- The effect of transition metal oxide doping on the sintering behaviour of yttria-stabilised tetragonal zirconia
H.C. Alexander Cheea, R.S.K. Singha,b and K.Y. Sara Leec,*
aFaculty of Engineering, University of Malaya, 50603 Kuala Lumpur, Malaysia
bFaculty of Engineering, Universiti Teknologi Brunei, Tungku Highway, Gadong BE1410, Brunei Darussalam
cTunku Abdul Rahman University College, Faculty of Engineering & Technology, Department of Mechanical Engineering, 53300, Kuala Lumpur, Malaysia
The effect of small amounts
(0.2 wt% and 0.5 wt%) of transition metal oxide (CuO) doping on the
sintering behavior of 3 mol% yttria-stabilized zirconia (Y-TZP) was
investigated over the temperature range of 1250 oC to
1500 oC. Sintered samples were characterized to determine the
phase present, relative density, microstructural evolution and Vickers
hardness. The studies revealed that the addition of 0.2 wt% CuO dopant was
most effective in enhancing the densification of the Y-TZP in particularly at
low temperatures below 1350 oC. The phase analysis revealed
that the tetragonal phase was disrupted as evident from the high monoclinic
phase formation in the 0.5 wt% CuO-doped zirconia. This phenomenon was
associated with mechanism involving transient liquid phase which believed to
have destabilized the tetragonal structure. Nevertheless, the study revealed
that compared to the undoped Y-TZP, the sample with addition of 0.2 wt%
CuO resulted in enhanced hardness and finer grain sizes when sintered at
relatively low temperatures.
Keywords: Y-TZP, Copper oxide, Doping, Sintering additives, Vickers hardness
Yttria-stabilized tetragonal zirconia polycrystals or
Y-TZP is known to exhibit attractive properties such as high
fracture toughness, strength and good wear properties suitable
for many industrial and biomedical applica-
tions. This has been attributed to its unique ‘self-healing’
mechanism known as transformation toughening. In this
mechanism, the energy of an advancing crack through the matrix is absorbed by
the tetragonal grains in the vicinity of the crack front and transform to a
monoclinic symmetry. This phase transformation is accompanied
by 3-5% volume expansion which induced a local compression
surrounding the crack tip; as a result more energy would be required for crack
pro- pagation and hence enhancing the
strength [1-3].
The widespread use of Y-TZP, however is hampered by the
undesirable tetragonal to monoclinic phase transformation when exposed to steam
environment at temperatures 60 oC to 500 oC, a
phenomenon known as hydrothermal ageing or low temperature degradation (LTD)
[4-8]. This devastating effect of zirconia was initially observed by Kobayashi
et al. [4], and the ageing process was documented to induce formation of
networks of micro- and macro-cracking, thus leading to deterioration of
mechanical properties. According to this study, when the Y-TZP was exposed to
humid environment at temperatures between 150 oC
and 400 oC, isolated tetragonal grains or
nuclei at the surface begin to transform to the monoclinic
symmetry by incorporating the hydroxyls in the oxygen
vacancies of the zirconia lattice. This induces localized residual stresses
which subsequently destabilized the tetragonal structure [7-10].
A nucleation and growth mechanisms have been proposed to
explain the ageing kinetics although a consensus on the nature of the
transformation in the interior of the sample i.e. whether the transformation
proceed linearly or exponentially with time and/or reached a saturation limit
after a certain depth have not been unequivocally resolved [11-15].
Nevertheless, factors such as grain sizes and the chemistry of the grain
boundaries have been associated with the LTD phenomenon [16-19]. For instance,
Hallmann et al. [17] reported that Y-TZPs with average grain size less than
0.3 μm was resistant against the ageing-induced phase transformation. The
authors also found that grain boundary modification using dopants such as alumina,
ceria and iron oxide were beneficial in suppressing the LTD in Y-TZP. Zhang et
al. [19] investigated the influence of 0.25 wt% alumina
addition and incorporating yttria coating of zirconia starting
powder on the LTD behavior of the ceramics when exposed in an autoclave
containing steam at 134 oC and 0.2 MPa up to 40 h.
Their TEM analysis revealed that the Y/Zr ratio of the yttria coated zirconia
was higher at grain boundary regions when compared to the grain core. Zhang et
al. suggested that the improved ageing resistant observed for their Y-TZP was
attributed to both, the segregation of Al3+ and the heterogeneously
distributed of Y3+ at the grain boundaries.
Improvement in the sintering conditions has been
demonstrated in the literatures to be viable in order to improve the
densification and properties of Y-TZP as well as to control the LTD in
zirconia. This includes using non-conventional sintering processes and the
manipulation of the sintering conditions such as field-assisted
sintering [20], hot-isostatic pressing [21], spark-plasma
sintering [22], cold sintering [23], two-step fast firing [24], microwave
sintering [25] and laser sintering [26]. All these methods have shown to be
effective in enhancing the properties of zirconia, however this improvement is
at the expense of high cost associated with the equipment used for powder
consolidation. A more economical approach would be to aid the conventional
sintering process using low melting sintering additives
or dopants, added to the zirconia during powder processing and subjecting the
doped powders to conventional sintering at low temperatures. This strategy has
been demonstrated to yield highly dense bodies in several ceramic systems
[27-33].
The present work aims to examine the effect of small
additions of transition metal copper oxide (0.2 wt% and 0.5 wt%)
on the mechanical properties and microstructural development
of Y-TZP ceramics prepared by the pressureless sintering method.
In the present work, 3 mol% yttria stabilized
zirconia powder from Kyoritsu Japan was used and the different amounts of high
purity CuO (0.2 wt% and 0.5 wt%) were mixed in an attritor-mill
contained zirconia beads (1 mm in diameter) as the milling media and
ethanol as the mixing medium. The speed of the rotation was kept constant at
600 rpm and each milling was carried out for 30 mins. After the
mixing process, the slurries were separated from the milling media by
filtration and then dried overnight in an oven at 60 oC.
Finally, soft, ready to press powders, were obtained by sieving through
212 µm mesh sieve. For the study, green disc samples (20 mm
diameter, 5 mm thick) were uniaxially compacted using a
metal mold and subsequently subjected to cold isostatic pressing at
200 MPa.
Powder consolidation was accomplished by pressureless
sintering under ambient conditions in a box furnace. The sintering was carried
out at different temperatures ranging from 1250 oC to
1500 oC at a ramp rate 10 oC/min and holding
time 2 h. The sintered samples were ground
successively on one face by using silicon carbide paper of
120 (rough), 240, 600, 800 and 1200 (fine) grades, followed by polishing using
6 µm and 1 µm diamond paste to obtain an optical reflective surface.
The bulk densities of sintered samples were determined by
the water immersion method based on the Archimedes principle, and the relative
densities were calculated by taking the theoretical density of tetragonal
zirconia as 6.07 g/cm3. Vickers Hardness (Hv) was measured
on polished samples using Vickers indention method. The
indention load was kept constant at 10 kgF with a loading time of 10
s. Phase analysis was conducted by X-ray diffraction (XRD) (Geiger-Flex,
Rigaku Japan) under room temperature conditions using Cu-Kα
as the radiation source. The fraction of monoclinic phase was determined using
the method of Toraya et al. [34]. The effect of temperature on the
microstructure evolution of the ceramic was examined by using scanning electron
microscope (SEM). The average grain size of the polished samples was
deter-mined by using the line
intercept method [35].
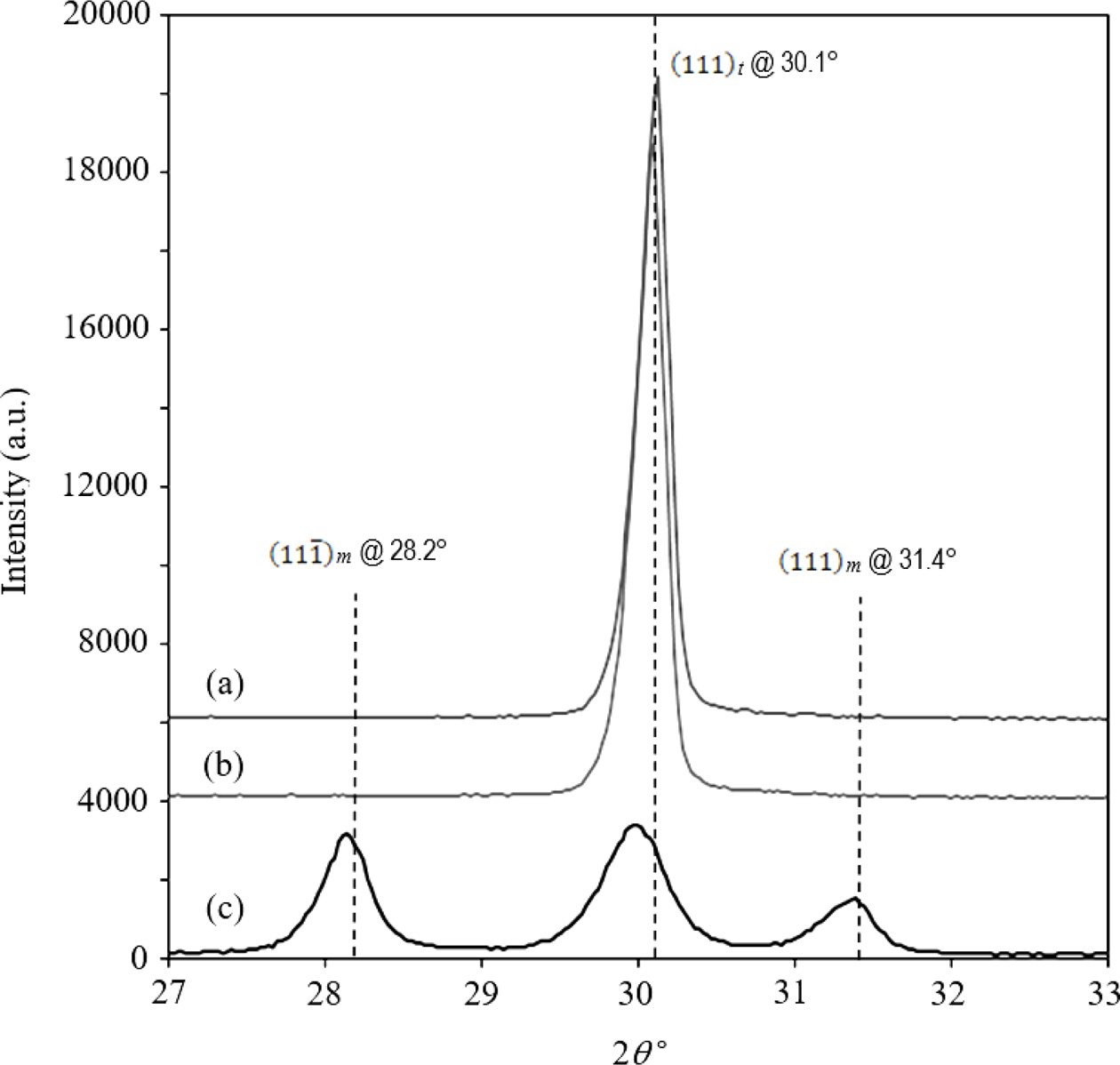
A typical XRD trace of the undoped and CuO-doped Y-TZP
sintered at 1400 oC is shown in Fig. 1. A similar trend was
observed at all temperatures. The undoped and 0.2 wt% CuO doped zirconia
exhibited a fully tetragonal structure, however, the XRD analysis indicated
that the addition of 0.5 wt% CuO has disrupted the tetragonal phase stability
of the Y-TZP throughout the sintering regime employed. A high percentage of
monoclinic phase was measured for the 0.5 wt% CuO addition. It
is believed that the addition of high amounts of dopant
could have developed excessive transient liquid phase
during sintering which caused the depletion of yttria
stabilizer from the zirconia lattice at around 1180 oC and on
cooling destabilised the tetragonal structure [36, 37]. It was determined that
the mono- clinic phase content for the
0.5 wt% CuO-doped zirconia samples varied between 50 to 55% as the
sintering temperature increased from 1250 to 1500 oC. In
addition, the tetragonal peaks at 2θ = 30.1° for this sample
shifted slightly to the left by about 0.10-0.13° when compared to the undoped
zirconia as shown by the inset picture in Fig. 1. This is an indication that
the high amount of CuO had affected the stability of the tetragonal zirconia
lattice.
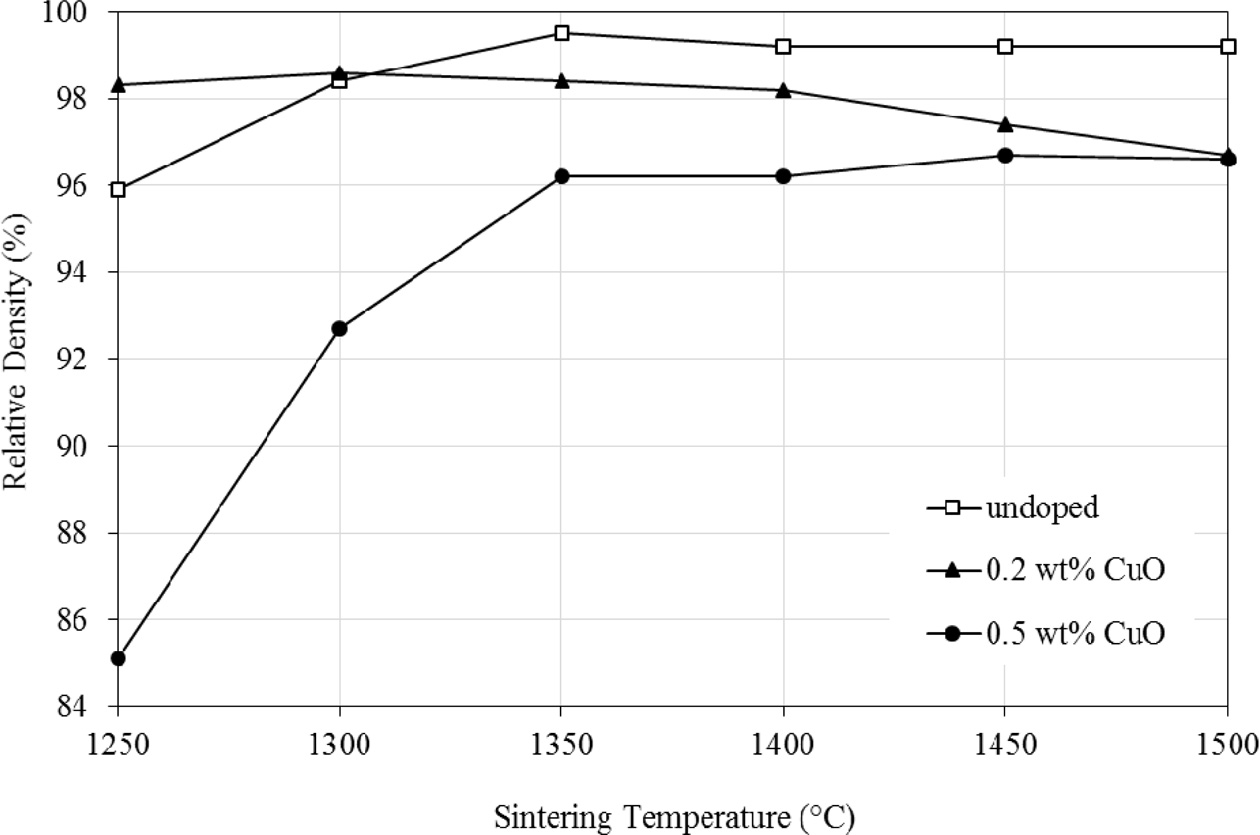
The effect of sintering temperature on the relative
density of undoped and CuO-doped zirconia is shown in Fig. 2. The result shows
that the addition of 0.2 wt% CuO was beneficial in enhancing the density
of Y-TZP sintered below 1350 oC. This sample
exhibited > 98% relative density when sintered at 1250 oC
when com- pared to 96% for the undoped
zirconia. However, the relative density of the undoped Y-TZP started to
increase and maintained above 98% as the temperature raised to 1350 oC
and above. In contrast, the 0.5 wt% CuO doped Y-TZP
exhibited the lowest density regardless of sintering temperature. It
started low at 85% dense @ 1250 oC and increased steadily to
96% at 1350 oC and then remained almost constant with further
increased in temperatures. This observation is in good agreement with the high
monoclinic content observed for the sintered body.
The beneficial effects of transition metal oxides such as
copper oxide and manganese oxide (MnO2) in promoting densification
and improvement in other properties were also observed in other ceramic systems
such as alumina [38], cadmium [39] as well as SnO2 [40] and ternary
based ferroelectrics [41]. It is known that ceria and the tantalates are not
easy to densify at moderate temperatures without sintering at elevated
temperature above 1500 oC. Shimada et al. [42] de- monstrated that 3 wt% MnO-doped LiTaO3
promoted densification at low temperatures of 1125-1190 oC.
They obtained a relative density of 85-90% when compared to 60-62% for the
undoped ceramic but at the expense of grain coarsening. The authors believed
that the enhancement in densification in these ceramics could be linked to the
reactive Mn-based liquid formation at low temperatures. In another research,
Corker et al. [43] studied the effect of CuO doping on the sintering of PZT and
they found the addition of 3 wt% of Cu2O/PbO with the eutectic ratio
of Cu2O : PbO (1 : 4) was effective in reducing the densification
temperature considerably by about 67%, from 1260 oC down to
850 oC. The authors attributed this remarkable improvement to
the presences of a Cu2O-PbO eutectic liquid at about 680 oC.
This finding was supported by TEM studies [44] which showed the formation of a
CuO-PbO rich grain boundary phase at 900 oC and the
crystallization of nanosized CuO precipitates near grain boundary regions after
sintering.
Zhang et al. [45] investigated the effect of transition
metal oxide (MnO2) doping in ceria and found that the addition of up
to 1.5 wt% MnO2 accelerated the densi- fication rate of ceria when sintered at low
temperatures. They went on to suggest the formation of a very thin Mn-amorphous
layer during heating could have facilitated particle
rearrangement thus leading to rapid consoli-
dation during the early-stage of sintering. Similar observation on the
effect of manganese oxide in aiding the sintering of zirconia was also reported
in the literatures [46, 47]. These studies concur that the presence
of a reactive liquid phase involving the transition metals during
sintering was responsible for the improve-
ment in sintered density. More recently, Watson et al.
[41] concluded that CuO actively reacted with PbO to form a reactive eutectic
liquid phase during sintering of PIN-PMN-PT ceramics, which reduces the
activation energy for sintering to proceed at temperatures as low as 790 oC
and hence retaining a fine grain micro-
structure on cooling. There have been different views on the sintering
mechanism involving CuO as additive. For instance, Nie et al. [48] who studied
the effect of CuO on the sintering of TiO2 ceramics inferred that
the enhancement in densification of CuO-doped TiO2 at low
temperatures was due to sub-eutectic activated sintering, rather than
liquid-phase sintering.
The fact that the melting point of copper oxide (1150 oC)
is lower than the sintering temperature employed in the
present work and based on the literature findings as well as the results
obtained, it is envisaged that a transient liquid phase mechanism was in
operative. The results indicated that the addition of 0.2 wt% CuO was the
optimum amount required to form sufficient reactive Y2O3/CuO-rich
transient liquid phase which facilitated particle rearrangement and coalescence
at low temperatures, below 1350 ºC. As the doping level increased to
0.5 wt%, the excess amount of Cu-rich liquid proved to be detrimental as
this has an effect to draw out too much of yttria from the zirconia matrix,
resulting in phase transformation upon cooling from sintering as evident from
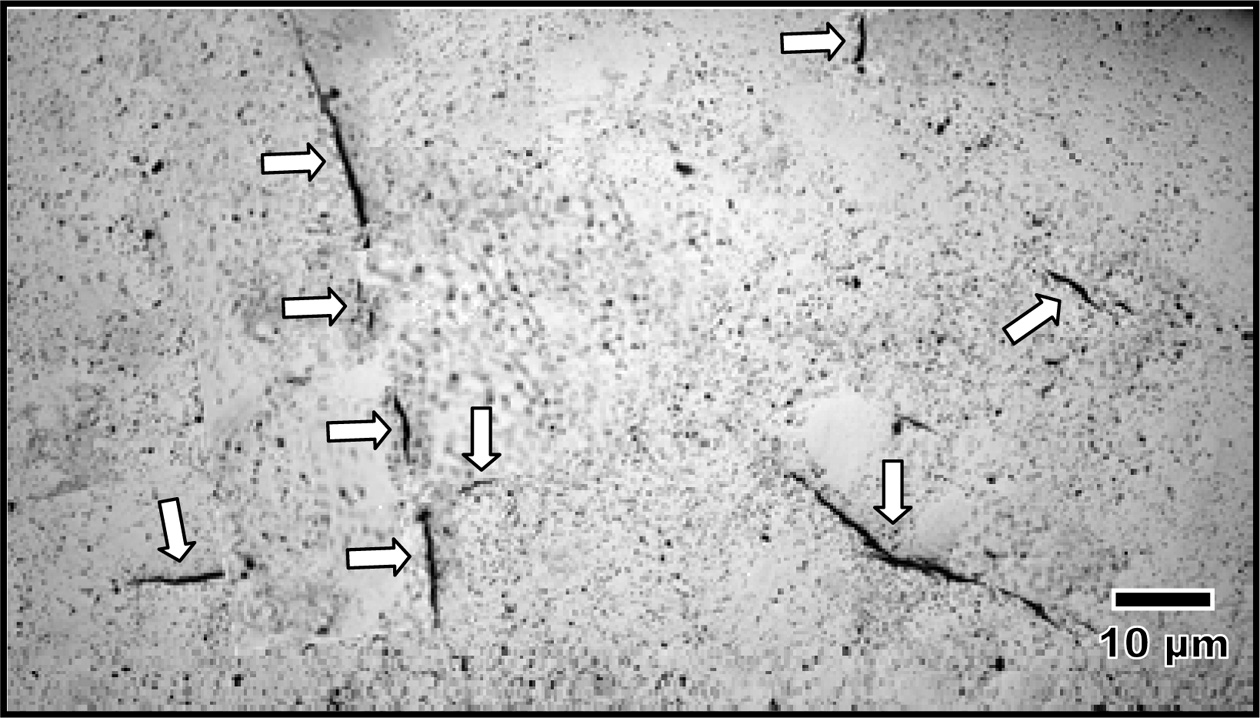
the XRD analysis. This tetragonal to monoclinic phase transformation was
accompanied by micro-and macro-cracking observed on the free surface of the
samples as typically shown in Fig. 3.
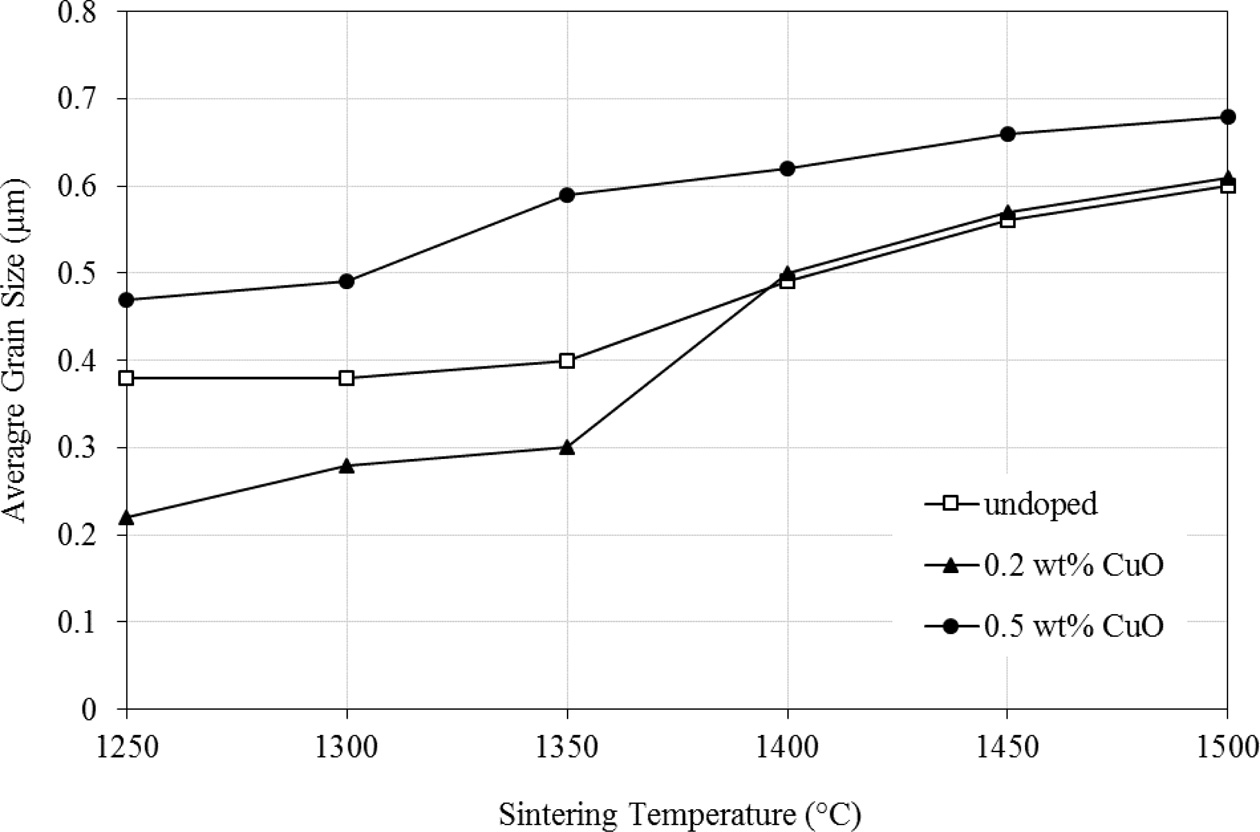
The effect of sintering and dopant addition on the average
grain size of Y-TZP is shown in Fig. 4. The general trend is that the grain
size increases with increasing sintering temperature for all samples. The
results showed that the 0.5 wt% CuO exhibited larger grain size when
compared to the 0.2 wt% CuO and undoped samples for all temperatures
investigated. The grain size of the 0.2 wt% CuO-doped zirconia was smaller
(below 0.3 µm) than the undoped when sintered at
1350 oC and below.
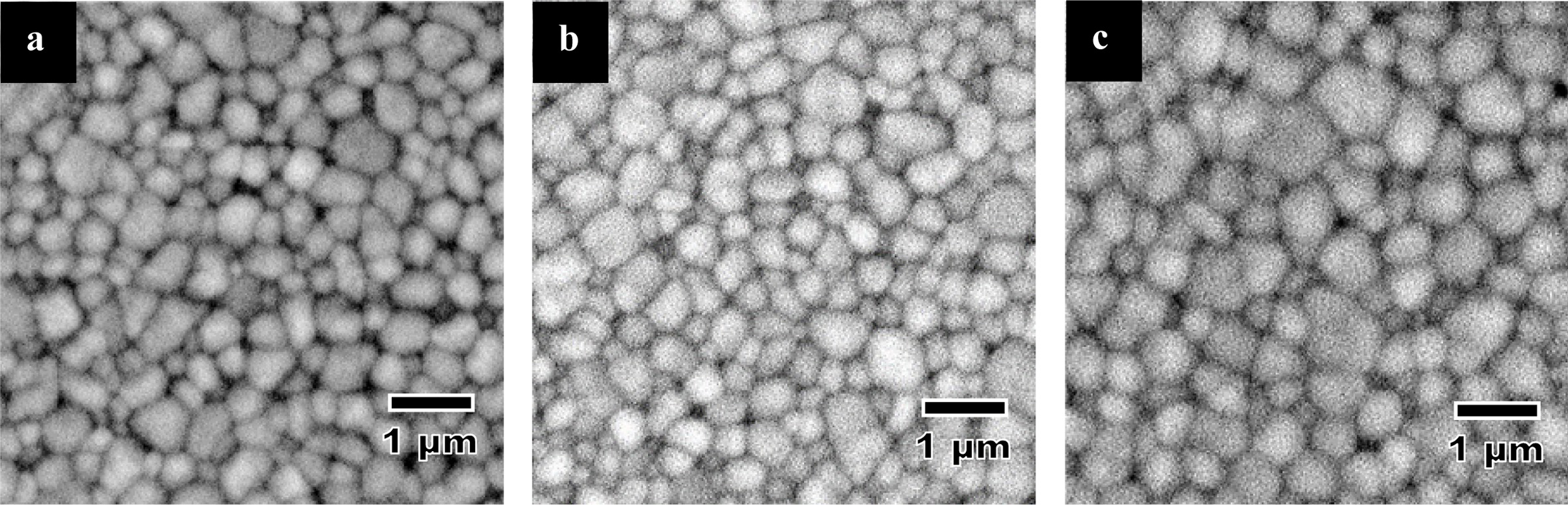
A typical SEM microstructure of the samples sintered
at 1450 oC is shown in Fig. 5. An equiaxed grain morphology was
observed for all samples regardless of dopant additions. The general
observation made was that the grain size increased with increasing CuO content.
This phenomenon of grain coarsening has also been noted by other researchers.
For instance, the effect of copper oxide doping on the electrical and
micro- structural properties of SnO2-based
varistors (SCNCr) was studied by Mahmoudi et al. [40]. The authors found
that the grain growth kinetics of the ceramic increased when the doping level
exceeded 0.25 mol%. This grain growth was accompanied by a reduction in
the activation energy from 594 kJ/mol (undoped) to 364 kJ/mol
(0.25 mol% CuO-doped). They suggested that a solute drag force was the
controlling mechanism of grain growth for samples with a low CuO content
(< 0.25 mol%) and this growth
mechanism changed to the Sn4+ solution-precipitation in CuO-rich
liquid phase for higher CuO doping. On the contrary, Kim et al. [49] who
studied the effect of CuO on the properties of (K,Na,Li)(Nb,Ta)O3 ceramics showed that the exaggerated grain growth observed for the CuO-doped ceramics
could be avoided by manipulating the sintering para- meters.
The authors initially performed normal sintering at temperatures above 900 oC and obtained an in inhomogeneous microstructures, consisting of a
bimodal distribution of small
(several microns) and exaggerated grains (20-40 µm). However, when a
two-step sintering (920-930 oC for 0 h in the first step and
880 oC for 1-12 h in the second step) was used, this
resulted in a homogeneous
fine-grained microstructure without com-promising on the piezoelectric properties of the
ceramics.
The variation in Vickers hardness as a function of
sintering temperature and dopant addition is shown in Fig. 6. The results show
that both the 0.2 wt% CuO-doped and undoped zirconia exhibited a similar
trend with increasing temperature. However, in all cases, the hardness of the
0.2 wt% CuO-doped was higher than the undoped ceramic and this agrees with
the density trend. The hardness of the 0.2 wt% CuO-doped zirconia
was > 13 GPa when sintered at 1350 oC and below.
Similar observation on the effect of CuO in enhancing the mechanical properties
of Sm-doped CeO2 ceramic was reported by Lu et al. [50]. The
researchers found that the addition of CuO lowers the densification
sintering temperature and enhances mechanical strength. They also
noted that the hardness dramatically increases from 4.9 ± 0.5 to
8.8 ± 0.4 GPa after doping with only 0.5 mol% CuO which was
attributed to a fracture transformation from intergranular to transgranular, as
well as enhanced density. In the present work, however, the hardness of the
0.5 wt% CuO was low regardless of sintering temperature. The sintered body
attained a maximum value of about 10 GPa when sintered at 1500 oC.
This was expected due to the lower bulk density as well as the high monoclinic
phase content measured in the sintered bodies as discussed earlier.
|
Fig. 1 XRD trace of Y-TZPs sintered at 1400 oC: (a) undoped, (b) 0.2 wt% CuO and (c) 0.5 wt% CuO. Note the shifting of the peaks resulting from copper doping. |
|
Fig. 2 The effect of sintering temperature on the relative density of Y-TZP. |
|
Fig. 3 Typical optical view of the free surface of the as-sintered 0.5 wt% CuO-doped Y-TZP revealing the presences of micro- and macro-cracks (as indicated by the arrows) associated with the tetragonal to monoclinic phase transformation upon cooling from sintering. |
|
Fig. 4 Average grain size variation as a function of sintering temperature and CuO doping. |
|
Fig. 5 Typical SEM microstructure of Y-TZPs sintered at 1400 oC: (a) undoped, (b) 0.2 wt% CuO and (c) 0.5 wt% CuO doped samples, respectively. |

|
Fig. 6 The effect of sintering temperature on the Vickers hardness of Y-TZPs. |
In this present work, the effect of small amounts of
transition metal oxide i.e. CuO addition as a sintering aid on the
densification and properties of Y-TZP ceramics were investigated. The results
revealed that the addition 0.2 wt% CuO was beneficial in aiding
densification with improved hardness when sintered at low temperatures, below
1350 oC when compared to the undoped ceramics. The tetragonal
phase stability was also not affected by the 0.2 wt% doping and the
sintered grain sizes were below 0.3 µm when sintered at lower temperature
regime. In contrast, the addition of 0.5 wt% CuO was found to be
detrimental as this excess liquid formation during sintering has disrupted the
tetragonal phase stability and resulted in lower hardness and low relative
density. This phase trans- formation
on cooling to room temperature was also accompanied by micro- and
macro-cracking thus rendering the material unfit for application.
Nevertheless, this study has shown the viability of aiding low
temperature sintering of Y-TZP through doping with low amounts of copper oxide.
- 1. R.C. Garvie, R.H. Hannink, and R.T. Pascoe, Nature 258 (1975) 703-704.
- 2. I. Birkby, and H. Hodgson, Proceedings of the 3rd European Symposium on Engineering Ceramics, edited by F.L. Riley (Elsevier Applied Science, Ltd., 1991) p.167.
- 3. M. Xue, S. Liu, X. Wang, and K. Jiang, Mater. Chem. Phys. 244 (2020) 122693.
-

- 4. K. Kobayashi, H. Kuwajima, and T. Masaki, Solid State Ionics 3-4 (1981) 489-493.
-

- 5. S. Ramesh, and C. Gill, Ceram. Int. 27 (2001) 705-711.
-

- 6. J. Chevalier, S. Deville, E. Münch, R. Jullian, and F. Lair, Biomater. 25 (2004) 5539-5545.
-

- 7. S. Ramesh, K.Y. Sara Lee, and C.Y. Tan, Ceram. Inter. 44 (2018) 20620-20634.
-

- 8. M. Cattani-Lorente, S. Durual, M. Amez-Droz, H.W.A. Wiskott, and S.S. Scherrer, Dent. Mater. 32 (2016) 394-402.
-

- 9. T. Sato, and M. Shimada, J. Am. Ceram. Soc., 68 (1985) 356-356.
-

- 10. G.K.R. Pereira, M. Amaral, P.F. Cesar, M.C. Bottino, C.J. Kleverlaan, and L.F. Valandro, J. Mech. Behav. Biomed. Mater. 45 (2015) 183-192.
-

- 11. H.Y. Lu, H.Y. Lin, and S.Y. Chen, Ceram Int, 13 (1987) 207-214.
-

- 12. S. Lawson, and P.A. Smith, J. Am. Ceram. Soc. 76 (1993) 3170-3172.
-

- 13. L. Gremillard, J. Chevalier, T. Epicier, S. Deville, and G. Fantozzi, J. Eur. Ceram. Soc. 24 (2004) 3483-3489.
-

- 14. M. Cattani-Lorente, S.S. Scherrer, P. Ammann, M. Jobin, and H.W.A. Wiskott, Acta Biomater. 7 (2011) 858-865.
-

- 15. M. Keuper, K. Eder, C. Berthold, and K.G. Nickel, Acta Biomater. 9 (2013) 4826-4835.
-

- 16. C.H. Ting, S. Ramesh, N. Lwin and U. Sutharsini, J. Ceram. Proc. Res. 17 (2016) 1265-1269.
- 17. L. Hallmann, P. Ulmer, E. Reusser, M. Louvel, and C.H.F. Hämmerle, J. Eur. Ceram. Soc. 32 (2012) 4091-4104.
-

- 18. S.M. Kwa, S. Ramesh, L.T. Bang, Y.H. Wong, W.J. Kelvin Chew, C.Y. Tan, J. Purbolaksono, H. Misran, and W.D. Teng, J. Ceram Proc. Res. 16 (2015) 193-198.
- 19. F. Zhang, K. Vanmeensel, M. Inokoshi, M. Batuk, J. Hadermann, B. Van Meerbeek, I. Naert, and J. Vleugels, J. Eur. Ceram. Soc. 34 (2014) 2453-2463.
-

- 20. K. Eltayeb, W. Hong, F. Chen, Y.-H. Han, Q. Shen, and L. Zhang, J. Ceram. Proc. Res. 18 (2017) 1-9.
- 21. X. Hu, X. Jiang, S. Chen, Q. Zhu, M. Feng, P. Zhang, J. Fan, B. Jiang, X. Mao, and L. Zhang, Ceram Int. 44 (2018) 2093-2097.
-

- 22. N. Obradović, and F. Kern, Ceram. Int. 44 (2018) 16931-16936.
-

- 23. H. Guo, T.J.M. Bayer, J. Guo, A. Baker, and C.A. Randall, Scripta Materialia 136 (2017) 141-148
-

- 24. Y. Wang, H. Huang, L. Gao, and F. Zhang, J. Ceram. Proc. Res. 12 (2011) 473-476.
- 25. S. Ramesh, N. Zulkifli, C.Y. Tan, Y.H. Wong, F. Tarlochan, S. Ramesh, W.D. Teng, I.Sopyan, L.T. Bang, and A.A.D. Sarhan, Ceram. Int. 44 (2018) 8922-8927.
-

- 26. F. Chen, J.-M. Wu, H.-Q. Wu, Y. Chen, C.-H. Li, and Y.-S. Shi, Int. J. Lightweight Mater. Manuf. 1 (2018) 239-245.
- 27. Z. Wu, N. Li, C. Jian, W. Zhao, and J. Yan, Ceram. Int. 39 (2013) 7199-7204.
-

- 28. S. Ramesh, S. Meenaloshini, C.Y. Tan, W.J.K. Chew, and W.D. Teng, Ceram. Int. 34 (2008) 1603-1608.
-

- 29. L. Holz, J. Macias, N. Vitorino, A.J.S. Fernandes, F.M. Costa, and M.M. Almeida, Ceram. Int. 44 (2018) 17962-17971.
-

- 30. A. Samodurova, A. Kocjan, M.V. Swain, and T. Kosmač, Acta Biomater. 11 (2015) 477-487.
-

- 31. Z. Feng, J. Qi, X. Guo, Y. Wang, X. Cao, Y. Yu, C. Meng, and T. Lu, J. Alloys Comp. 787 (2019) 254-259.
-

- 32. S.S. Mishra, D. Chaira, and S.K. Karak, Ceram. Int. 45 (2019) 20555-20565.
-

- 33. L. Fu, H. Engqvist, and W. Xia, J. Eur. Ceram. Soc. 38 (2018) 2110-2119.
-

- 34. H. Toraya, M. Yoshimura, and S. Somiya, J. Am. Ceram. Soc. 67 (1984) C-119-C-121.
- 35. M.I. Mendelson, J. Am. Ceram. Soc. 52 (1969) 443-446.
-

- 36. L. Lemaire, S. M. Scholz, P. Bowen, J. Dutta, H. Hofmeister, and H. Hofmann, J. Mater. Sci. 34 (1999) 2207-2215.
-

- 37. M.M. Khan, S. Ramesh, L.T. Bang, Y.H. Wong, S. Ubenthiran, C.Y. Tan, J. Purbolaksono, and H. Misran, J. Mater. Eng. and Perform. 23 (2014) 4328-4335.
-

- 38. H.R. Pasaribu K.M. Reuver, D.J. Schipper, S. Ran, K.W. Wiratha, A.J.A. Winnubst, and D.H.A. Blank, Inter. J. Refractory Metals & Hard Mater. 23 (2005) 386-390.
-

- 39. A.A. Menaze, A.M. Mostafa, and E.A. Al-Ashkar, Optical Mater. 100 (2020) 109663.
-

- 40. P. Mahmoudi, A. Nemati, and M.M. Shahraki, J. Alloys Comp. 770 (2019) 784-791.
-

- 41. B.H. Watson III, M.J. Brova, Y. Chang, S.T. Misture, M.A. Fanton, R.J. Meyer Jr., and G.L. Messing, J. Eur. Ceram. Soc. 39 (2019) 4719-4726.
-

- 42. S. Shimada, K. Kodaira, and T. Matsushita, J. Mater. Sci. 19 (1984) 1385-1390.
-

- 43. D.L. Corker, R.W. Whatmore, E. Ringgaard, and W.W. Wolny, J. Eur. Ceram. Soc. 20 (2000) 2039-2045.
-

- 44. Y.H. Kim, H. Ryu, Y.-K. Cho, H.-J. Lee, and S. Nahm, J. Am. Ceram. Soc. 96 (2013) 312-317.
-

- 45. T. Zhang, P. Hing, H. Huang, and J. Kilner, Mater. Lett. 57 (2002) 507-512.
-

- 46. N. Kimura, S. Abe, Y. Hayashi, J. Morishita, and H. Okamura, Sprechsaal 122 (1989) 341-343.
- 47. Y. Sakka, T. Ishii, T.S. Suzuki, K. Morita, and K. Hiraga, J. Eur. Ceram. Soc., 24 (2004) 449-453.
-

- 48. J. Nie, J.M. Chan, M. Qin, N. Zhou, and J. Luo, Acta Materialia 130 (2017) 329-338.
-

- 49. J.H. Kim, D.S. Kim, S.H. Han, H-W. Kang, H.-G. Lee, J.S. Kim, and C.I. Cheon, Mater. Letts. 241 (2019) 202-205.
- 50. Q. Lü, X. Dong, Z. Zhu, and Y. Dong, Ceram. Int. 40 (2014) 15545-15550.
-

 This Article
This Article
-
2020; 21(4): 495-500
Published on Aug 30, 2020
- 10.36410/jcpr.2020.21.4.495
- Received on Mar 2, 2020
- Revised on Apr 7, 2020
- Accepted on Apr 14, 2020
 Services
Services
Shared
 Correspondence to
Correspondence to
- K.Y. Sara Lee
-
Tunku Abdul Rahman University College, Faculty of Engineering & Technology, Department of Mechanical Engineering, 53300, Kuala Lumpur, Malaysia
Tel : +(6)03-41450123
Fax: +(6)03-41423166 - E-mail: leeky@tarc.edu.my






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.