- Hydrogen production from methane steam reforming over Mg modified nickel-based catalyst: Process optimization
Shian Lia, Zhiyu Yaob, Facai Yanga, Guogang Yanga,* and Qiuwan Shena
aMarine Engineering College, Dalian Maritime University, Dalian, China
bHarbin Guanghan Gas Turbine Co. Ltd., Harbin, China
Methane steam reforming (MSR)
reaction is a promising industrial hydrogen production technology. Mg-Ni/CeO2
catalysts at different Mg/Ni ratios of 6/4, 7/3, 8/2 and 9/1 with total loading
of 10 wt% were used as catalysts for hydrogen production. The
characterization techniques such as XRD, SEM and EDS were carried out on fresh
and spent samples. Results showed that the optimum Mg addition content for
Mg/Ni-CeO2 is 2%. The process optimization of reaction parameters
were conducted by evaluating the catalytic activity. The stability of optimal
Mg-Ni/CeO2 catalyst at 700 oC is examined for
8 h on-stream reaction. It reveals that Mg/Ni-CeO2 still
maintains a relatively high catalytic activity after 8 h stability test.
Keywords: hydrogen production, Methane steam reforming, Mg addition, Ni-CeO2
An increase in the global policies related to the
generation of green energy has stimulated the use of alternative energy sources
and the development of several technologies that will help to replace the
dependency on fossil fuels [1, 2]. Compared with new energy sources such as
solar energy and wind energy, hydrogen is a secondary energy source that can be
produced in a wide variety of ways, such as photolysis of water,
electrolysis of water, chemical looping hydrogen production
and hydrocarbon reforming [3]. The most mature
hydrogen production technology is the hydrocarbon reforming
which has the characteristics of low cost and high hydrogen production rate.
Methane reforming is a widely used technique in industries
to convert natural gas to hydrogen or syngas [4]. According to different
reforming raw materials, it can be divided into partial oxidation of methane
[5], carbon dioxide reforming of methane [6-7] and steam reforming of methane
[8-9]. Methane steam reforming (MSR) reaction is an endothermic reaction and is
a fairly mature industrial hydrogen production technology.
Noble metal catalysts such as Rh, Ru, Pd and Pt with high
activity, stability and carbon deposition resistance have been investigated
intensity [10-12]. Among many traditional metal catalysts, Ni-based catalysts
stand out due to their lower cost, good catalytic and higher stability
performance at high temperatures [13-15]. The support is one of the essential
components of the catalyst. The nature of the support is crucial in the
catalytic performance of supported metal catalysts. For some
specific reactions, besides affecting metal dispersion and
providing stability to the metal particles, the support can also participate in
the reactions [16-17]. The support is required to have high
mechanical strength, large specific surface area and strong
anti-sintering ability. In general, ceria has been widely used in heterogeneous
catalysis due to its unique properties [18].
Several types of materials have been studied for MSR. And
it was reported that intermediate metal loading can
significantly improve the catalytic performance [19-21].
Nickel-based catalysts with different metallic contents supported on
ceria-zirconia were studied by Dong et al. [19]. They found that the optimal
amount of nickel loading is 15%.Roh et al. studied the effect of nickel content
on a Ce-ZrO2/Al2O3 support. The result showed
that when the nickel content is 12% wt., the conversion rate of methane is the
maximum [20].
In this work, we have studied different Ni contents and Mg
addition to optimize their composition as catalysts in
MSR. A series of catalysts were synthesized by wet
incipient wetness impregnation method and thoroughly characterized by XRD, SEM
and EDS. The effects of the Ni loading and Mg addition on reactivity of the
catalysts were investigated to achieve a deeper understanding on Mg-Ni/CeO2
catalysts. In addition, the optimal reaction conditions were
studied by evaluating the effect of
reactivity temperature, the gas hourly space velocity
(GHSV) and H2O/CH4 (S/C) molar ratios.
Samples
preparation
Preparation of different wt% Ni loadings catalysts
Wet incipient
wetness impregnation method was used to
prepare the catalysts. First, Ni(NO3)2·6H2O
having a corresponding Ni loading amount was weighed into a beaker, and CeO2
was used as a support. Deionized water was added to Ni(NO3)2·6H2O
according to the measured water absorption rate of the support to prepare a
precursor solution. When the Ni(NO3)2·6H2O was
completely dissolved, the precursor solution and the CeO2 support
were immersed, mixed vigorously with a stirrer for 15 minutes to achieve
homogenous absorption of Ni solution over the surface of CeO2.
Standing at room temperature for 12 hours, then the composite was aged at
110 oC for 12 h inside, taken out and ground, and finally
calcined at 500 oC for 5 hours in a muffle furnace. The Ni loadings were 6.0 wt%,
8.0 wt%, 10.0 wt% and 12.0 wt% respectively.
Preparation of Mg-Ni/CeO2
Mg(NO3)2·6H2O having a
corresponding Mg doping amount was weighed into a beaker and dissolved in a
certain amount of deionized water. Then immerse the solution in the prepared
Ni/CeO2. Standing at room temperature for 12 hours, then the
composite was aged at 110 oC for 12 h inside. The composite was aged at 110 oC
for 12 h inside, taken out and ground, and finally
calcined at 500 oC for 5 hours in a muffle furnace. The Mg doping were 1.0 wt%,
2.0 wt%, 3.0 wt% and 4.0 wt% respectively.
Evaluation
of catalytic activity
The catalytic activity of the catalysts was carried out in
a fixed bed system at atmospheric pressure. The catalyst was pretreated for
2~3 hours at 500 oC under hydrogen stream. The reducing
mixture gas was composite of H2 and Ar (H2: Ar=1:9,
300 mL/min) and brought in at a ramp rate of 10 oC/min
from room temperature to 500 oC. The schematic diagram of the
fixed bed quartz reactor is shown in Figure 1. The system is divided into three
parts: a feeding unit, a methane steam reforming reactor and an analysis part.
Characterization
Powder X-ray diffraction data (XRD) were collected in the
Philips X’Pert PRO of PANalytical B.V. with Cu Kα radiation (λ = 0.1542 nm)
and a 2θ range of 10-90° to study the crystalline structure of the samples. The
morphologies of the synthesized catalysts were studied by scanning electron
microscopy (SEM, SUPRA 55 SAPPHIRE).
Experimental
result evaluation and analysis method
The following definitions were used for evaluation of the
catalyst performance:
Conversion of methane was calculated as follows: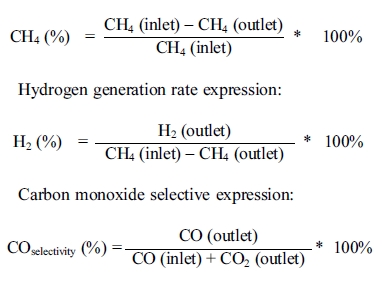
|
Fig. 1 Schematic diagram of the fixed bed quartz reactor system (1. Methane 2. Nitrogen 3. Hydrogen 4. Mass flow meter 5. Three-way valve 6. Preheating pipe 7. Reactor 8. Tube furnace 9. Exhaust gas treatment device 10. Online infrared gas composition and calorimeter 11. Computer Control 12. Water Pump 13. Steam Generator). |
Characterizations
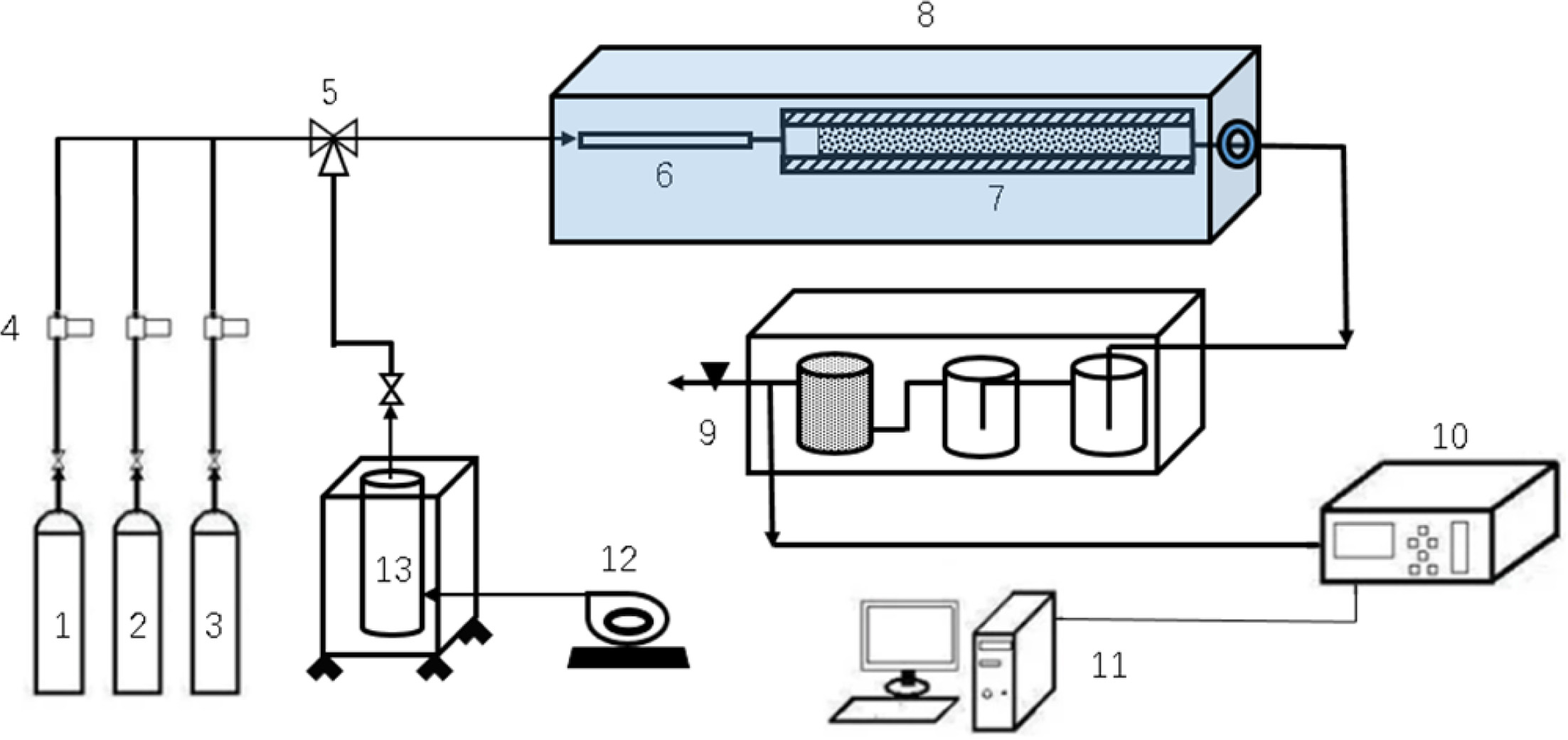
XRD diffraction results of Ni/CeO2 with
different Ni loading of 6-12 wt% are shown in Figure 2. It can be
seen that there are characteristic peaks of NiO at 2θ = 43.5°, 50.8°, and
75°, and characteristic peaks of CeO2 exist at 2θ = 33.1°,
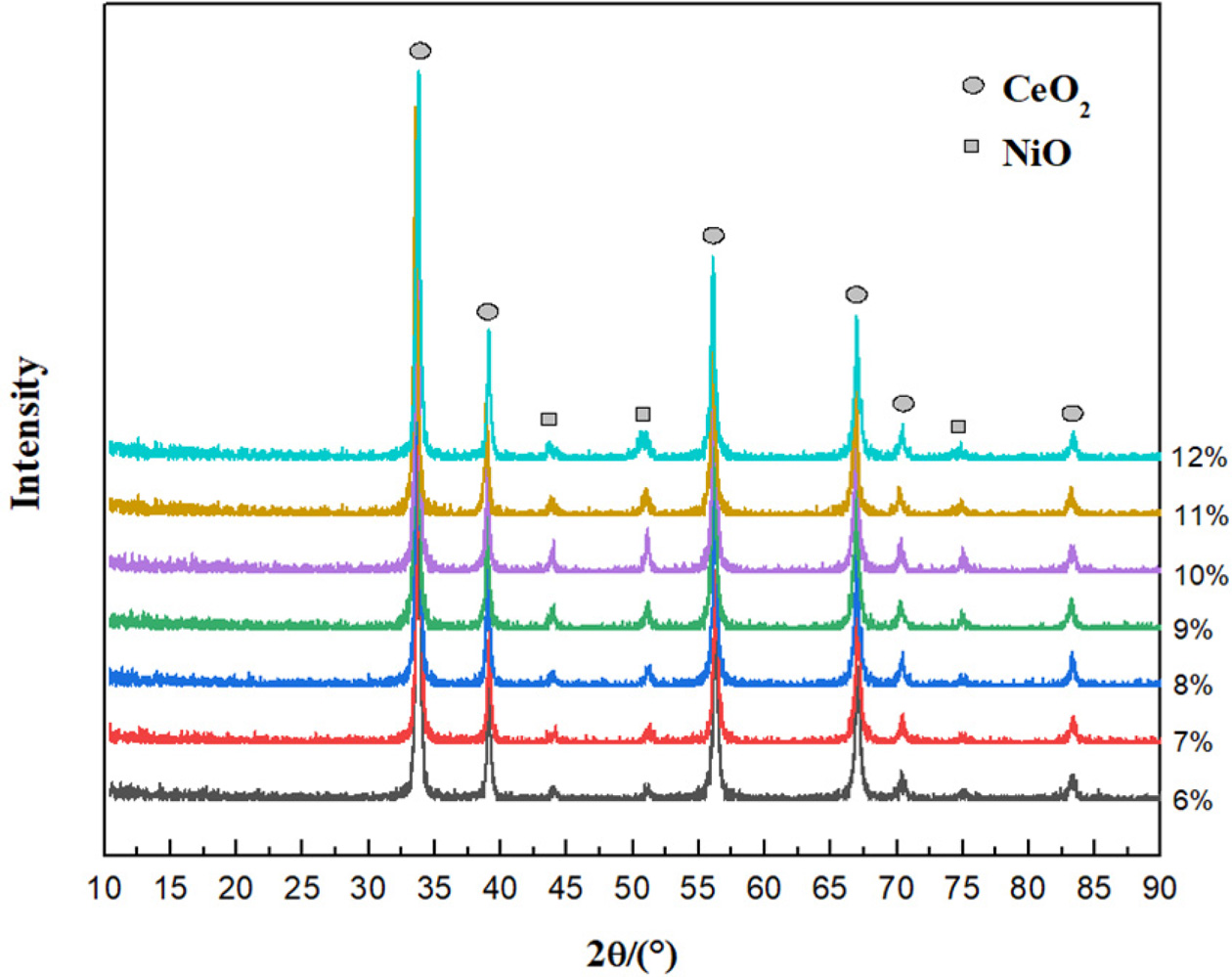
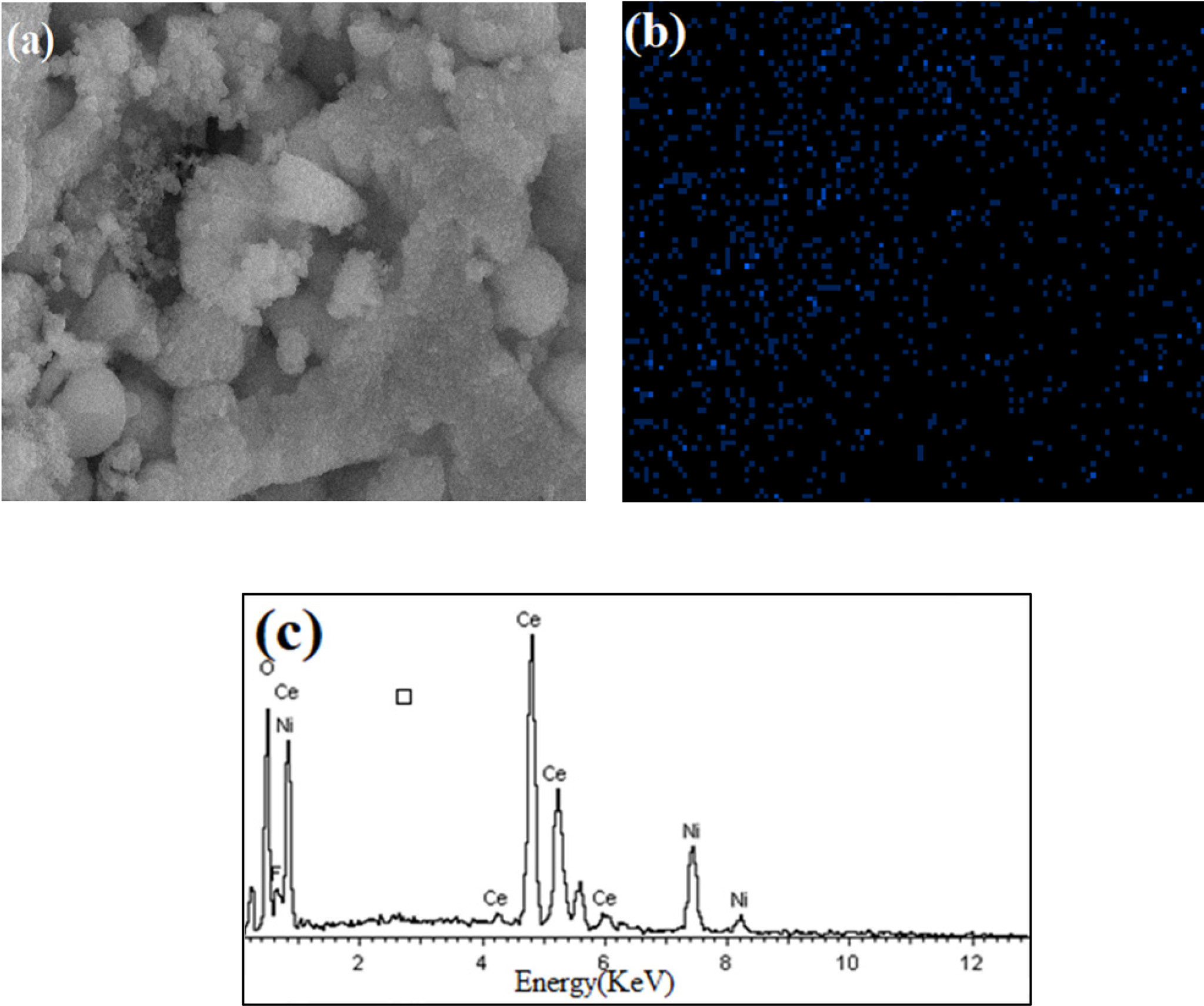
38.5°, 56.6°, 67.7° and 83.7°. Figure 3(a)-3(c) displayed the SEM image,
elemental mapping and EDS analysis of 10% Ni/CeO2. It
validates the existence of Ni on the surface of CeO2.
The EDS spectrum and chemical composition of Ni/CeO2 are given in
Table 1. It demonstrates that the fraction of Ni in Ni/CeO2 is
smaller than CeO2.
Catalytic
performance
Effect of loading amount on catalytic activity of Ni/CeO2
This part mainly investigates the effect of different Ni
loadings on the catalytic activity of Ni/CeO2 with Ni content
varying from 6% to 12% (Temperature = 700 oC; n(H2O):
n(CH4) = 3; and GHSV = 1000 h-1).
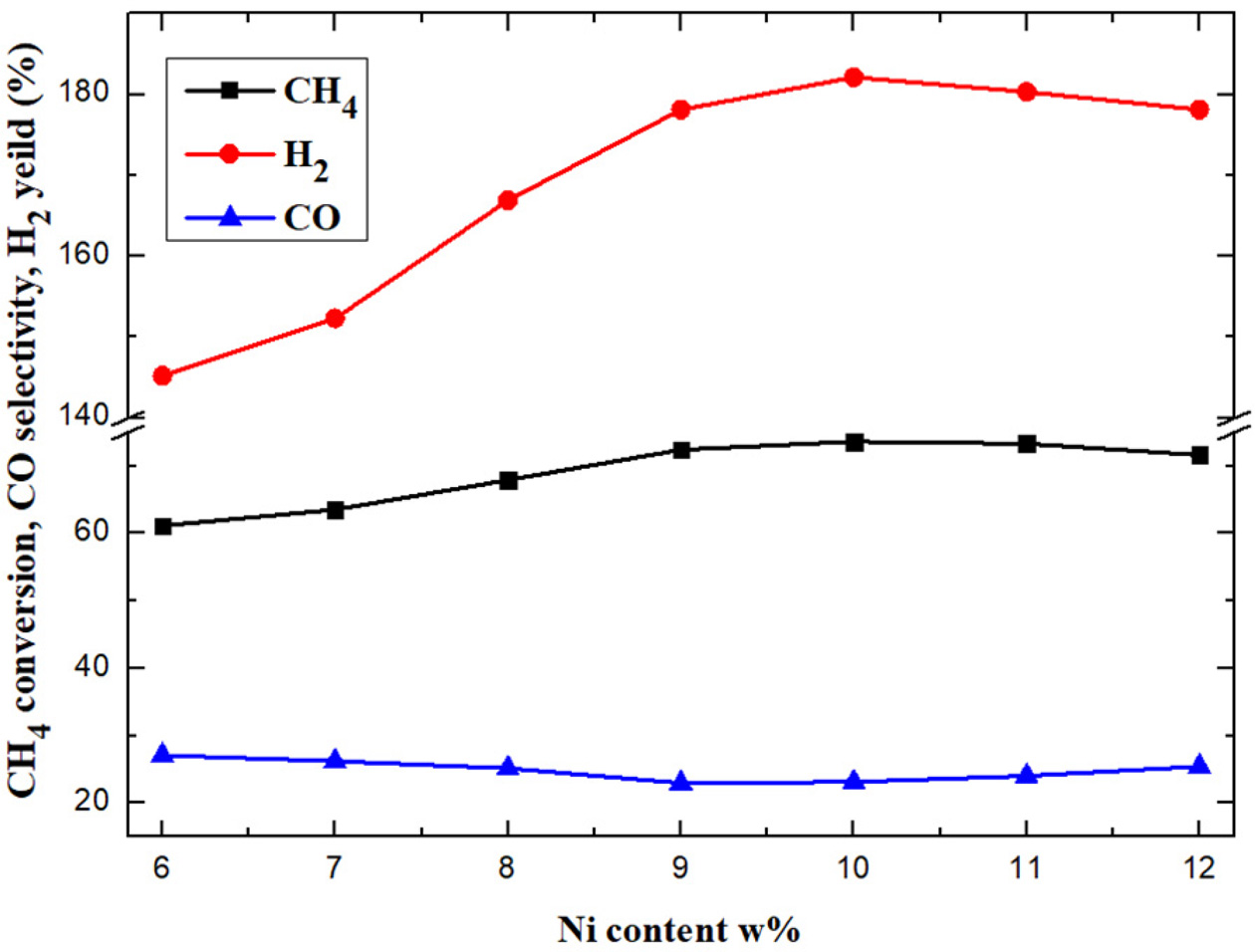
Figure 4 shows the comparison of catalytic capability
with different Ni loadings. It can be concluded that the conversion rate of CH4
increase with the increase of Ni loading from 6% to 10%, the hydrogen
yield also increase obviously. When the value of the Ni loading reaches 10%,
the hydrogen yield reaches the highest value of 182.1%, and the CH4
conversion rate reaches to 73.6%. However, CH4 conversion rate and
the hydrogen yield all decrease when the Ni loading continued increasing until
12%. For the CO selectivity, it decreases first and then increases with the
increase of Ni loading. The minimum value of CO selectivity is 22.97% for the
catalyst with 9% Ni loading. The above results demonstrate that the Ni content
and dispersion have significant effect on the catalytic properties. There is
an optimum nickel content in the nickel based catalyst, which
allows all the active components to disperse uniformly on the active site of
the support surface without aggregation [20].
Effect of Mg and Sr addition
In this part, comparison of catalytic performance of
Mg/Ni-CeO2 and Sr/Ni-CeO2 with different Mg/Sr additions
are studied (Temperature = 700 oC; n(H2O):
n(CH4) = 3; and GHSV = 1000 h-1).
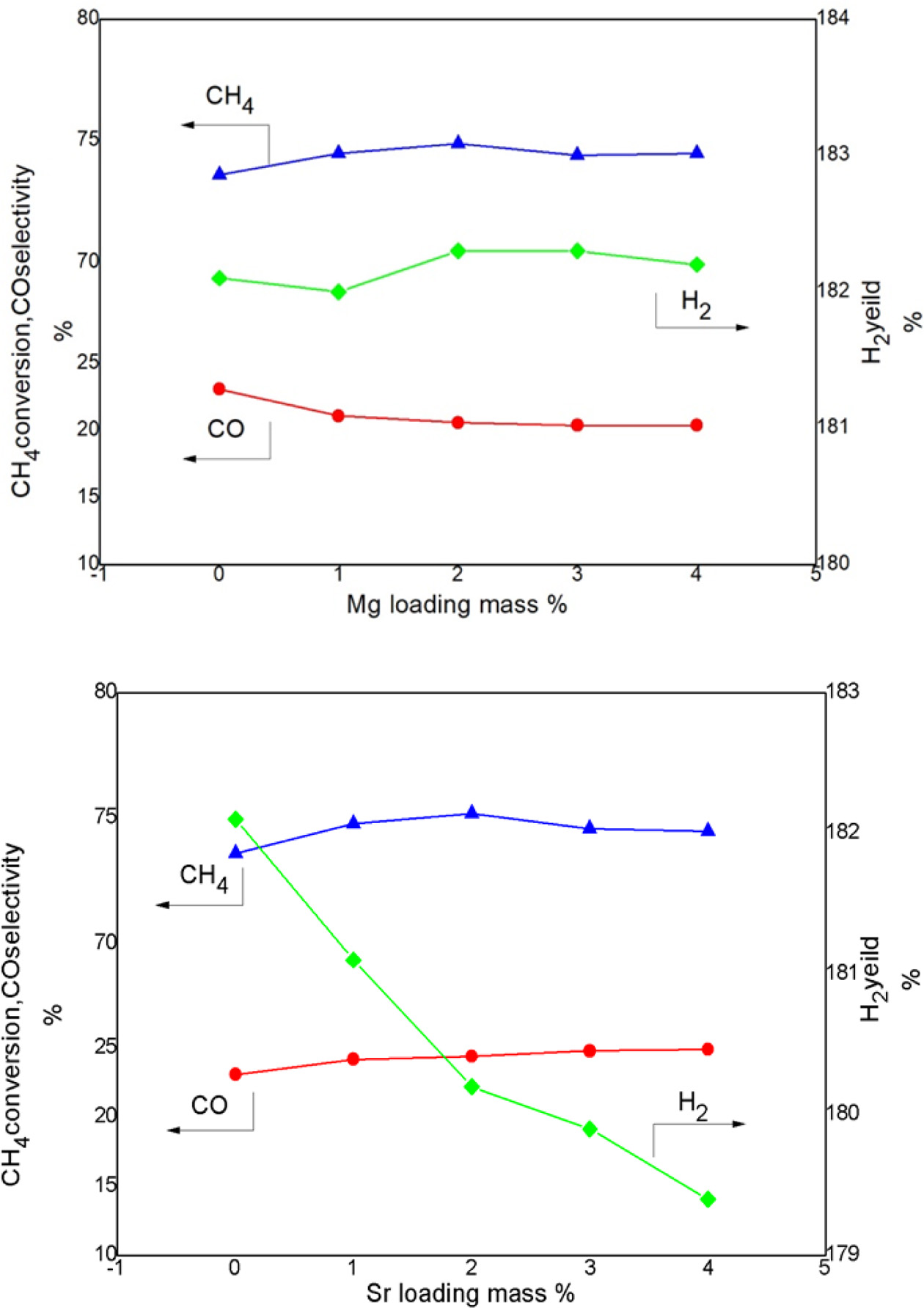
It can be seen from Figure 5 that the addition of Mg
contributes the methane conversion rate of Ni-CeO2 from 73.6% to
74.9%. However, the addition of Mg deceases the CO selectivity from 23.1% to
20.4%. The hydrogen yield has the optimal value when 2% of Mg was
added. The addition of Mg inhibits the formation of CO2, thereby
reducing the water vapor reaction, mean-
while the methane conversion rate increases signifi- cantly. Thus the value of
hydrogen production also shows an increasing trend. Overall, the
addition of Mg has a definite influence for the activity
of the catalyst. According to the above analysis, it is
conclude that the optimum value of Mg addition for Ni/CeO2 catalysts
is 2%.
It can be seen from Figure 5 that the nickel-based
catalyst with the addition of 2% Sr has a methane conversion rate from 73.6% to
75.2%. However, with the increase of the Sr addition amount, the H2
yield shows a downward trend and the CO selectivity shows an upward trend,
indicating that the addition of Sr increases the poisoning of the catalyst,
which is not conducive to long-term work of the catalyst.
Morphology characterization of Mg/Ni-CeO2
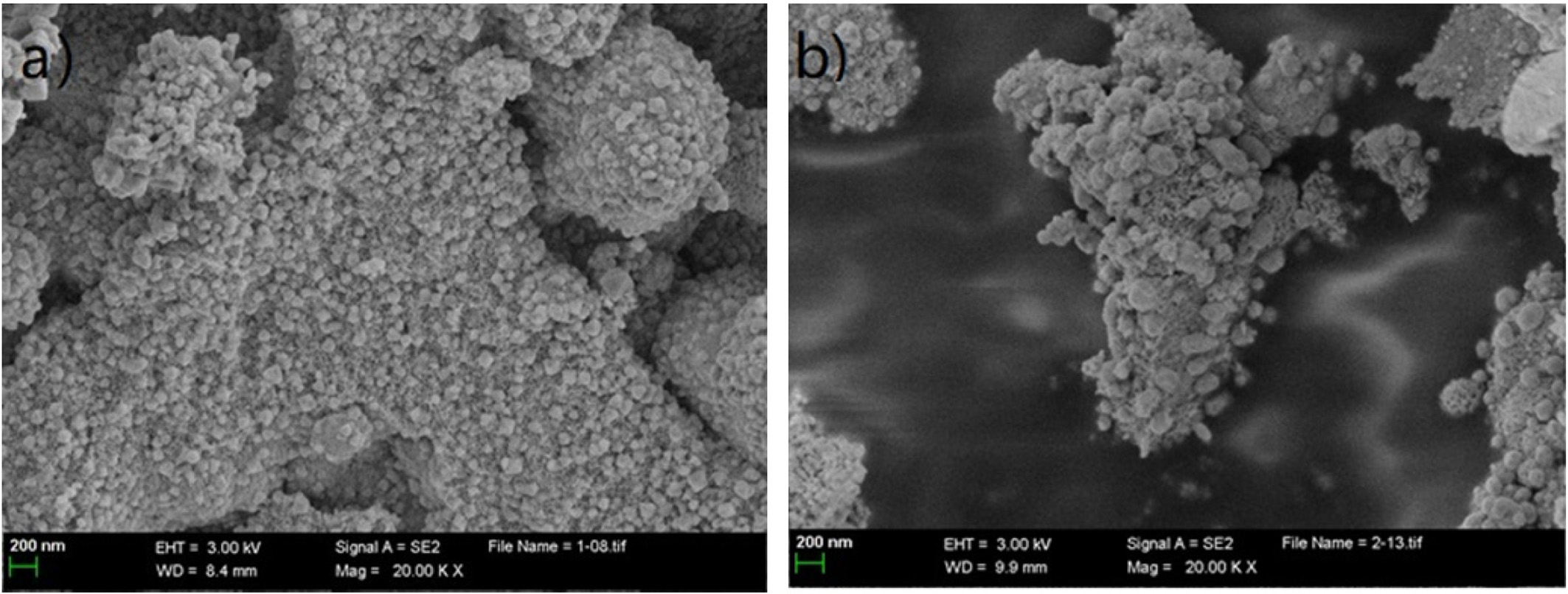
Figure 6a shows that the surface morphology of Mg/Ni-CeO2
is porous. The dispersion is high and there is no agglomeration or sintering.
From Figure 6b, it can be seen that the crystal of NiO can still be observed on
the surface of the Mg/Ni-CeO2 after the reaction. No carbon
filaments are observed in the SEM. It can be inferred that the carbon diffusion
rate at the surface metal-carbon interface of the catalyst is at a low level,
so that Mg/Ni-CeO2 is considered to have high carbon deposition
resistance and stability [20].
Effect of Temperature
The effect of temperature on the activity performance of
catalysts are investigated at the following conditions: Mg-Ni/CeO2
with a Ni loading of 10%, Mg addition of 2%, 1 atm, n(H2O)/n(CH4) = 3,
and GHSV = 1000 h-1.
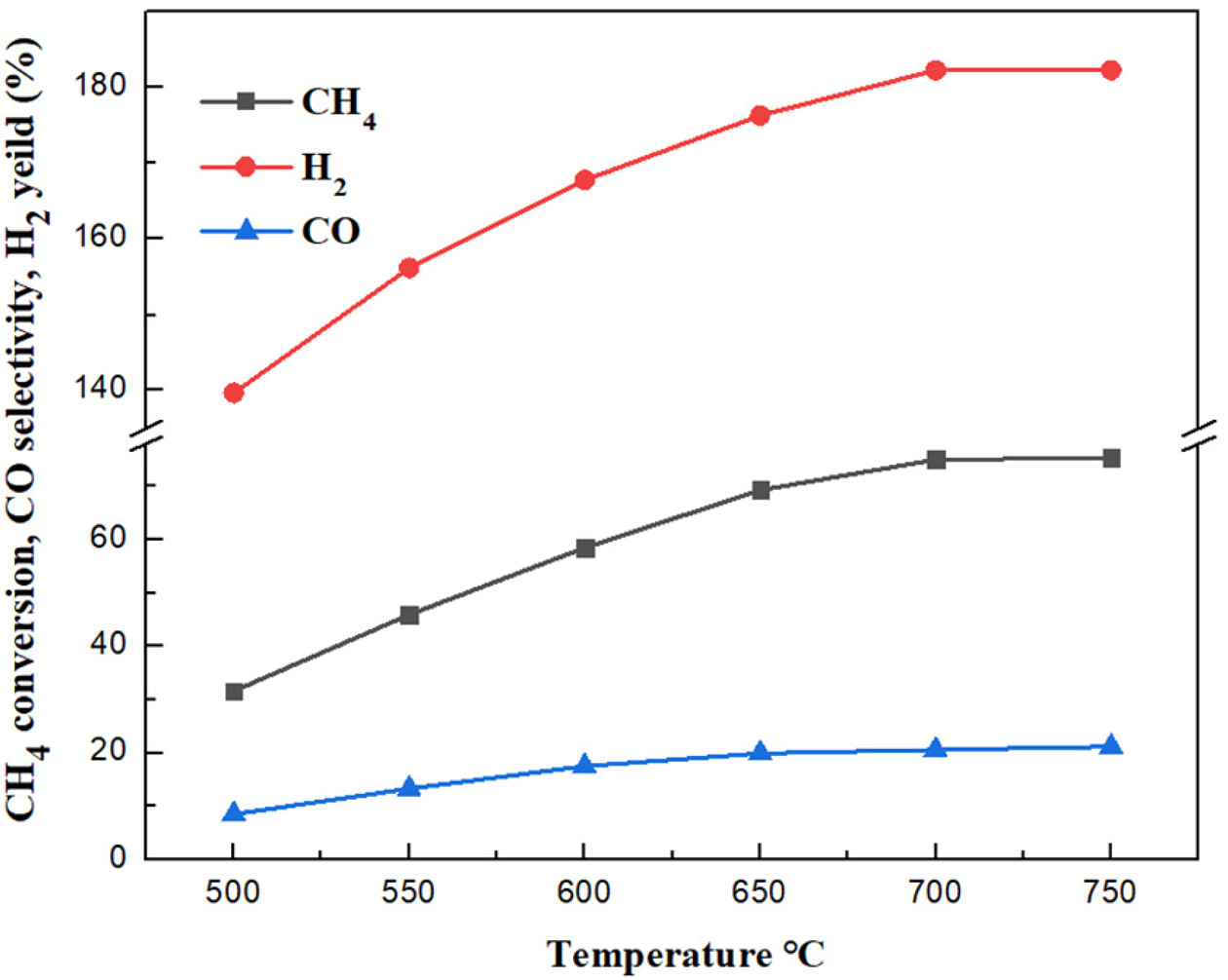
As shown in Figure 7, it is clear that the CH4
con- version rate, H2 yield
and CO selectivity all increase with the increasing temperature. CH4
conversion rate increases from 31.6% to 75.2%, and reaches the maximum value at
750 oC. The methane conversion rate changes slightly at the
temperature above 700 oC. The hydrogen yield increases from
139.7% to 182.3%, reaching the maximum value at 700 oC. CO
selectivity increases from 8.5% to 21.2%, reaching the maximum value of 21.2%
at 750 oC. Considering the life of pipelines and the energy
consumption, 700 oC is the optimum reaction temperature in the
study. Under this reaction condition, the CH4 conversion rate is
74.9%, the H2 production rate is 182.3%, the CO selectivity is
20.6%.
Effect of S/C
The effect of H2O/CH4 ratios (S/C)
on the activity performance of catalysts are investigated at the
following conditions: Mg-Ni/CeO2 with a Ni loading of
10%, Mg addition of 2%, 700 oC, 1 atm, and
GHSV = 1000 h-1.
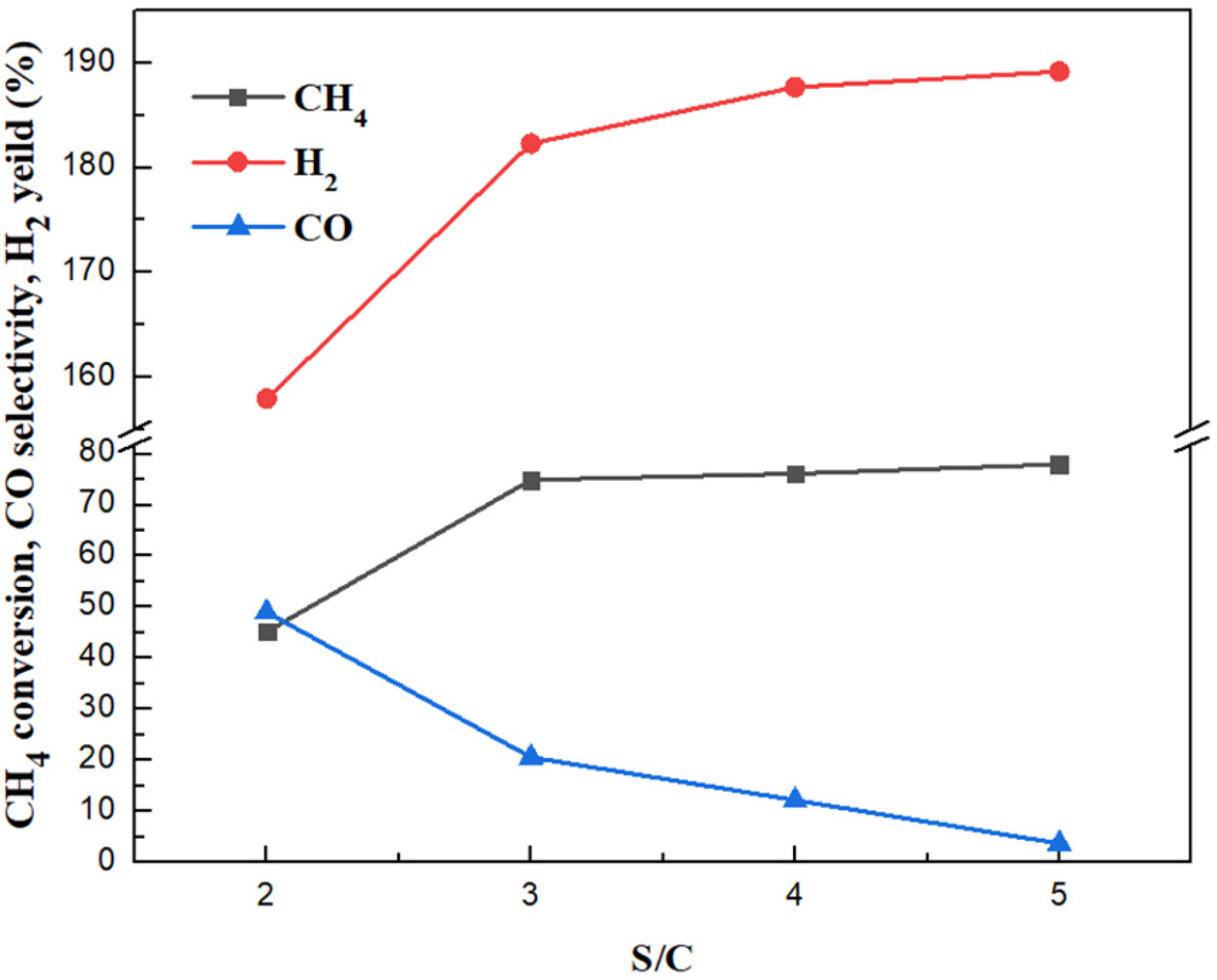
It can be seen from Figure 8 that an increase of the S/C
ratio results in an increase of methane conversion rate,
hydrogen yield, and a decrease of carbon monoxide selectivity.
When the S/C ratios increase from 2 to 3, the CH4 conversion rate
increases from 45.2% to 74.9%, the hydrogen yield increases from 157.9% to
182.3%, and the CO selectivity ranges from 49% to 20.6%; When the S/C ratios
increase from 3 to 5, the CH4 conversion rate and hydrogen yield
continue to increasing but they change slightly, while CO
decreased to 3.66%.
Increasing the amount of water vapor in the reaction
leading the active oxygen atom which on the surface of the nickel-based
catalyst reacting with the active quaternary carbon atom and carbon monoxide to
form CO2. The cleavage of reactive functional groups such as methine
and methylene groups accelerating the methane cracking. Previous studies have
confirmed the enhancement of water vapor partial pressure on the MSR reaction,
indicating that the water vapor promoted the rapid decline of CO
selectivity [17]. However, excessive water vapor consumption
requires a large amount of energy. From the economic point of view, the S/C
ratio is not as high as possible. In this study, the S/C ratio of 3 is
considered as the optimum value.
Effect of GHSV
In this part, Mg-Ni/CeO2 with a Ni loading of
10%, Mg addition of 2% was studied under the reaction conditions: 700 oC;
S/C = 3; 1 atm. The gas hourly space velocity (GHSV) of methane
is used as the standard, and the methane input is =100 SCCM. The GHSV of each
experiment is controlled by changing the catalyst loading. The calculation
formula is:

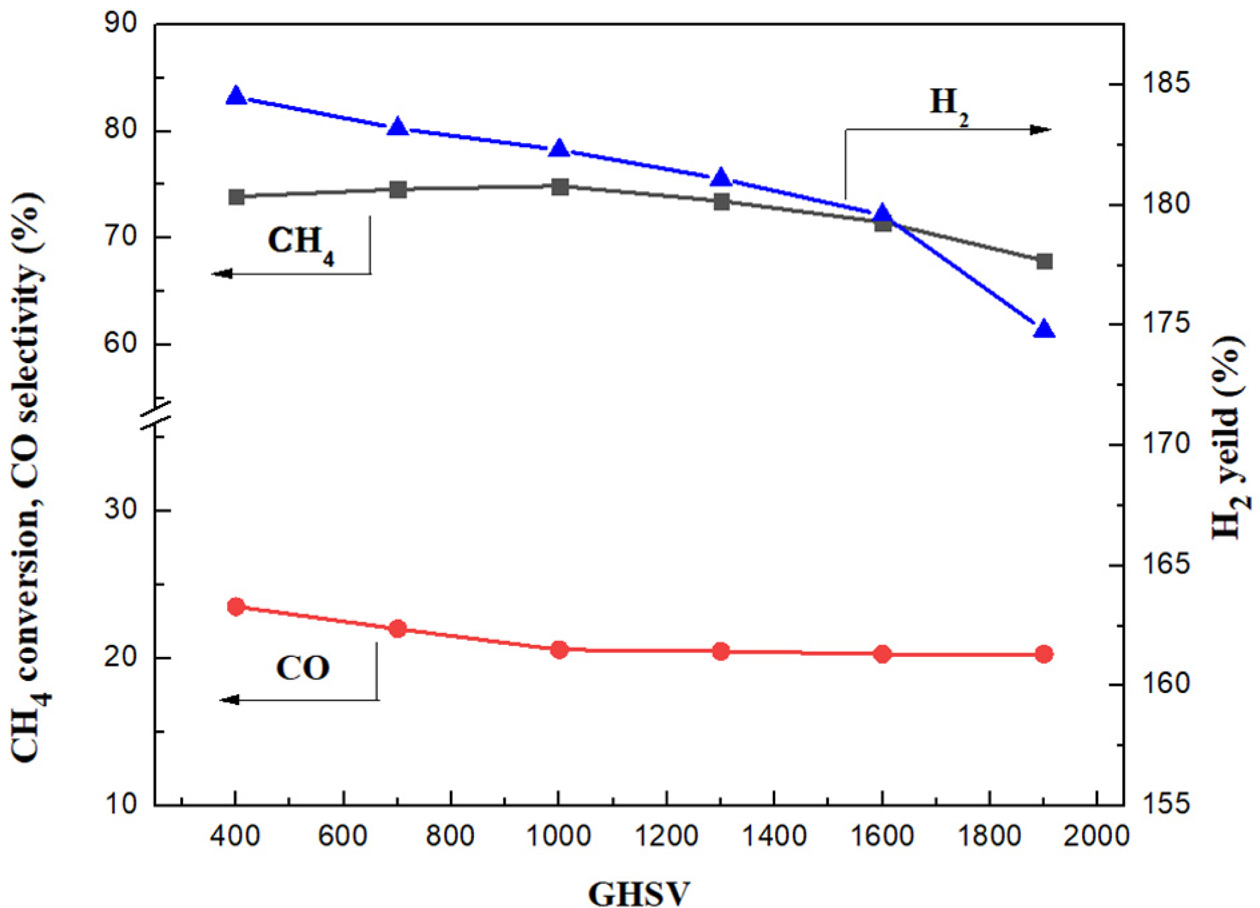
It can be seen from Figure 9 that in the lower GHSV region
(400 h-1~1000 h-1), the CO selectivity decreases
from 23.51% to 20.6% with the increase of GHSV, and the methane conversion rate
fluctuates between 73.9% and 74.9%. The hydrogen yield dropped from 184.5% to
182.3%. The selectivity of CO remained unchanged when the GHSV continued to
increase. However when the GHSV increases to 1900 h-1,
the methane conversion rate decreases to 67.9%, and the
hydrogen yield has the same trend. In this study, the GHSV of 1000 h-1
is considered as the optimum value.
Stability tests
Catalyst with a Ni loading of 10% and a Mg addition content
of 2% was used for the stability test. Experiment was
conducted to evaluate the stability of the catalyst at 700 oC
for 8 hours under flow (n(H2O): n(CH4) = 3,
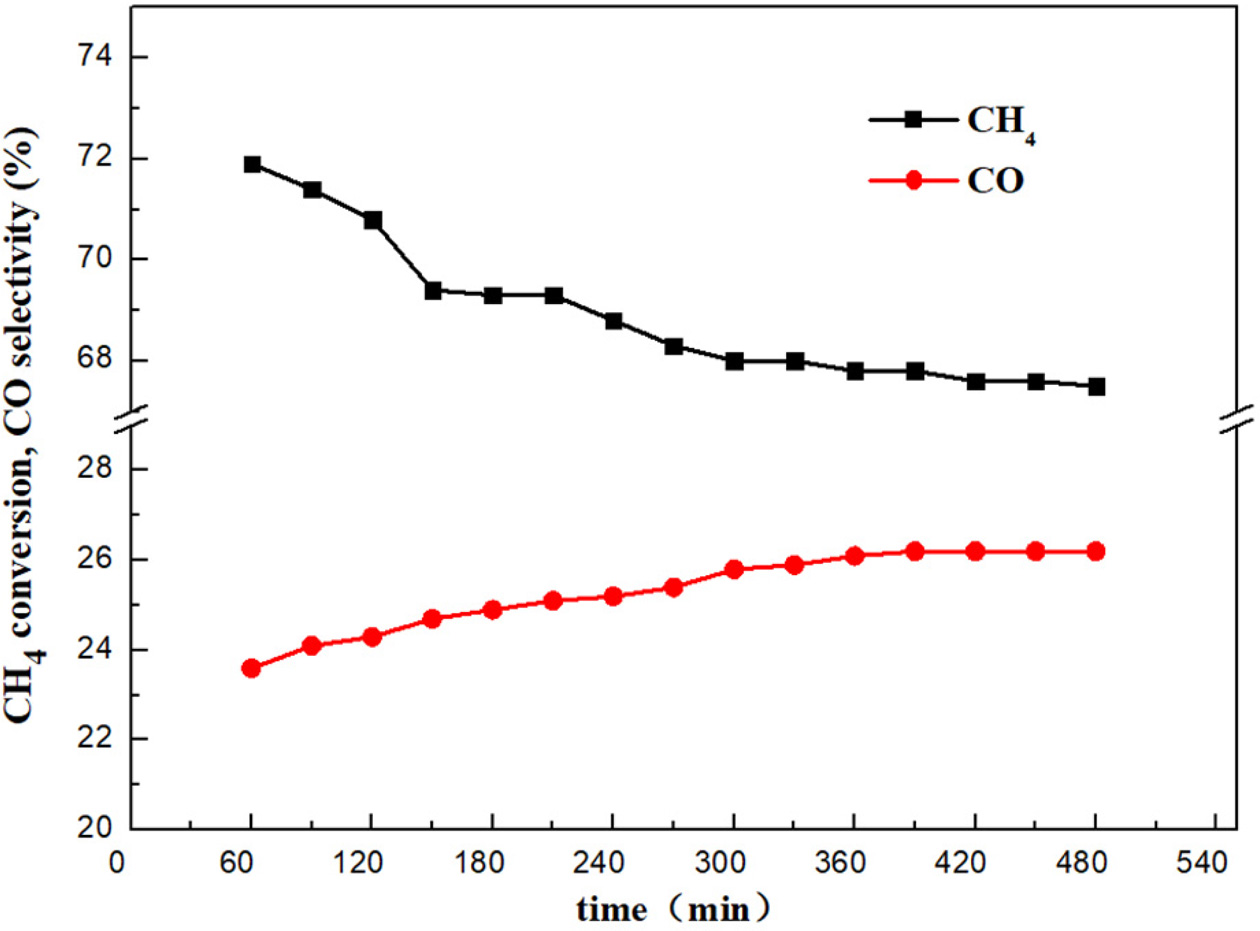
and GHSV = 1000 h-1). Results
are shown in Figure 10. During this period, the catalysts show good stability
at 700 oC where the beginning of highest CH4
conversion is observed. It can be concluded that Mg/Ni-CeO2 still
maintains a relatively high catalytic activity after 8 h stability test.
|
Fig. 2 XRD patterns of catalysts with different Ni loadings. |
|
Fig. 3 (a) SEM image of Ni in 10% Ni/CeO2; (b) Elemental mapping; (c) EDS analysis of 10% Ni/CeO2. |
|
Fig. 4 Catalytic properties of catalysts with different Ni loadings. |
|
Fig. 5 Comparison of catalytic performance Ni-CeO2 with different Mg/Sr additions. |
|
Fig. 6 SEM image of the Mg/ Ni-CeO2 catalysts (a) fresh sample (20000×); (b) spent sample (20000×). |
|
Fig. 7 Effect of temperature on the activity performance of catalysts. |
|
Fig. 8 Effect of S/C on the activity performance of catalysts. |
|
Fig. 9 Effect of GHSV on the activity performance of catalysts. |
|
Fig. 10 Stability of Mg-Ni/CeO2 catalysts for SRM, Reaction conditions: T = 700 oC (8 h), GHSV = 1000 h-1, S/C = 3. |
Mg-Ni/CeO2 catalysts at different Mg/Ni ratios
of 6/4, 7/3, 8/2 and 9/1 with total loading of 10 wt% were synthesized via
a wetness impregnation method and applied for MSR. The characterization
techniques such as XRD, SEM and EDS were carried out on fresh and spent samples.
Results showed that the optimum Mg addition content for Mg/Ni-CeO2
is 2%. The process optimization of reaction parameters were conducted by
evaluating the catalytic activity. Finally, the stability test for 8h at
700 oC demonstrated the excellent thermal stability of the
resulted Mg/Ni-CeO2 catalysts.
This work was supported by the National Natural Science
Foundation of China Funding (No.51779025 and No.51606013), China Postdoctoral
Science Foun- dation Funding
(No.2019M651097 and No. 2019M651094),
Fundamental Research Funds for the Central Universities
of China (No.3132019187, No.3132019191 and No.3132019327), and
Natural Science Foundation of Liaoning Province (No.2019-BS-026 and
No.2019-ZD-0154).
- 1. S.A. Li, J.L. Yuan, G.N. Xie, and B. Sunden, Int. J. Hydrog. Energy 43 (2018) 8451-8463.
-

- 2. Q.W. Shen, S.A. Li, G.G. Yang, J.L. Yuan, N.B. Huang, J. Ceram. Process. Res. 20 (2019) 152-157.
-

- 3. J.H. Hwang, and K.T. Lee, J. Ceram. Process. Res. 21[1] (2019) 57-63.
-

- 4. T.L. LeValley, A.R. Richard, and M. Fan, Int. J. Hydrog. Energy 39 (2014) 16983-17000.
-

- 5. A.C.W. Koh, L. Chen, W.K. Leong. B.F.G. Johnson, T. Khimyak, and J. Lin, Int. J. Hydrog. Energy 32 (2007) 725-730.
-

- 6. M. Rezaei, S.M. Alavi, S. Sahebdelfar, P. Bai, X. Liu, and Z.F. Yan, Appl Catal, B. 77(2008)346-354.
-

- 7. M.S. Fan, A.Z. Abdullah, and S. Bhatia, Int. J. Hydrog. Energy 36 (2011) 4875-4886.
-

- 8. S. Ali, M.J. Almarri, A.G. Abdelmoneim, A. Kumar, and M.M. Khader, Int. J. Hydrog. Energy 41[48] (2016) 22876-22885.
-

- 9. V.K. Jaiswar, S. Katheria, G. Deo, and D. Kunzru, Int. J. Hydrog. Energy 27 (2017) 18968-18976.
-

- 10. A. Berman, R.K. Karn, and M. Epstein, Appl. Catal. Gen. 282[1-2] (2005) 73-83.
-

- 11. R. Craciun, W. Daniell, H. Kno zinger, Appl. Catal. Gen. 230 (2002) 53-68.
-

- 12. V.P. De Souza, D. Costa, D. Dos Santos, A.G. Sato, and J.M.C. Bueno, Int. J. Hydrog. Energy 37[13] (2012) 9985-9993.
-

- 13. N. Salhi, C. Petit, A.C. Roger, A. Kienemann, S. Libs, and M.M. Bettahar, Catal. Today 113[3-4] (2006) 187-193.
-

- 14. X. Fang, X. Zhang, Y. Guo, M. Chen, W. Liu, and X. Xu, Int. J. Hydrog. Energy 41 (2016) 11141-11153.
-

- 15. R. Martınez, E. Romero, C. Guimon, R. Bilbao, Appl. Catal. Gen. 274[1-2] (2004) 139-149.
-

- 16. M. Cargnello, P. Fornasiero, and R.J. Gorte, Catal. Lett. 142 (2012) 1043-1048.
- 17. M. Ahmadi, H. Mistry, and B.R. Cuenya, J. Phys. Chem. Lett. 7 (2016) 3519 -3533.
- 18. T. Montini, M. Melchionna, M. Monai, P. Fornasiero, Chem. Rev.116 (2016) 5987-6041.
- 19. W.S. Dong, H.S. Roh, K.W. Jun, S.E. Park, and Y.S. Oh, Appl. Catal. A Gen. 226 (2002) 63-72.
-

- 20. H.S. Roh, K.W. Jun, S.E. Park, Appl. Catal. A Gen. 251 (2003) 275-283.
-

- 21. Y.Y. Ji, W.Z. Li, and Y.X. Chen, Natural Gas Chemistry 9[4] (2000) 291-303.
 This Article
This Article
-
2020; 21(4): 508-514
Published on Aug 30, 2020
- 10.36410/jcpr.2020.21.4.508
- Received on Mar 18, 2020
- Revised on Mar 27, 2020
- Accepted on Apr 2, 2020
 Services
Services
- Abstract
introduction
materials and methods
results and discussion
conclusions
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Guogang Yang
-
Marine Engineering College, Dalian Maritime University, Dalian, China
Tel : +86-13050561150
Fax: +0411-84728659 - E-mail: yanggg@dlmu.edu.cn






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.