- Synthesis and properties of ultrafine YAG powder via low-temperature microwave hydrothermal method
Yue Liua,b, Jieguang Songa,b,*, Lin Chenb,*, Huihui Luob, Guojian Lina, Peng Chenb, Chenhui Weia and Jingjing Liua
aSponge City Research Institute of Pingxiang University, Pingxiang 337055 China
bKey Laboratory for Industrial Ceramics of Jiangxi Province, Pingxiang University, Pingxiang 337055 China
Yttrium aluminum garnet (YAG)
has good optical properties and has been widely used in engineering. The
preparation of conventional YAG powder is costly due to its high synthesis
temperature, so lowering the synthesis temperature is the most effective way to
save cost. The ultrafine oxide composite powder was rapidly synthesized using
the microwave hydrothermal method at a low temperature. The effect of
temperature and its performance. Differential thermal analysis and XRD phase
analysis showed the mixed solution of Al(NO3)3 and Y(NO3)3
at pH=9 and C(Al3+)=0.42 mol·L−1. SEM, zeta
potential, and particle size analysis revealed that the microwave hydrothermal
reaction temperature was 170 oC, and the high-purity ultrafine
YAG powder was calcined at 928 oC, which was lower than
conventional calcination temperature. The synthesized ultrafine spherical YAG
powder had a small particle size and a uniform distribution.
Keywords: Ultrafine YAG powder, Synthesis temperature, Composite powder, Microwave hydrothermal method
Ceramics, as an important part of industrial ceramics, has
been extensively investigated because of its high-temperature performance. The
most common ones are Al2O3 ceramics, SiC ceramics, and Si3N4
ceramics [1-3], but the sintering temperature of several ceramics is usually
high, accompanied by high cost, long preparation period,
and limited existing research. YAG is a complex oxide
formed by the reaction of Y2O3 and Al2O3,
which belongs to the cubic system and has a garnet structure [4]. YAG ceramics
have excellent high-temperature mechanical properties and chemical stability.
YAG powder, which is the main material of YAG ceramics, has been widely
explored. In optics, YAG is the best choice for the selection of laser matrix
materials [5-6].
YAG is prepared mainly through hydrothermal, coprecipitation,
mixed calcination, and sol-gel methods, et al. [7-10]. These
synthetic methods have their own advantages and disadvantages. Although the
cycle of ultrafine YAG powder preparation via coprecipitation is short, it
results in large locally precipitated particles because of the influence of
diffusion time when a precipitant is added during
precipitation. The hydrothermal method yields ultrafine YAG powder
with a small particle size and a uniform distribution, but the pre- paration cycle is longer and the error is
larger than those of other methods.
Coprecipitation and hydrothermal methods result in YAG
powder with good dispersibility. However, in comparison with coprecipitation,
the hydrothermal method has the advantages of yielding YAG powder with a small
particle size and a uniform distribution, but the preparation cycle is long and
costly [11-12]. Therefore, finding a method with a short period, low cost, and
excellent powder performance is the current research direction concerning
ultrafine YAG powder preparation. In terms of the shortcomings of the
hydrothermal method, this study adopts the microwave hydrothermal method to
help prepare ultrafine YAG powder. This method results in ultrafine YAG powder
with a small particle size, a uniform distribution, and a considerably
shortened preparation cycle [13-15].
In this paper, ultrafine YAG powder was prepared through
microwave hydrothermal synthesis technology with a considerably shorter
preparation cycle than the traditional hydrothermal method, and the process conditions
in the YAG ultrafine powder synthesis process were
started. The lowest synthesis temperature in the range was used to analyze the
variables and determine the optimum conditions for the lowest synthesis
tem- perature of
ultrafine YAG powder to reduce the sintering temperature
and cost of YAG ceramics. The findings are also the innovation of this paper.
The main raw materials were aluminum nitrate Al(NO3)·9H2O
(analytical grade), yttrium nitrate Y(NO3)· 6H2O
(analytical grade), and ammonia water. Analytically pure Al(NO3)3·9H2O
(purity: 99.99%) was weighed with a beaker, deionized, dissolved,
and stirred thoroughly with a magnetic stirrer to obtain
Al (NO3)3 at a concen-
tration of 2.0826 mol·L−1. The pure Y(NO3)3·6H2O
(purity: 99.99%) solid was weighed into a beaker, dissolved in deionized water,
and fully dissolved by stirring with a magnetic stirrer to obtain an Y(NO3)3
solution with a concentration of 2.4866 mol·L−1. The precursors
of YAG, Al(OH)3, and Y(OH)3 were obtained
by adjusting the pH of the mixed solution of Al(NO3)3-Y(NO3)3
by using the aqueous ammonia solution to carry out precipitation reaction and
obtain Al(OH)3 and Y(OH)3. Different concentrations of
Al(NO3)3 and Y(NO3)3 mixed
solutions were prepared according to a Y:Al ratio of 3:5 and adjusted to
different pH values by using a pH-adjustable aqueous ammonia solution. The
mixed precipitates of the YAG precursors Al(OH)3 and Y(OH)3
obtained after precipitation were subjected to microwave hydrothermal
decomposition by using a microwave hydrothermal synthesizer to obtain a mixed
precipitate of Al2O3-Y2O3.
Thereafter, the obtained mixed precipitate was filtered to
obtain white composite powder that was then subjected to
differential thermal analysis. The temperature of the YAG crystal phase was
determined using a DSC-TG curve via a STA 449 F3-type comprehensive thermal analyzer
(NETZSCH, Germany) and calcined in a programmed furnace to analyze the
temperature. After calcination, the phase was conducted by using a D8 Advance
X-ray powder diffractometer (Bruker, Germany). Surface morphology and particle
size (D50) were examined using a Zetasizer Nano Zeta
potential and particle size analyzer (Malvern, UK),
respectively.
Effect
of pH on the properties of YAG ultrafine powder
The prepared mixed solution of C(Al3+)=0.42 mol·
L−1 and C(Y3+)=0.25 mol·L−1 was adjusted
to different pH values. The microwave hydrothermal temperature was 180 oC
and the holding time was 5 min (Table 1). White precipitated powder was
filtered and dried to obtain a composite powder.
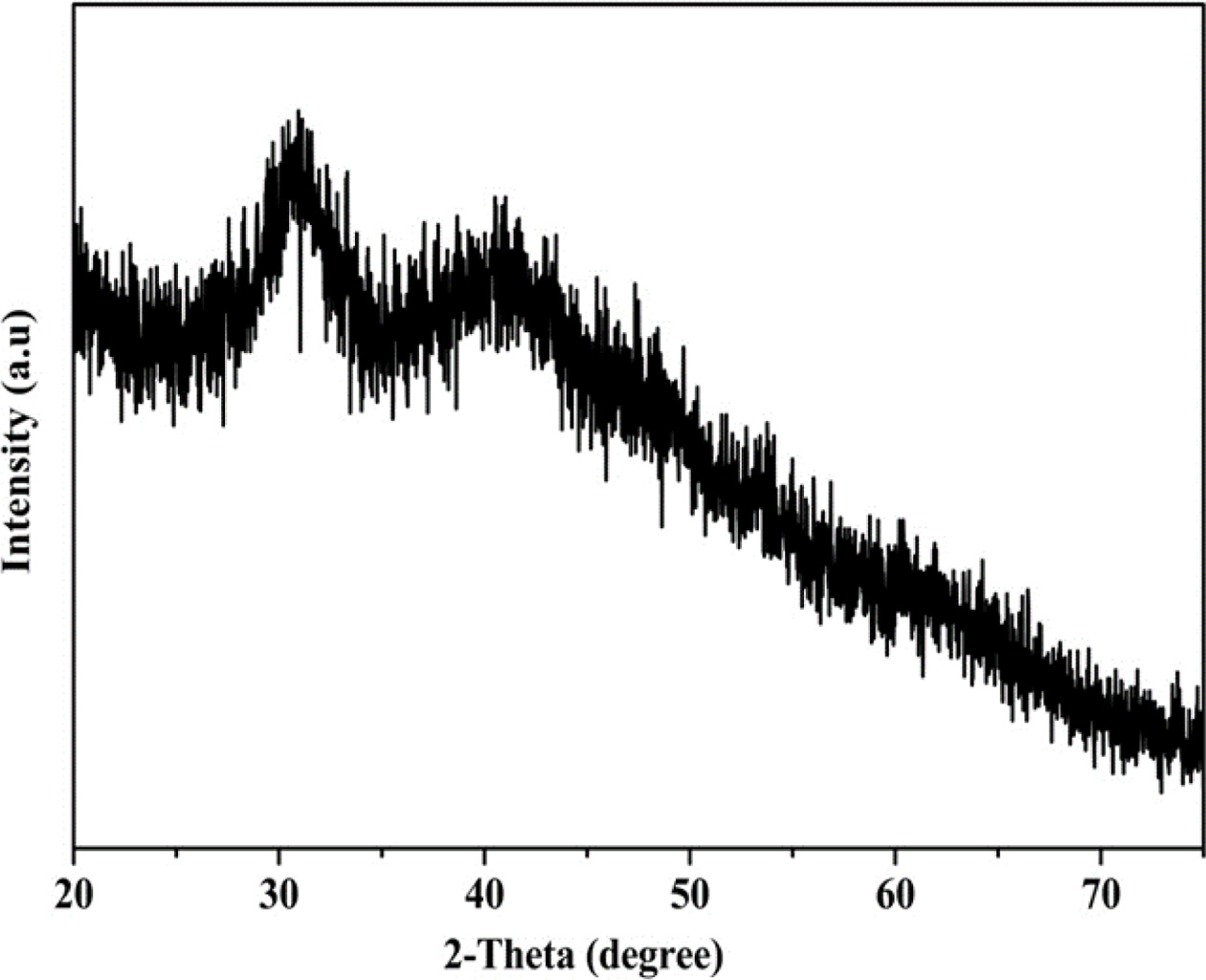
Fig. 1 shows the XRD pattern of the YAG precursor in a Cu
target with a source of Ka-ray (λ = 1.5405 nm) and a scan range
of 2θ from 10° to 80°. The XRD pattern of the YAG precursor after thermal
decom- position by microwave. The YAG
precursor did not decompose into an oxide at a hydrothermal temperature of
180 oC (Fig. 1).
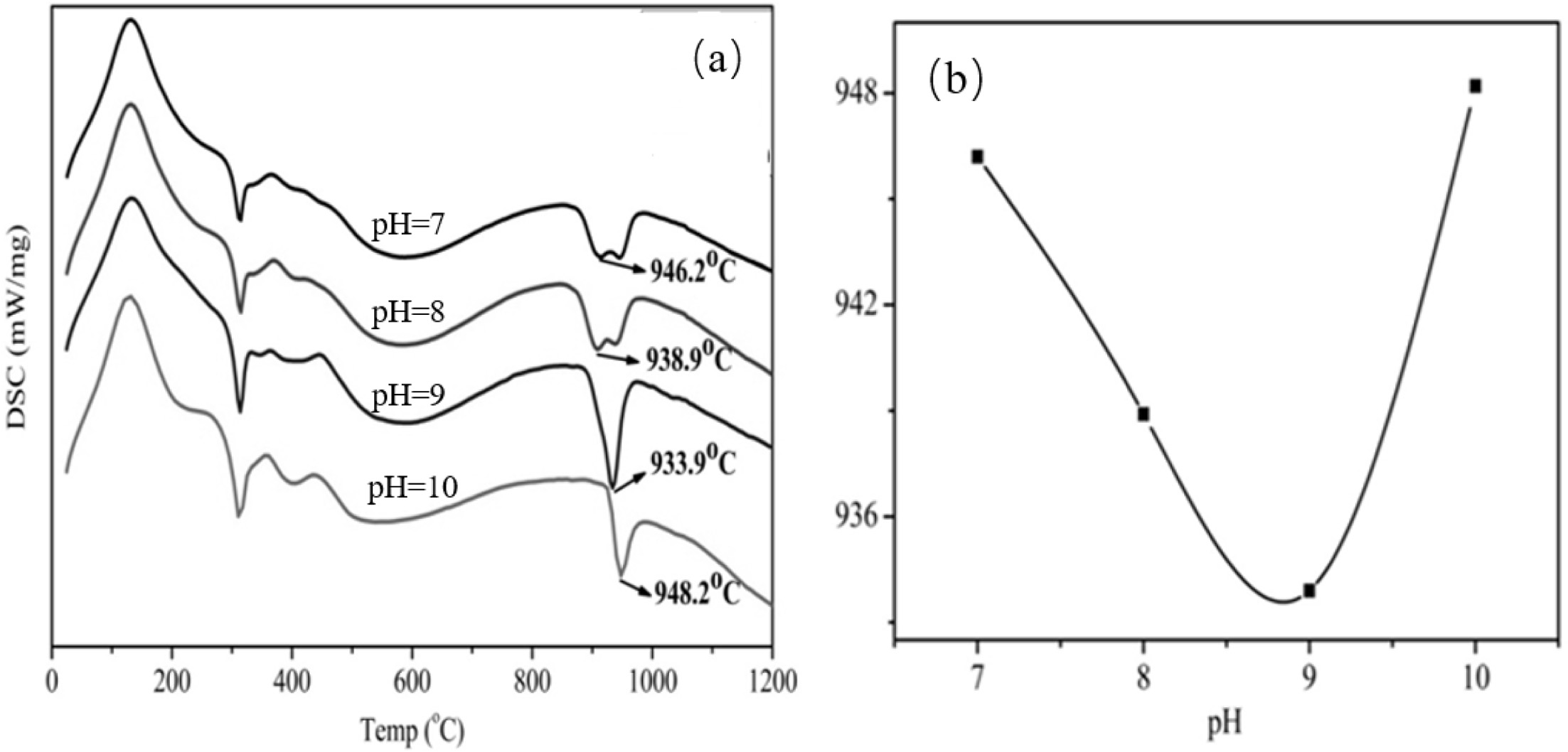
Fig. 2a is a DSC curve of YAG precursor after microwave
hydrothermal decomposition in an air atmosphere at a heating rate of
15 K/min from 25 oC to 1200 oC.
An endothermic peak appeared at approximately 150 oC,
and this observation was caused by the dehydra- tion
decomposition reaction of Y and Al hydroxides and the evaporation of adsorbed
water. An exotherm occurred at approximately 940 oC. The peak
did not lose weight because of the crystallization of the amorphous
powder into the YAG phase. Fig. 2b illustrates the
synthesis temperature and pH of YAG obtained by preparing the ultrafine YAG
powder under different pH conditions. The relationship curve shows that the
tem- perature of the YAG crystal phase
initially decreases and then increases as the pH of the mixed mother salt
solution increases. At pH=9, the temperature of the YAG crystal phase is
relatively low, and the tem- perature
is 933.9 oC.
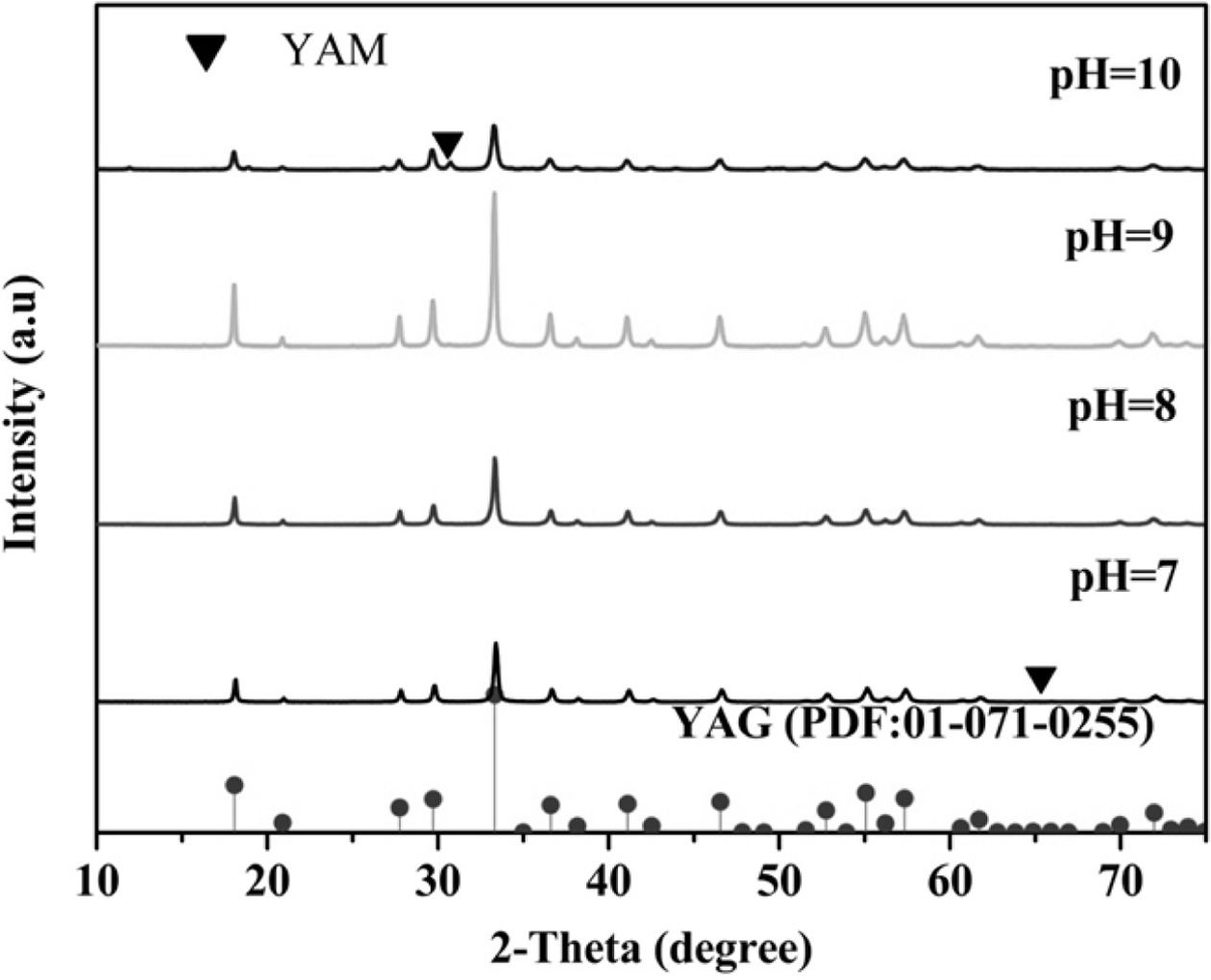
Fig. 3 is the XRD pattern of the YAG precursor after
calcination. At pH=8 and 9, pure cubic phase ultrafine YAG powder
can be synthesized, but the low calcination temperature
is observed at pH=9. ultrafine YAG powder and high
crystallinity. At this time, Y:Al=3:5 may result in the lower potential energy
of YAG synthesis and lower synthesis temperature. When pH=10, the intermediate
phase Y4Al2O9 (YAM) appears, and Y:Al= 2:1
mainly because a certain content of AlO2− is formed at a high
concentration of OH− so that the ratio of Y:Al is greater than 1,
leading to the appearance of the intermediate YAM phase of YAG
during calcination. When pH=7, the YAM phase appears because of the low
concentration of OH−, and Al3+ cannot be completely precipitated,
so the Al(NO3)3-Y(NO3)3 mixed salt
solution is not completely converted into hydroxide precipitate
[16-18]. The ratio of Y:Al does not reach 3:5, resulting in the occurrence of
the intermediate YAM phase of YAG during calcination. At pH=9 of the mixed
mother salt solution, the pure cubic phase ultrafine YAG powder
was synthesized at a low temperature (933.9 oC) under the
condition of Y:Al=3:5.
Effect
of Concentration on the Properties of YAG Ultrafine Powder
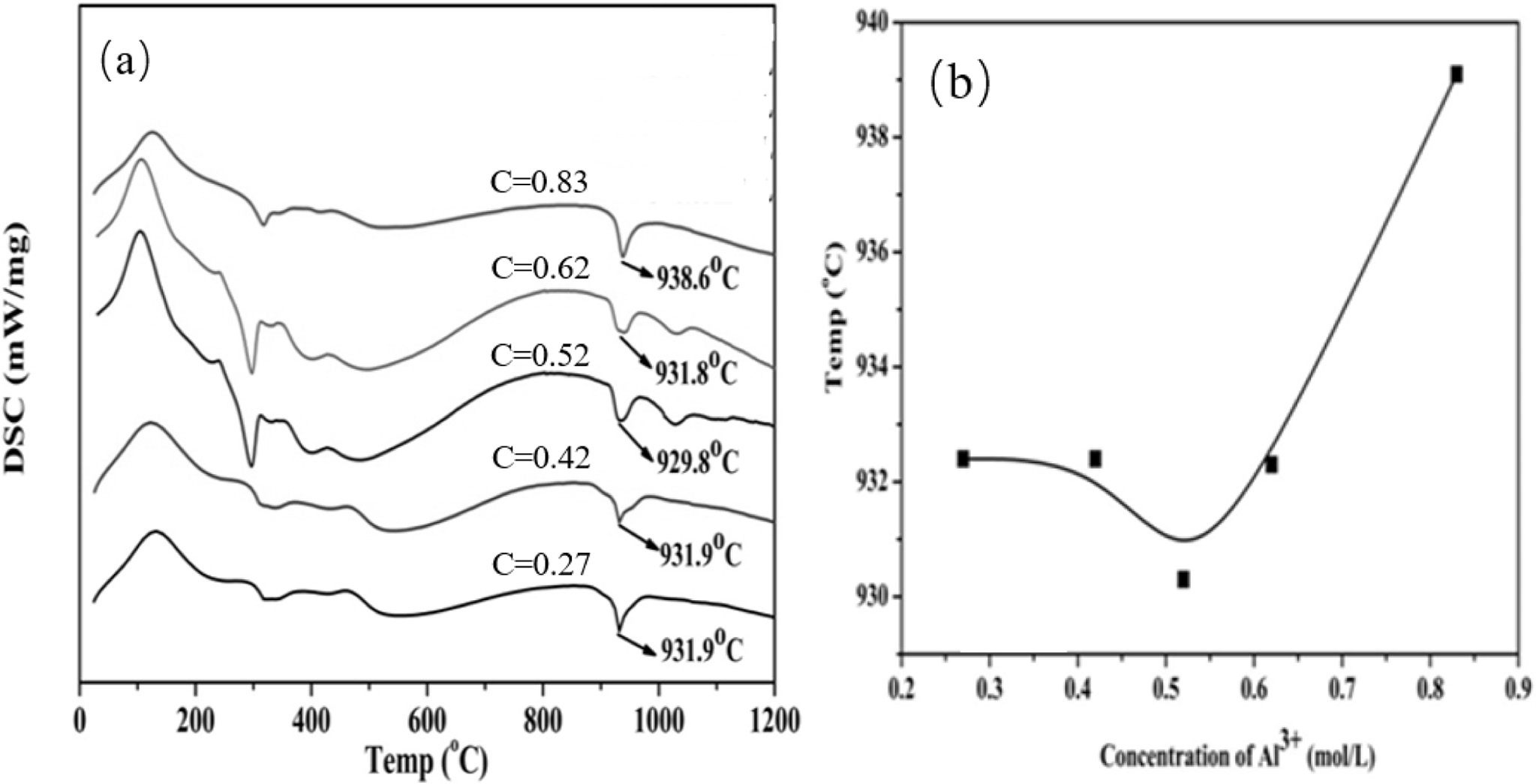
Fig. 4a shows the DSC curve of the synthetic powder.
An endothermic peak appears at approximately 150 oC, and this
peak is caused by the decomposition of the hydroxide of Y and Al and the
adsorbed water. An exothermic peak appears at approximately 940 oC,
and no loss of weight is observed because of the crystalli- zation of the amorphous powder into the YAG
phase. Fig. 4b shows the synthesis temperature of YAG and the Al3+
concentration in the mixed parent salt solution. The relationship curve (Fig.
4b) illustrates that the temperature of the YAG crystal phase
initially decreases and then increases as the concentration of Al3+
in the mixed mother salt solution increases. At 0.52 mol·L−1 concentration,
the YAG crystal phase is produced at a lower temperature, and the temperature
is 929.8 oC.
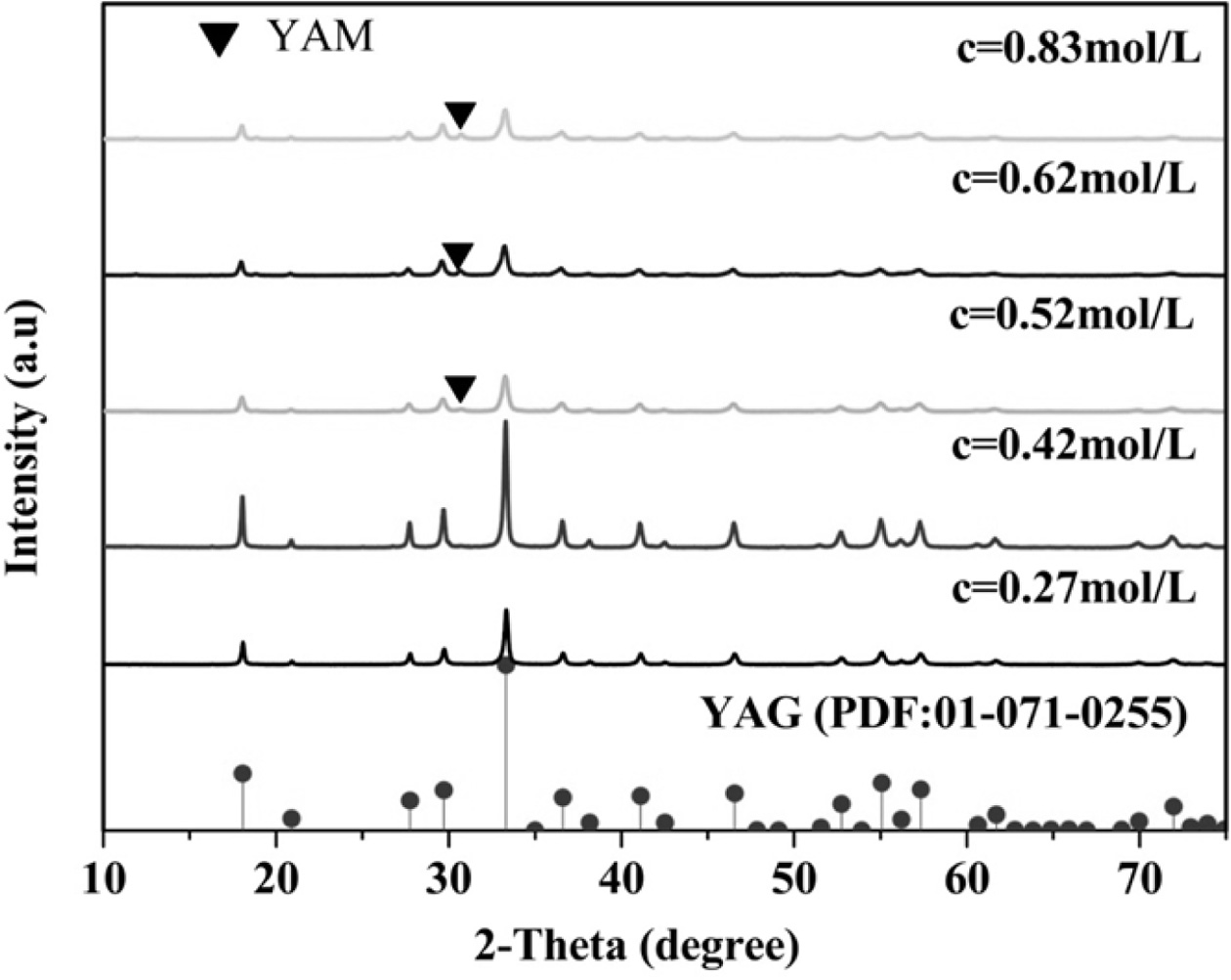
Fig. 5 shows the XRD pattern of the YAG precursor after
calcination. When the Al3+ concentration in the mixed salt solution
is less than 0.52 mol·L−1, a pure cubic-phase ultrafine YAG
powder can be produced, and the Al3+ concentration in the mixed salt
solution is 0.42 mol·L−1, which is synthesized. The ultrafine
YAG powder has a strong peak and a high degree of crystallinity. When the
concentration of Al3+ in the mixed salt solution is greater than or
equal to 0.52 mol·L−1, the YAM phase is produced, and the synthesis
temperature of the ultrafine YAG powder increases as the concentrations
increase. In the reaction of a dilute solution system, Al3+
precipitates earlier than Y3+ does because Ksp of the solubility
products Y3+ and Al3+ is different. However, the formed
Al(OH)3 cannot be nucleated, making Y3+ precipitate in
the outer layer of Al3+ to form a unique inclusion structure in high
con- centration systems. This
phenomenon may be due to the incomplete conversion of Al3+ to Al(OH)3
precipi- tation or AlO2−,
resulting in the precipitation of Al(OH)3 in the
solution [19-21]. Less, eventually Y:Al=2:1, and the YAM phase
is produced after calcination. Therefore, under the
same conditions, when the concentration of Al3+ in the mixed salt
solution is 0.42 mol·L−1, a pure cubic-phase ultrafine YAG
powder can be synthesized at a calcination temperature of 931.9 oC.
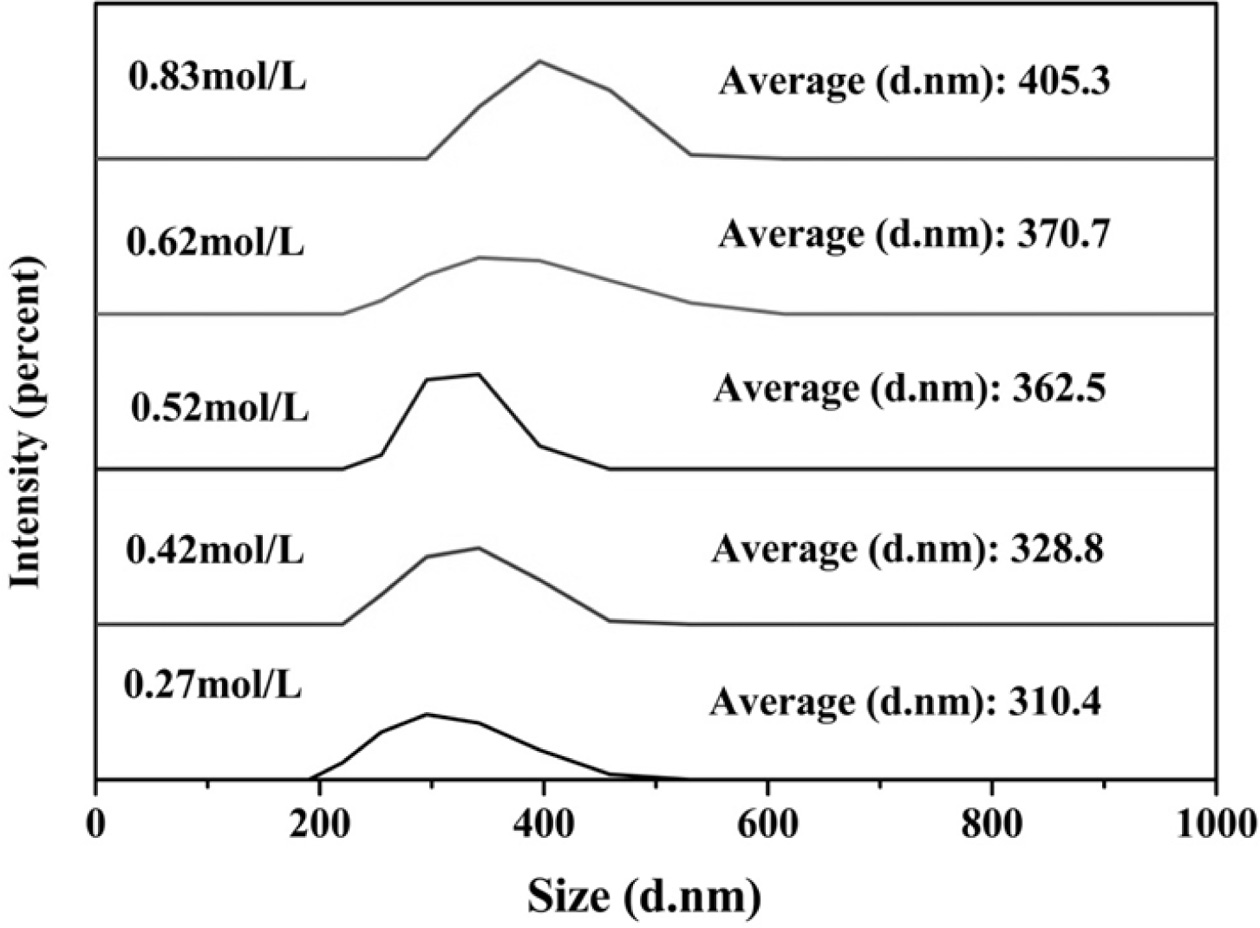
Fig. 6 is a comparison of the particle size of the
ultrafine YAG powder prepared at different Al3+ concentrations. Fig.
6 shows that the particle size of the prepared ultrafine YAG powder also
increases as the concentration of the reaction mother salt solution increases.
Thus, the higher the concentration is, the higher the formation temperature of
the YAG crystal phase will be.
Fig. 7 shows the XRD pattern of the YAG precursor after
microwave hydrothermal decomposition. YAG precursors have crystal phases of γ-Al2O3
and Y2O3 under the experimental parameters of the
microwave hydrothermal temperatures of 190 oC and 200 oC
and the holding time of 5 min, respectively.
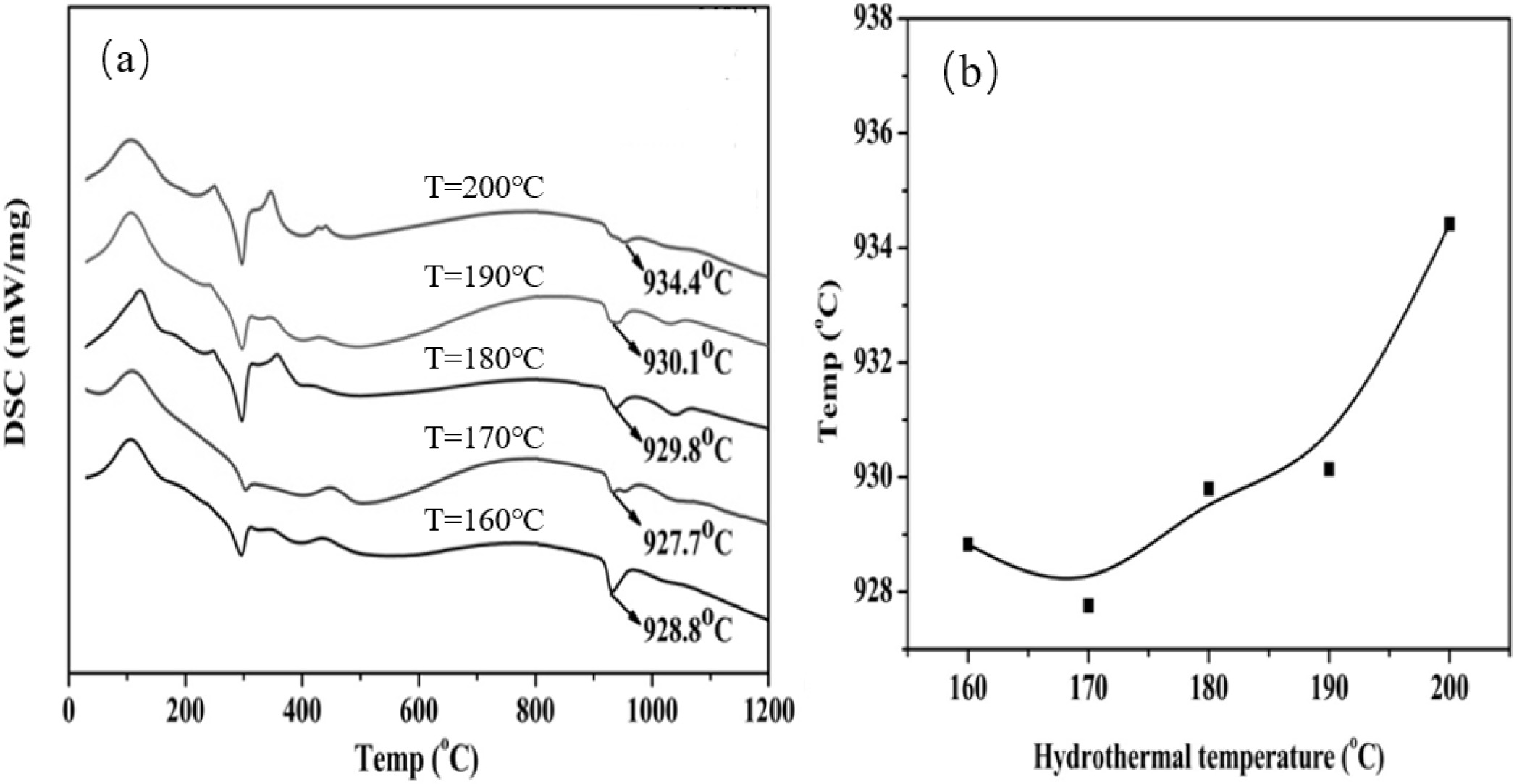
Fig. 8a is the DSC curve of the powder. An endothermic
peak appears at about 150 oC because of the decomposition of
the hydroxide of Y and Al and the evaporation of the adsorbed water. An
exothermic peak appears at approximately 940 oC, and no weight
loss is observed because of the crystallization of amorphous powder into the
YAG phase. Fig. 8b shows the relationship between the synthesis temperature of
YAG and the temperature of microwave hydrothermal reaction. When the
temperature of microwave hydro- thermal
reaction is 170 oC, the YAG crystal phase produces a low
temperature of 927.7 oC.
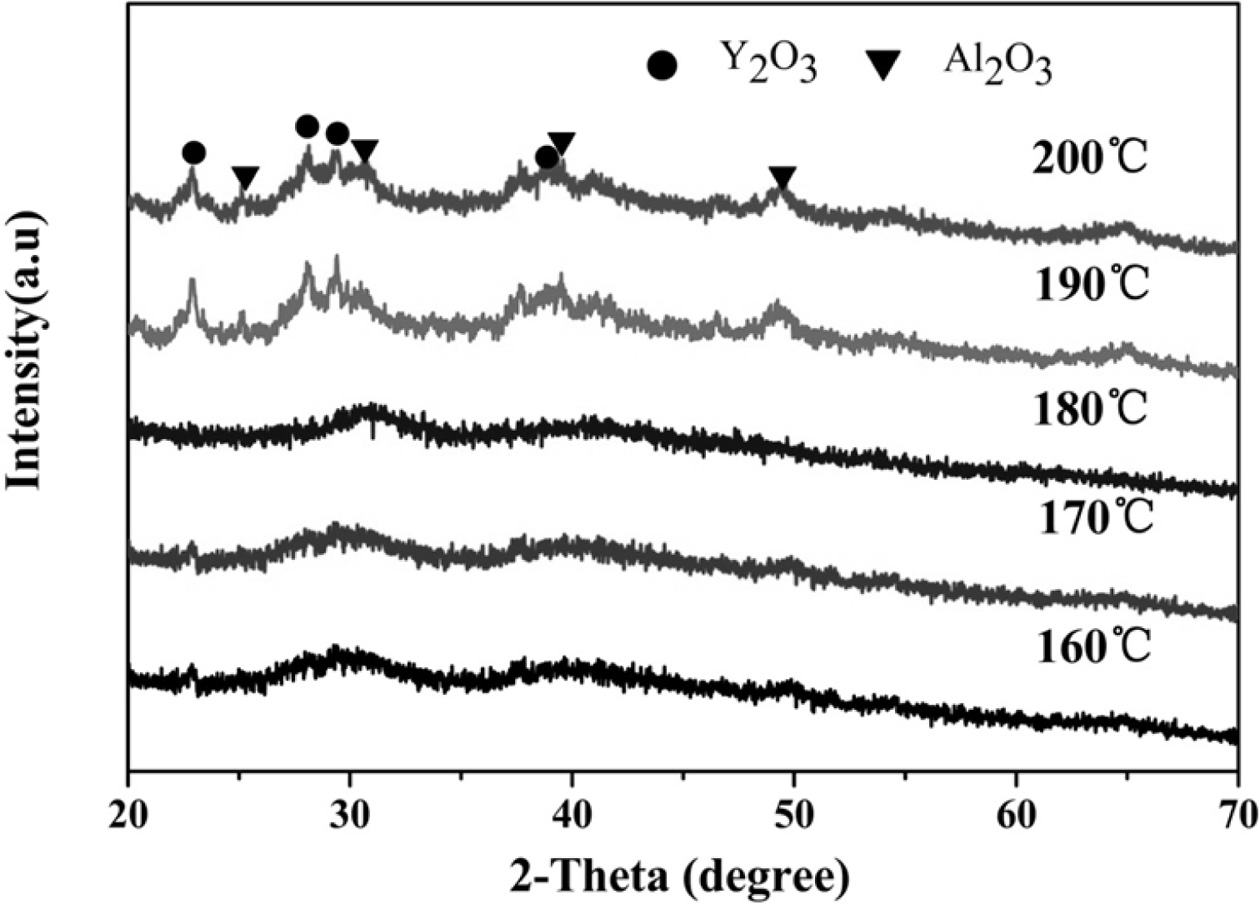
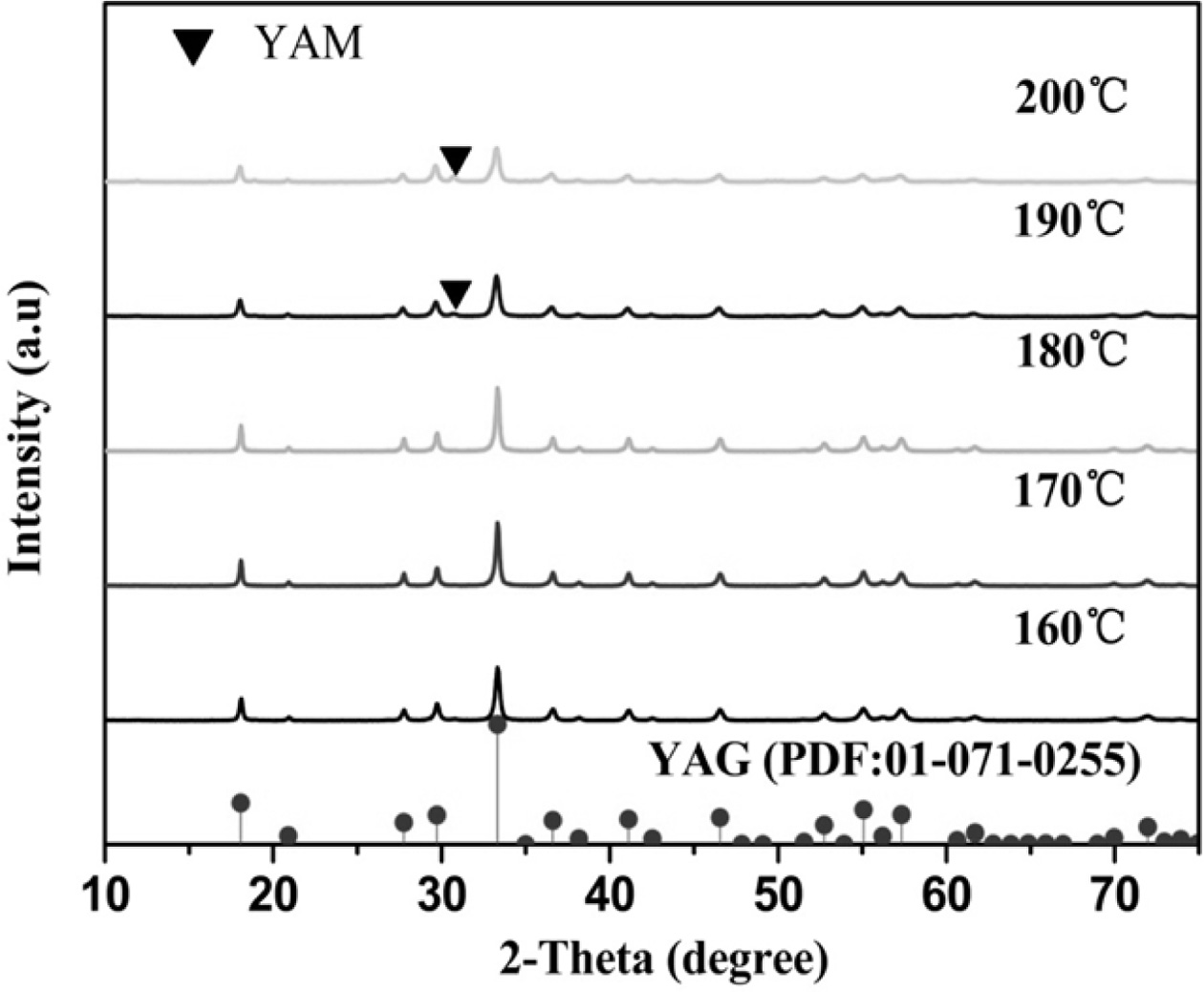
Fig. 9 illustrates the XRD pattern of the YAG precursor
after calcination via microwave hydrothermal decomposition. The
intermediate-phase YAM of YAG occurs when the hydrothermal temperatures are
190 oC and 200 oC, and only the pure
cubic-phase ultrafine YAG powder can be produced at the hydrothermal
temperatures of 170 oC and 180 oC,
respectively, and the water is hot. The ultrafine YAG powder prepared at 170 oC
has a lower synthesis temperature and a calcination temperature
of only 927.7 oC. Therefore, at 170 oC, it can
provide the energy required for the decomposition of the YAG precursor
hydroxide precipitate into the corresponding oxide to form a crystal phase
[22-24]. As the temperature continues to increase, the energy also increases,
so the formed grains grow and the particle size becomes coarse.
Consequently, the calcination temperature of the ultrafine YAG
powder further increases. When the hydrothermal temperatures are 190 oC
and 200 oC, the intermediate-phase YAM of YAG appears because
the crystal phases of Al2O3 and Y2O3
appear in microwave hydrothermal reaction in the YAG precursor. Large amounts
of Al2O3 and Y2O3 decompose, and
solid phase reaction is formed during calcination. The intermediate-phase YAM
of YAG inevitably occurs because the solid phase reaction in YAG is gradual.
Under other conditions, at the micro-
wave water temperature of 170 oC, a pure cubic-phase
ultrafine YAG powder can be synthesized at a low calcination temperature
(927.7 oC).
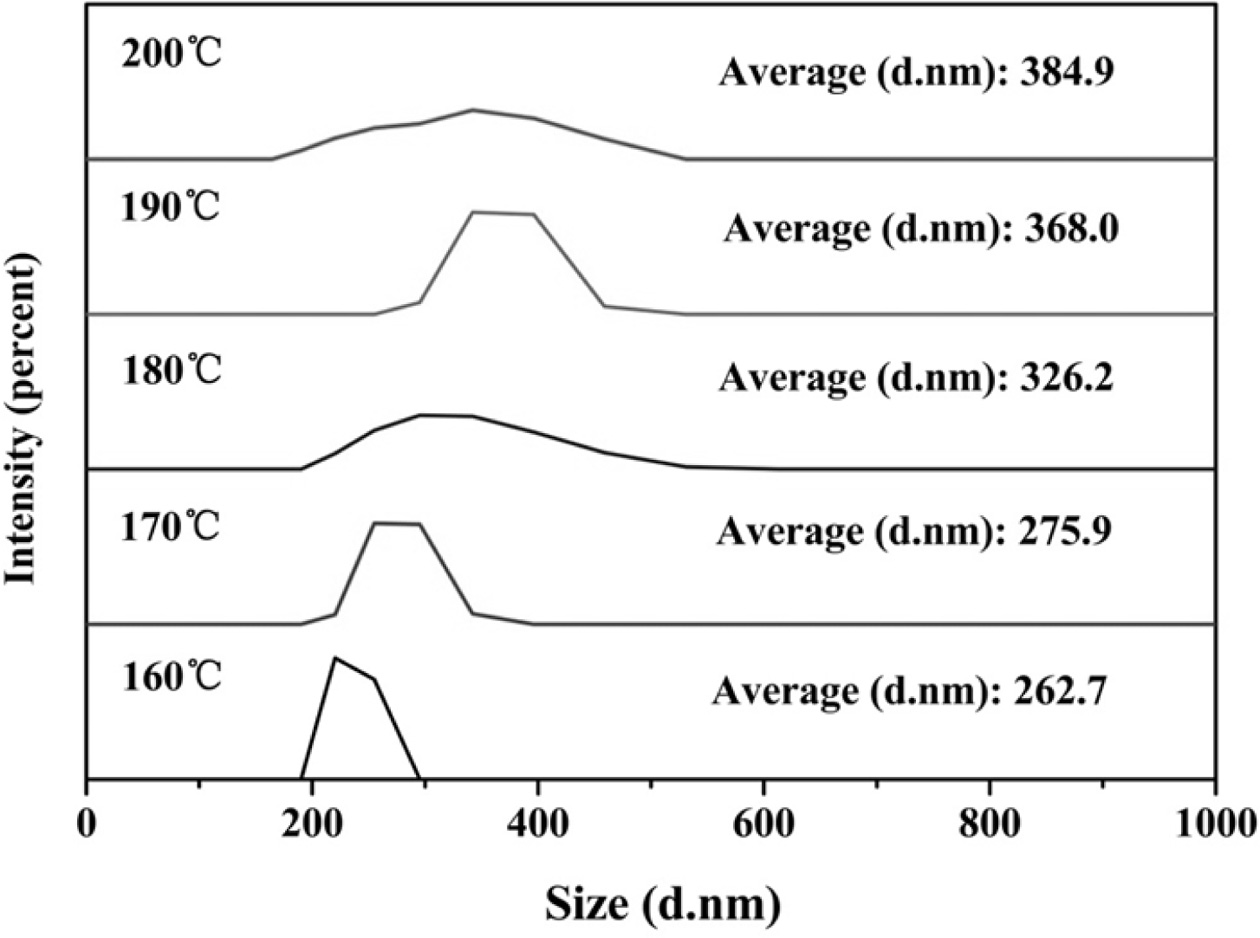
Fig. 10 shows a chart of the analysis of the particle size
of the ultrafine YAG powder, indicating that the particle size of the prepared
ultrafine YAG powder also continuously increases as the microwave water tem- perature increases because in the microwave.
During the hydrothermal process, an increase in temperature causes
the crystal grains to grow continuously, resulting in a large
particle size of the synthesized ultrafine YAG powder and a high temperature of
the crystal phase.
Fig. 11 illustrates a mixture of Al(NO3)3
and Y(NO3)3 calcined at 927.7 oC
under the experimental conditions of pH=9, C(Al3+)=0.42 mol·L−1,
and a microwave hydrothermal reaction temperature of 170 oC.
Fig. 11 also shows the scanning electron micrograph of the pure cubic-phase
ultrafine YAG powder. The ultrafine YAG powder synthesized under the
experimental con- ditions is
approximately spherical, and the particle diameter is approximately
200 nm. It can be seen from the figure that the particle size of the
powder is very small and has a spherical shape. Due to the small particle size,
the powder has a large specific surface area, a high specific surface energy,
and is prone to agglomeration. Table 2 Table 3
|
Fig. 1 XRD pattern of YAG precursor after microwave hydrothermal decomposition. |
|
Fig. 2 DSC curves of ultrafine powders (a- prepared at different pH; b-the relation of synthesis temperature and pH curve). |
|
Fig. 3 XRD patterns of calcined ultrafine powders prepared at different pH. |
|
Fig. 4 (a) DSC curves of ultrafine powders prepared at different concentrations;(b) YAG synthesis temperature-concentration curve. |
|
Fig. 5 XRD patterns of calcined ultrafine powders prepared at different reaction concentrations. |
|
Fig. 6 Effect of different concentration of Al3+ on particle size of YAG ultrafine powder. Effect of hydrothermal temperature on the properties of YAG ultrafine powder. |
|
Fig. 7 XRD spectra of YAG precursor after microwave hydrothermal decomposition. |
|
Fig. 8 (a) DSC curves of powders prepared at different hydrothermal temperatures; (b) YAG synthesis temperature-hydrothermal temperature curve. |
|
Fig. 9 XRD patterns of calcined ultrafine powders prepared at different hydrothermal temperatures. |
|
Fig. 10 Effect of different hydrothermal temperature on particle size of YAG ultrafine powder. |

|
Fig. 11 SEM and TEM patterns of YAG ultrafine powder. |
|
Table 1 Microwave hydrothermal synthesis conditions at different pH conditions. |

|
Table 2 Microwave hydrothermal synthesis conditions of YAG mixed solutions at different Al3+ concentrations. |

|
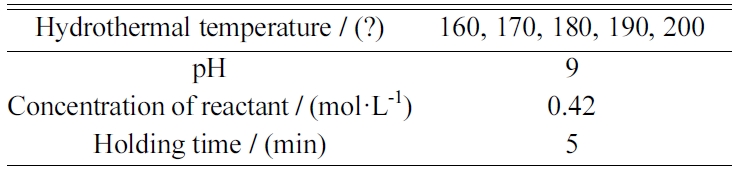
Table 3 Microwave hydrothermal synthesis conditions of YAG at different hydrothermal temperatures. |
YAG precursor oxide was synthesized using the microwave
hydrothermal method. After calcination, the powder was analyzed, and the
following conclusions were drawn: as the Al3+ concentration in the
mother salt solution increased, the particle size of the prepared ultrafine
YAG powder also increased. As the microwave hydrothermal
temperature increased, the particle size of the prepared ultrafine YAG powder
increased. With microwave hydrothermal synthesis, the process
conditions of high-purity ultrafine YAG powder were pH=9, C(Al3+)=0.42 mol·L−1,
microwave hydrothermal reaction temperature=170 oC,
and calcination=928 oC. The body had a particle size of about
200 nm, and its shape was almost spherical. This study provided a reference for
the low-temperature synthesis of high-purity and high-performance ultrafine YAG
powder.
The authors are thankful for the financial support provide
by the Science and Technology Found of the Educational Department of Jiangxi
Province, China (GJJ181107), Teaching Reform Research Fund for Higher Education
of Jiangxi Province (JXJG-18-22-2), The 13th Five-Year Plan for Educational
Science in Jiangxi Province (20YB259), The Science and Tech- nology Support Project of Pingxiang City
(PST2019-01) and the Science and Technology Found of Jiujiang University, China
(2019D0207).
- 1. Y. Hu, Z. Xiao, and H.P. Wang. Ceram. Int. 45 (2019) 3710-3714.
-

- 2. J.G. Song, L. Chen, and Y. Xiang. Solid State Phen. 279 (2018) 104-108.
-

- 3. Q.M. Liu, S.Z. Huang, and A.J. He. J. Mater. Eng. 47 (2019) 1-10.
- 4. L.X. Wang, D.F. Sun, and Q. Li, X. Wang. Chin. J. Lumin. 41 (2020) 160-167.
-

- 5. H. Wang, X. Zhang, and N. Wang. Sci. Adv. 3 (2017) 1-9.
- 6. N. Arai, and K.T. Faber. Script. Mater. 162 (2019) 72-76.
-

- 7. X.L. Ma, Z.L. Lv, and H.B. Tan. J. Phys. Chem. Solid. 130 (2019) 276-281.
-

- 8. J.G. Song, F. Wang, and M.H. Xu. J. Ceram. Proc. Res. 13 (2012) 154-157.
- 9. M. Rahmani, O. Mirzaee, and M. Tajally. Ceram. Int. 44 (2018) 23215-23225.
-

- 10. Y. Liu, X.Q. Yang, and J.G. Song. J. Ceram. Proc. Res. 20 (2019) 436-441.
- 11. T.D. Afolabi, M. Ariff, and A.H. Mazlan. Int. J. Appl. Ceram. Tech. 15 (2018) 1060-1071.
-

- 12. J.G. Song, M.H. Xu, and G.C. Ji. J. Ceram. Proc. Res. 14 (2013) 27-30.
- 13. Z.T Wang, V. Valtchev, and X.M. Li. Nano. Meta. Chem. 49 (2019) 44-50.
-

- 14. G. Zhang, B.X. Jiang, and L. Zhang. Ceram. Int. 44 (2018) 18949-18954.
-

- 15. J. Kraxner, J. Chovanec, and K. Haladejova. Mater. Lett. 204 (2017) 181-183.
-

- 16. G.H. Zhou, G.C. Xu, and J. Liu. Int. J. Adv. Manuf. Tech. 95 (2018) 1677-1684.
- 17. M. Singlard, F. Remondiere, and S. Oriol. J. Sol-Gel Sci. Tech. 87 (2018) 496-503.
- 18. J. Hostasa, V. Necina, and T. Uhlírova. J. Eur. Ceram. Soc. 39 (2019) 53-58.
- 19. X. Wang, Y.J. Zhong, and D. Wang. J. Am. Ceram. Soc. 100 (2017) 1-7.
- 20. J.G. Song, L.M. Zhang, and J.G. Li. Synt. Reac. Inorg. M. 36 (2006) 529-533.
-

- 21. Z.N. Magdalena, and H. Krzystof, Process. Appl. Ceram. 1 (2007) 69-74.
- 22. Y. Liu, Z.F. Zhang, and J. Halloran. J. Am. Ceram. Soc., 81 (1998) 629-645.
-

- 23. S. Bhattacharyya, and S. Ghatak. Ceram. Int. 35 (2009) 29-34.
-

- 24. P. Palmero, and R. Traverso. Mater. 7 (2014) 7145-7156.
-

 This Article
This Article
-
2020; 21(4): 488-494
Published on Aug 30, 2020
- 10.36410/jcpr.2020.21.4.488
- Received on Feb 25, 2020
- Revised on May 16, 2020
- Accepted on Jun 10, 2020
 Services
Services
- Abstract
introduction
experimental materials and methods
results and discussion
conclusion
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Jieguang Song a,b, Lin Chen b
-
aSponge City Research Institute of Pingxiang University, Pingxiang 337055 China
bKey Laboratory for Industrial Ceramics of Jiangxi Province, Pingxiang University, Pingxiang 337055 China
Tel : +86 799 6682251
Fax: +86 799 6682171 - E-mail: sjg825@163.com, rymw27@163.com






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.