites,
electrostatic force, and chemical affinity with glutaraldehyde. Then,
glutaraldehyde formed another peptide bond with the amine group of NTA, which
could attach with several metal ions, such as Ni and W. However, PAA-treated Fe3O4
particles contained carboxylic acid, which could form peptide bonds with the
amine group. Therefore, PAA-treated Fe3O4 could be conjugated
with NTA directly, and glutaraldehyde was not required as a cross-linker. The
absence of a cross-linker and the reversal in the pH value and surface charge were different
for the APTES- and PEI-treated particles, and these could be affected after the
NTA conjugation.
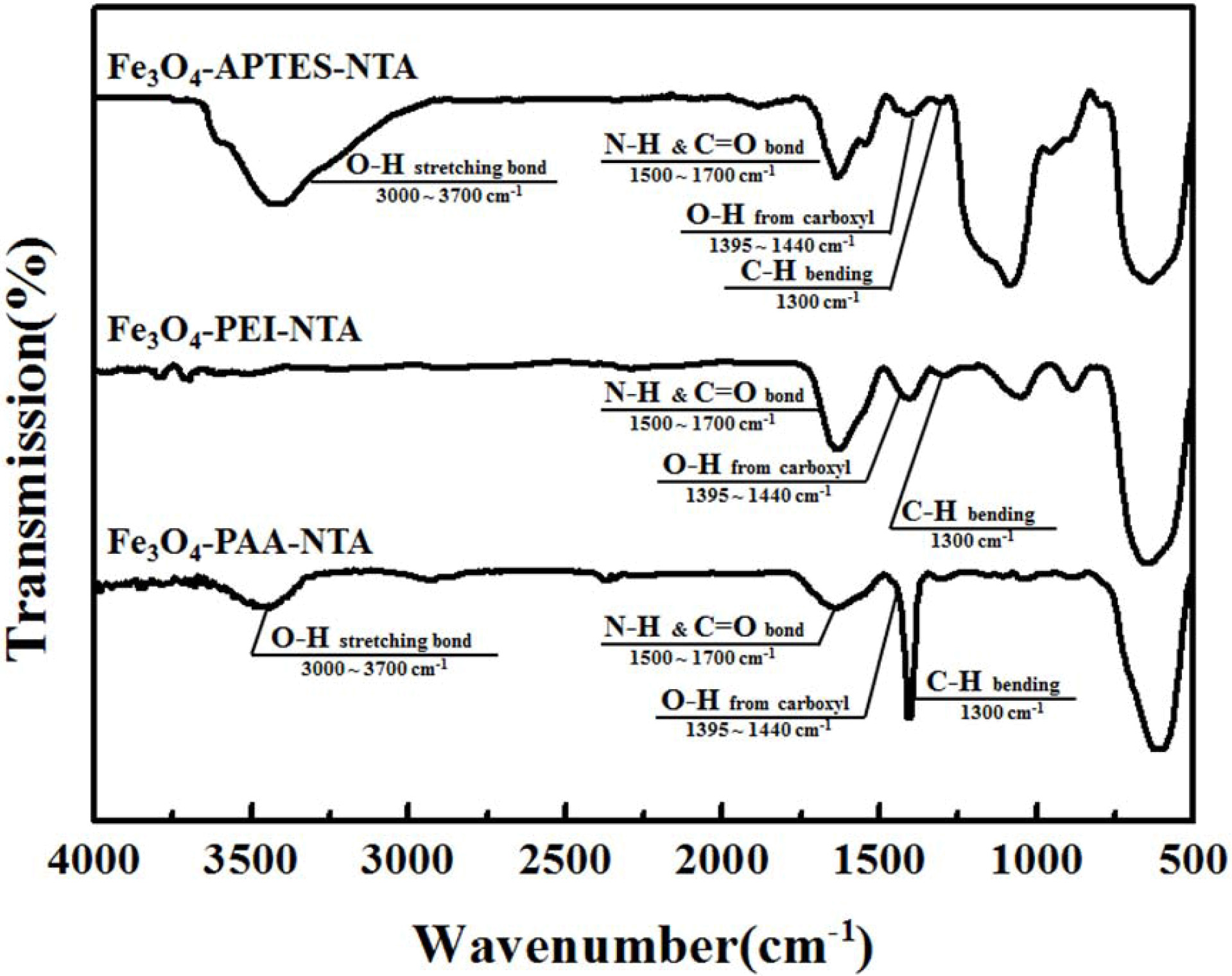
Morphological
changes of NTA conjugated Fe3O4
The FT-IR spectra of NTA-attached Fe3O4
particles obtained via various types of intermediate
functionalization are shown in Fig. 4. An emphasized peak was
observed in the spectrum of the NTA-attached particles, in contrast
to the APTES-attached particles. Distinctive peaks, which were observed in the
FT-IR spectrum of Fe3O4 with APTES shown in Fig. 2, were
also observed at 600 cm-1 (Fe–O)
and 1,300 cm-1 (C–H).
A C=O peak, the representative bonding of a carboxyl group, co-existed with the
N–H peak at 1,500 ~ 1,700 cm-1
[23, 24]. These distinctive peaks of NTA were also observed
in the FT-IR spectra of the NTA-attached particles on the PEI. These peaks were
in contrast to the spectrum of PEI-attached Fe3O4
nanoparticles. Normally, most of differences on FTIR spectra of each NTA
attached particle could be found almost same with that of amino-functionalized
specimen [14, 22, 23]. In contrast, NTA-attached
Fe3O4 nanoparticles obtained via carboxylic
functionalization with PAA had distinctive NTA peaks at 1,300 cm-1
(C–H), 1,395 ~ 1,440 cm-1 (O–H),
and 1,500 ~ 1,700 cm-1
(N–H and C=O co-exist), similar to other APTES- and PEI-treated particles;
however, their intensities were lower than those of other samples [22].
Additionally, the O–H stretching bond was shifted to 3,000 ~ 3,700
cm-1,
which was 2,700 ~ 3,300 cm-1
from the PAA-treated particles.
Considering these differences between the amino- or
carboxylic-functionalized and NTA-conjugated particles, it could be suggested
that NTA was successfully attached to the surface of the Fe3O4
particles.
Functional group on the NTA conjugated Fe3O4
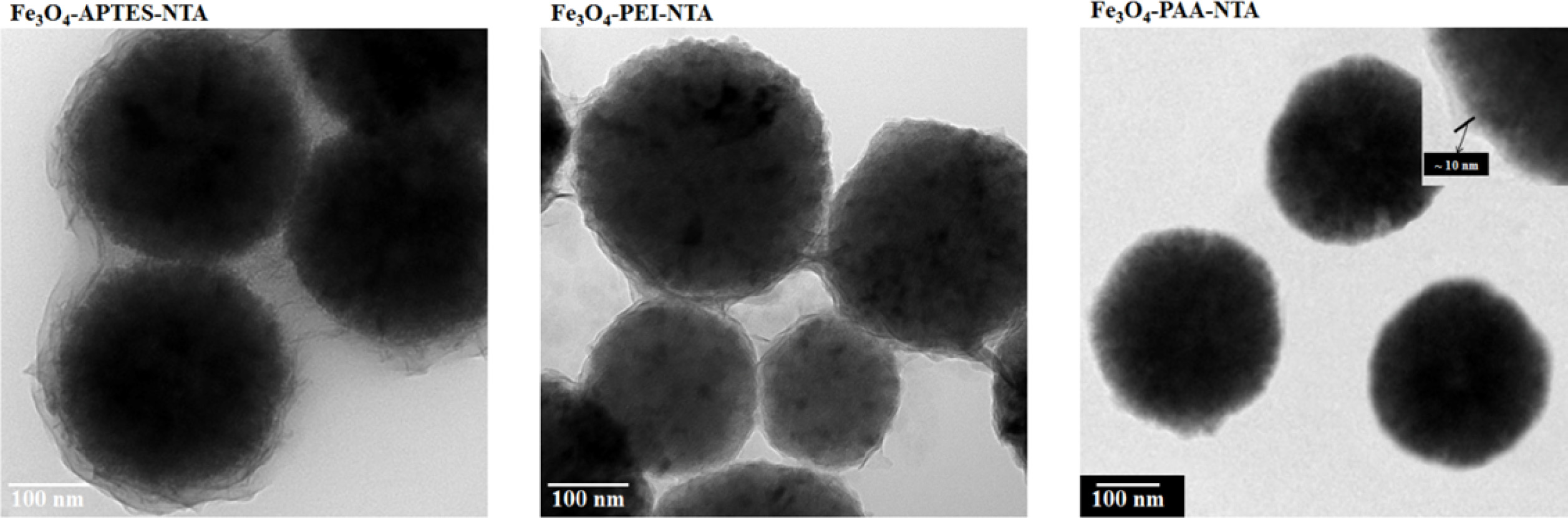
In the TEM image of NTA attached on the APTES used Fe3O4
particle from Fig. 5, core-shell structure was observed spherical shaped Fe3O4
core and fluffy shell. The size of the core material was approximately 300
nm, and the surface of the coated layer was observed to be wavy.
However, the particles were covered with a coated layer under the agglomerated
state, instead of being individually coated. The thickness of the coated layer
was measured to be 15 ~ 30 nm, which was greater than that of
APTES-treated particle. In contrast, in the image of the PEI-treated Fe3O4
particles, round particles with a size of 300 nm were observed along with other
small particles (150 ~ 250 nm in size). These particles were
agglomerated and linked with a thin coated layer (8 ~ 15 nm). In the
case of the PAA-treated particles, the sizes of the core Fe3O4
particles were observed to be around 300 nm, similar to APTES- and
PEI-treated particles; however, unlike other samples, these
PAA-treated particles remained separated. The coated layer
covered the Fe3O4 nanoparticles individually
and observed around 10 nm.
Differences
about NTA conjugated Fe3O4 with various kind of
intermediate functionalization
Thus, the NTA was successfully attached to the surface of
the Fe3O4 nanoparticles, with the appearance of O–H and
C–H peaks in the FT-IR spectrum of NTA-attached particles
obtained via APTES or PEI treatment; hence, it was difficult to
identify the C=O bonds owing to the existence of N–H peaks.
However, these distinctive peaks were also observed in the
FT-IR spectrum of the NTA-attached particles with PAA. Because NTA was
conjugated with the same peptide bonding, it could be assumed that the pH
values of APTES-, PEI-, and PAA-treated particles or the existence of a
cross-linker affected the formation of the peptide bond. Hence, owing to these
differences in the FT-IR spectra, the thickness of the coated layer of each
NTA-attached particle increased; thus, NTA was observed to be successfully
conjugated on the surface of PAA-treated Fe3O4. However,
their coated status varied for different intermediate
functional groups. In the case of the amino-functionalized
particles with APTES or PEI, agglomeration occurred.
However, for carboxylic functionalization with PAA,
the particles remained separated. Additionally, because
difference of pH values and used cross linker could be the cause of this
result.
Surface
charges of NTA conjugated Fe3O4 with various kind of
intermediate functionalization
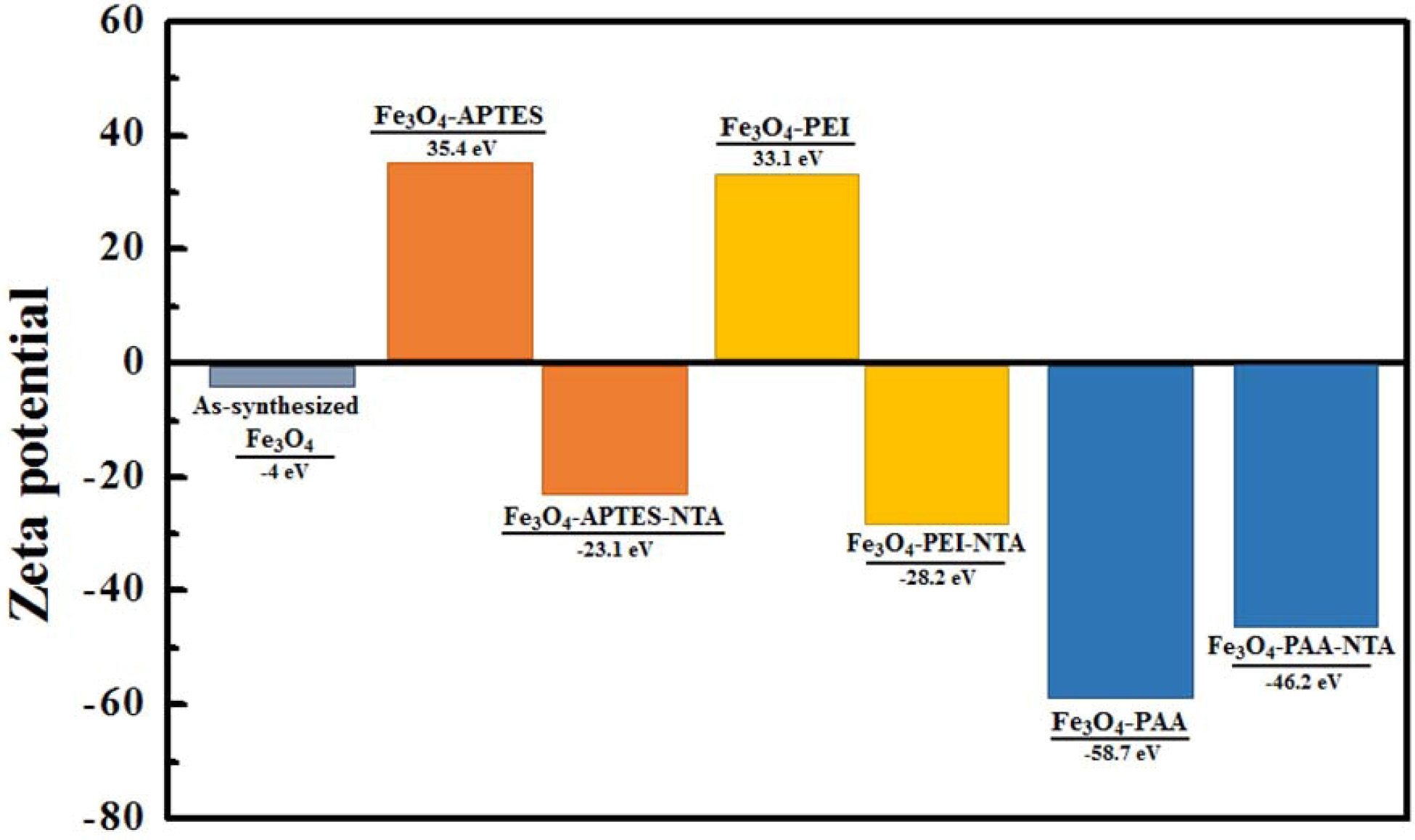
In order to analyze repulsion force on the particle
surface charge of each functionalization stage of Fe3O4
samples were exhibited at Fig. 6. As-synthesized Fe3O4
had a slight negative charge that could not produce a repulsive
force sufficient for stable dispersion. However, after
the APTES treatment, the surface charge increased to 35.4 eV
owing to the positive amine group [26]. However, the NTA-conjugation positive
charge was changed into a negative charge of -23.1 eV. A similar observation
was made for the PEI-treated particles, the surface charge of which changed
from 33.1 eV to -28.2 eV. However, after PAA treatment, a strong negative charge
(-58.7 eV) was formed on the surface of the particle, which changed into -46.2
eV upon NTA attachment.
Magnetic
properties of NTA conjugated Fe3O4 with various kind of
intermediate functionalization
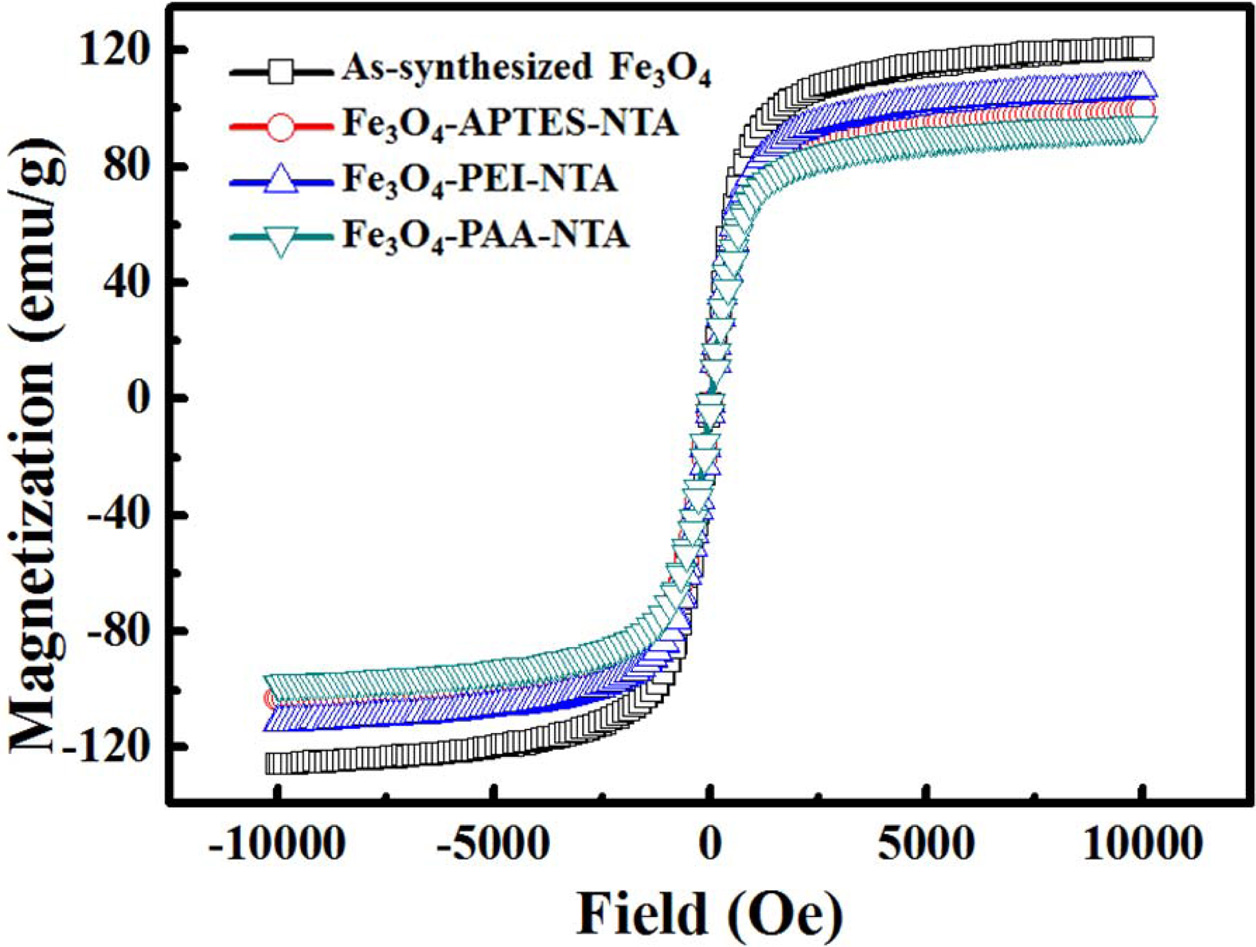
The hysteresis curves of as-synthesized Fe3O4
and NTA-attached samples obtained via APTES, PEI, and PAA treatment are shown
in Fig. 7. No coercivity or remnant magnetization was found in
any of the hysteresis curves, and each particle exhibited
paramagnetic curves. However, the saturated magnetization
of as-synthesized Fe3O4 was the highest, with a value of
120 emu/g. This was followed by the APTES- and PEI-treated samples, which
exhibited values of 106 and 98 emu/g, respectively; these
values were 88.3% and 81.7% of largest value of saturated magnetization,
respectively. The PAA-treated particles exhibited the lowest value, 94 emu/g,
which was 78.3% of that of as-synthesized Fe3O4.
Review
about NTA conjugated Fe3O4 with various kind of
intermediate functionalization
NTA was observed to be attached properly with the negative
surface charge after the NTA-attaching process. However,
the inversion of surface charge occurred in the amino-functionalized samples
with APTES and PEI. During this inversion, owing to the pH inversion and
changed functional group, the electrostatic force was weakened, and it was
difficult to maintain their dispersion stability; thus,
agglomeration occurred, which can be observed in the TEM images
mentioned above. The PAA-treated particles, however, underwent a slight decline
during the NTA conjugation process, and this charge was still strong enough for
the dispersion. Meanwhile, the magnetization of the NTA-attached
specimens was directly related to the reversible separation
and re-dispersion, considering the high saturated
magnetization and low remnant magnetization of these
specimens. The magnetic hysteresis curves of each specimen originated from its
Fe3O4 core particle and differed
owing to the coated layer or phase variation resulting
from external circumstances. During NTA conjugation via amino or carboxylic
functionalization, the Fe3O4 nanoparticles underwent
basic or acidic condition, which could damage the magnetic particles. However,
their paramagnetic properties remained intact, and their
saturated magnetization was sufficiently high for magnetic separation after the
NTA attachment.
|
Fig. 1 TEM images of as-synthesized Fe3O4 and intermediate functionalized Fe3O4 particles. |
|
Fig. 2 FT-IR spectra of as-synthesized Fe3O4 and intermediate functionalized Fe3O4 particles. |
|
Fig. 3 Scheme of chemical structures of NTA conjugated Fe3O4 particles through various types of intermediate functionalization. |
|
Fig. 4 T-IR images of NTA conjugated Fe3O4 particles through various types of intermediate functionalization. |
|
Fig. 5 EM spectra of NTA conjugated Fe3O4 particles through various types of intermediate functionalization. |
|
Fig. 6 urface charge of as-synthesized, intermediate functionalized, and NTA conjugated Fe3O4 nanoparticles. |
|
Fig. 7 Hysteresis loop of as-synthesized and NTA conjugated
Fe3O4 nanoparticles. |
In this study, chelate-functionalized Fe3O4
nanoparticles were fabricated through intermediate
functionalization with APTES, PEI, and PAA, and characterized for further
applications, which can be derived using metal-ion chelating. The peaks of the
FT-IR spectra indicated the alternation of the surface
functional group according to the
progression from the Fe3O4 nanoparticle synthesis to
the NTA attachment. Specifically, after NTA attachment, distinctive
peaks of NTA were observed, which were derived from the carboxyl group, amine
group, and peptide bonding. After NTA attachment, the surface charge
of the Fe3O4 nanoparticles was slightly weakened,
and electronic inversion occurred through amino func- tionalization. Owing to the
temporary lack of electrostatic repulsive force due to the
electronic inversion, the APTES- and PEI-treated particles were covered with an
NTA layer under an agglomerated state, unlike the PAA-treated particles.
In conclusion, NTA was successfully attached to the surface
of Fe3O4 nanoparticles through functionalization
using APTES, PEI, and PAA. Owing to the various functionalization chemicals,
the dispersion properties of the NTA-attached Fe3O4
particles were different. By further analyzing metal-ion chelating and
separating biomaterials, such as his-tag or DNA, NTA-attached superparamagnetic
nanoparticles can be used in various applications.
This work was supported by “Human Resources Program
in Energy Technology” of the KETEP and MOTIE of the
Republic of Korea (No. 20194030202450) and by the National Research
Foundation of Korea (NRF) grant funded by the Korea government (MSIP)
(2018R1A5A6075959).
- 1. M.T. Gabr and F.C. Pigge, Dalton T. 45 (2016) 14039-14043.
-

- 2. G.R. You, G.J. Park, S.A. Lee, K.Y. Ryu, and C. Kim, Sensor Actuat. B-Chem. 215 (2015) 188-195.
-

- 3. N.V. Petrochenkova, A.G. Mirochnik, T.B. Emelina, A.A. Sergeev, A.A. Leonov, and S.S. Voznesenskii, Spectrochim Acta A Mol. Biomol. Spectrosc. 200 (2018) 70-75.
-

- 4. F. Tudorache, I. Petrila, T. Slatineanu, A.M. Dumitrescu, A.R. Iordan, M. Dobromir, and M.N. Palamaru, J. Mater. Sci.: Mater. 27 (2015) 272-278.
-

- 5. S.-L. Kao, P. Venkatesan, and S.-P. Wu, New J. Chem. 39 (2015) 3551-3557.
-

- 6. N.E. Boland and A.T. Stone, Geochim. Cosmochim. Acta 212 (2017) 176-195.
- 7. W. Dong, Y. Bhide, S. Marsman, M. Holtrop, T. Meijerhof, J. de Vries-Idema, A. de Haan, and A. Huckriede, Biotechnol. J. 13 (2018) 1700645.
-

- 8. L. Li, Z. Huang, X. Fan, Z. Zhang, R. Dou, S. Wen, Y. Chen, Y. Chen, and Y. Hu, Electrochim. Acta 231 (2017) 354-362.
-

- 9. J. Li, L. Zhang, G. Wei, Y. Zhang, and Y. Zeng, Biosens. Bioelectron 69 (2015) 316-320.
-

- 10. A. Dutta Chowdhury, N. Agnihotri, R.A. Doong, and A. De, Anal Chem 89 (2017) 12244-12251.
-

- 11. Y. Xin, X. Fu-bing, L. Hong-wei, W. Feng, C. Di-zhao, and W. Zhao-yang, Electrochimi. Acta 109 (2013) 750-755.
-

- 12. C. Zhang, S. Si, and Z. Yang, Biosens. Bioelectron 65 (2015) 115-120.
-

- 13. S. Hu, W. Ouyang, L. Guo, Z. Lin, X. Jiang, B. Qiu, and G. Chen, Biosens. Bioelectron 92 (2017) 718-723.
-

- 14. H. Guo, M. Li, S. Tu, and H. Sun, IOP Conf. Ser.: Mater. Sci. Eng. 322 (2018) 022017.
-

- 15. Y. Wang, J. Dostalek, and W. Knoll, Anal. Chem 83 (2011) 6202-6207.
-

- 16. Q. Wu, Y. Sun, D. Zhang, S. Li, X. Wang, and D. Song, Biosens. Bioelectron 86 (2016) 95-101.
-

- 17. S. Yao, X. Yan, Y. Zhao, B. Li, and L. Sun, Mater. Lett. 126 (2014) 97-100.
-

- 18. G. Harris, T. Palosaari, Z. Magdolenova, M. Mennecozzi, J.M. Gineste, L. Saavedra, A. Milcamps, A. Huk, A.R. Collins, M. Dusinska, and M. Whelan, Nanotoxicology 9 (2015) 87-94.
-

- 19. G.S. An, S.W. Choi, D.H. Chae, H.S. Lee, H.-J. Kim, Y. Kim, Y.-G. Jung, and S.-C. Choi, Ceram. Int. 43 (2017) 12888-12892.
-

- 20. G.S. An, J.S. Han, J.R. Shin, D.H. Chae, J.U. Hur, H.-Y. Park, Y.-G. Jung, and S.-C. Choi, Ceram. Int. 44 (2018) 12233-12237.
-

- 21. S. Li, K. Yang, L. Liu, B. Zhao, Y. Chen, X. Li, L. Zhang, and Y. Zhang, Anal. Chim. Acta 997 (2018) 9-15.
-

- 22. G.S. An, S.W. Choi, T.G. Kim, J.R. Shin, Y.-I. Kim, S.-C. Choi, and Y.-G. Jung, Ceram. Int. 43 (2017) 157-161.
-

- 23. G.S. An, J.R. Shin, J.U. Hur, A.H. Oh, B.-G. Kim, Y.-G. Jung, and S.-C. Choi, J. Alloy. Compd. 798 (2019) 360-366.
-

- 24. G.S An, J.S. Han, J.R. Shin, J.H. Cha, B.-G. Kim., Y.-G. Jung, and S.-C. Choi, J. Alloy. Compd. 792 (2019) 1008-1012.
-

- 25. J.S Han, G.S. An, B.G. Park, and S.-C. Choi, J. Korean. Ceram. Soc. 55 (2018) 80-84.
-

- 26. J.U. Hur, J.S. Choi, S.-C. Choi, and G.S. An, J. Korean. Ceram. Soc. 57 (2020) 80-84.
-

- 27. A.H. Oh, H.-Y. Park, Y.-G. Jung, S.-C. Choi, and G.S. An, Ceram. Int. 46 (2020) 10723-10728.
-

 This Article
This Article
-
2020; 21(5): 515-523
Published on Oct 31, 2020
- 10.36410/jcpr.2020.21.5.515
- Received on Feb 3, 2020
- Revised on Mar 31, 2020
- Accepted on Apr 7, 2020
 Services
Services
- Abstract
introduction
experimental procedure
results and discussion
conclusion
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Gye Seok An
-
Hanyang university 04763 Division of Materials Science and Engineering, Hanyang University, 222 Wangsimni-ro, Seongdong-gu, Republic of Korea
Tel : +8222200505
Fax: +8222916767 - E-mail: faustmaro@hanyang.ac.kr






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.