- Synthesis of zinc substituted cobalt ferrites via standard double sintering ceramic technique: A study on their structural, magnetic and dielectric properties
Tamanna Mariama,b, I. N. Eshab, M. N. I. Khanc, Shamima Choudhuryb and Kazi Hanium Mariab,*
aDepartment of Physics and Astronomy, University of Toledo, Toledo, Ohio, USA
bDepartment of Physics, University of Dhaka, Dhaka-1000, Bangladesh
cMaterial Science Divisions, Atomic Energy Centre, Dhaka-1000, Bangladesh
A series of Co1-xZnxFe2O4
ferrites with x = 0.0, 0.1, 0.2, 0.3, 0.4, 0.5 were synthesized
using the standard double sintering ceramic technique. The single-phase cubic
spinel structure of the samples was confirmed from the X-ray diffraction
patterns and the sharp high intensity peak revealed that the samples are in
good crystalline form. The lattice parameters were observed to increase with
the increasing of Zn concentration. By increasing the concentration of Zn
content, a significant increase in the density and a subsequent decrease in
porosity and grain size were observed. The dielectric constant (ε′) of the
sample is found to decrease with the increase in frequency. Dielectric relaxation
peaks were observed for the frequency dependence of dielectric loss curves. The
dc resistivity at different temperatures was studied and all the samples were
of semiconductor nature. The saturation magnetization was found to be reduced
as the Zn substitution was increased and consequently permeability decreases.
Keywords: X-ray diffraction, SEM, Permeability, Dielectric, Anisotropy Constant
The spinel ferrite, MFe2O4 (M=Co,
Mn, Ni, Zn etc.) with general formula (MδFe1−δ)[M1−δFe1+δ]O4
where, δ represents degree of inversion [1, 2] have remarkable
electrical and magnetic properties that can be tuned by ion substitution. Such
materials are considered as promising candidates for microwave and
magneto-optic devices, high-frequency catalysis, magnetic drug delivery,
magnetic resonance imaging (MRI), radio-frequency hyperthermia and high-density
recording medium [1-3]. Cobalt ferrite (CoFe2O4)
has a high cubic magneto-crystalline anisotropy, high coercivity and moderate
saturation magnetization [3]. These properties of cobalt ferrite
can be tuned by substituting various metal ions in the ferrite lattice.
Substitution of small amount of non-magnetic Zn ions generates cation
redistribution in the octahedral [B] and tetrahedral [A] sites of the spinel
structure which makes changes in the saturation magnetization and coercivity of
the materials [4]. In CoFe2O4 ferrites Co2+
ions occupy octahedral [B] sites and Fe3+ ions are equally
distributed between tetrahedral and octahedral sites. It is reported that Zn2+
can continuously migrate to tetrahedral and octahedral positions which has
significant impact on the magnetic and electrical properties of ferrites [4].
Therefore, sub- stitution of Zn2+
in CoFe2O4 makes it a hard magnetic material
with high Curie temperature and good chemical stability
[5]. Co-Zn ferrite is suitable in high frequency magnetic applications,
magnetic recording applications (audio–video tapes) and high-density digital
recording disks etc. [6] because of its high electrical resistivity and
negligible eddy current losses. There are many reports on Co-Zn ferrites
synthesis in the literature. A. Khan et al. [7] by ceramic method, S. Singhal
et al. [8] by sol-gel-route, S. Tapdiya and A.K. Shrivastava [9], M.T.
Jamil et al. [10] by chemical co precipitation method, R. Rani et al. [11] by
solution combustion method, S.T. Alone and K.M. Jadhav, [12] by wet chemical
co-operation technique prepared the samples. In this paper, Co1-xZnxFe2O4(x=0.0-0.5)
ferrites are synthesized by cost effective double sintering solid state
reaction method [7] which involves amalgamating the powdered particles together
through solid state diffusion process at an elevated temperature below the
melting points of the constituents. This is a two-step process
where the pre-sintering stimulates phase formation and
sintering expedites grain growth with densification. As such, the prepared
samples are less porous, denser, harder and has greater
flexural strength [7, 13]. Thus, the sintering
process gives a microstructure of the samples with conductive grains and
improves grain boundaries. But the temperature and duration need to be chosen carefully in order to
attain the particular characteristics of the samples.
Moreover, sintering process prevents
deformation and cracking of samples. Here, we have
carried out direct substitution of nonmagnetic Zn2+ for Co in cobalt
ferrite by double sintering process in order to investigate the effect on
magnetic behavior. Zn2+ substitution is likely to affect the
cation distribution and thereby significantly alter the magnetic
properties.
All ferrite samples were synthesized by double sintering
ceramic process. The materials used in this study were CoO, Fe2O3
and ZnO and mixed together to form the composition of Co1-xZnxFe2O4(x = 0.0-0.5)
where “x” is the Zn concentration in CoFe2O4 ceramic
compound. Mixtures of these raw materials were grinded into extremely fine
powder for 4 hours. The prepared powder was kept in programmable furnace for
calcination process. The calcination temperature was 750 oC
and it was calcined for 3 hours. After calcination, they were
grinded again. Finally, the pellets were made from the powders using hydraulic
pressure. Then the samples were sintered for 2 hours at 1050 oC.
Characterization
The crystalline and cubic spinel structure of the ceramic
samples was studied by an X-ray Diffracto-
meter (XRD) using PHILLIPS PRO XRD SYSTEM in the 2θ range from 20o
to 70o. The specimens were exposed to CuKα
radiation of wavelength, λ = 1.54178 Å with a
primary beam of 40 kV and 30 mA with a sampling pitch of 0.02o
and time for each step data collection was 1.0 sec. The lattice parameter
of the synthesized samples was estimated using the relation [2]
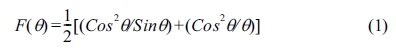
where, q
is the Bragg’s angle [14].
The X-ray densities of the samples were estimated using
the relation [2]

where Z is the number of molecules per unit cell of spinel
lattice, M is the molecular weight of the samples, N is the Avogadro’s number
and a is the lattice parameter of the samples.
The bulk density of the samples was calculated by using
the formula

And we have measured the porosity of the samples using the
formula given below,

The surface morphology and compositional features were
studied using a scanning electron microscope (FEI SEM). dielectric and
permeability measurements were conducted by Impedance Analyzer (WAYNE KERR
PRECISION-6500B). The real part of dielectric constant was calculated using the
formula,

Here, C is the capacitance of the pellet, L is the
thickness or height of the pellet, A is the area of cross-section of the flat
surface of the pellet, ![]() is the
dielectric constant at free space.
is the
dielectric constant at free space.
The imaginary part of the dielectric constant is,

with tand
being the loss tangent.
The quality factor was measured using the formula

The initial permeability was measured by using the formula
[15]

Where, Ls is the inductance in Henry, N is the
number of turns of copper wire in the torroid, Douter indicates the
outer diameter and Dinner stands for the inner diameter of the
torroid.
To determine the temperature dependent resistivity,
“KEITHLEY 6514 SYSTEM ELECTROMETER” a small oven and a thermocouple-based
thermometer had been used.
The resistivity was calculated using the formula,

Where, r
denotes the specific resistance, L is the length of the specimen and A
indicates the area of cross section.
Magnetic properties of the samples were measured by a
Vibrating Sample Magnetometer (EV9 MICROSENSE VSM). All
the hysteresis magnetic measurements were carried out at room temperature.
Anisotropy constant has been calculated by using the formula,

with Hc is Coercive field and Ms
denotes Saturation Magnetization.
Structural
Properties
The structural characteristics of Zn doped Cobalt ferrites
with compositions of Co1-xZnxFe2O4(x = 0.0-0.5)
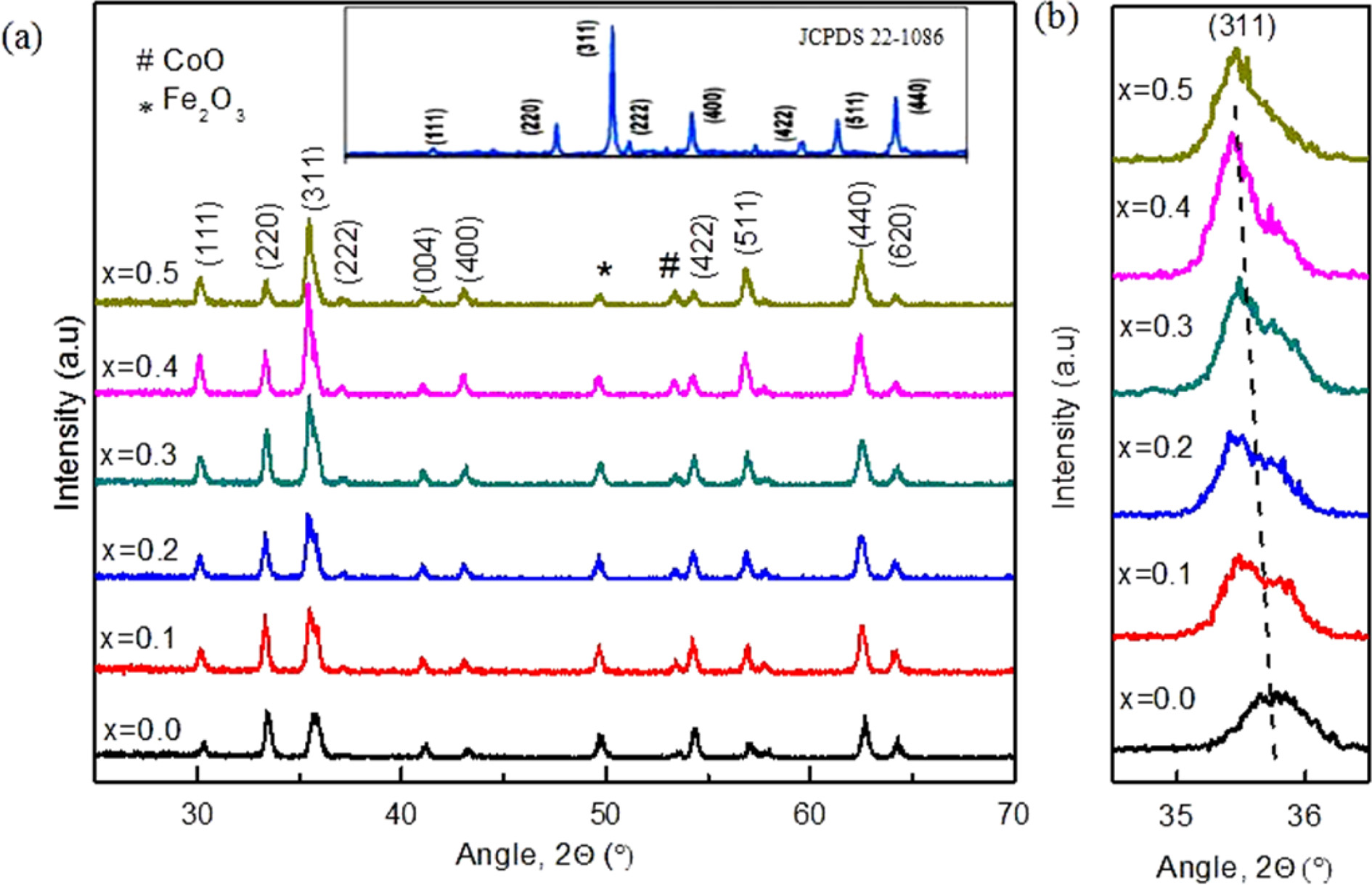
were measured by X-ray diffraction technique shown in Fig. 1. The miller
indices were indexed according to JCPDS card no 22-1086 [16].
The fundamental reflections from the planes of (220), (311),
(511), (440) are charac-
terizing the single-phase cubic spinel structures [17]. The
sharp peak reveals that the samples are in good crystalline form. Reflection
from the other planes (400), (422) has also been observed
with weak intensities. Impurity peaks for Fe2O3 and
CoO were detected which suggests the occurrence
of a few unwanted chemical reactions at the time of sintering.
In this sample, it is observed that the highest intensity peak was shifted to
lower angle with the increase of Zn content (Fig. 1(b)). This shifting
of highest intensity peak towards lower angle is attributed to have higher
inter-planar distance and increase in cell volume. The intensity of the highest
peak is found to increase with increase of Zn content which indicates the
crystalli- zation and spatial position
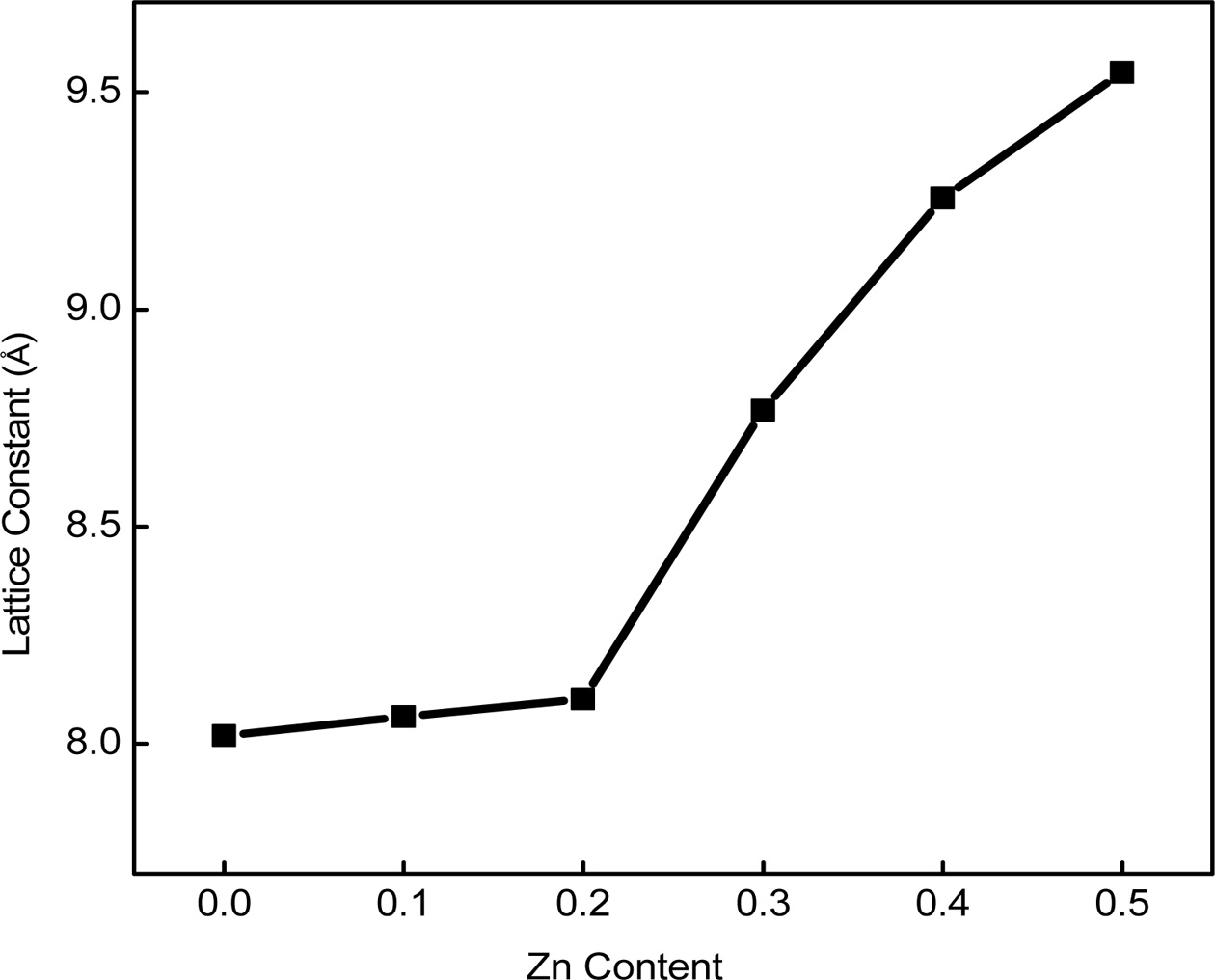
of the substituted atom. To determine the exact value of lattice parameter,
Nelson-Riley (N-R) extrapolation method was used [2]. The effect of Zinc substitution
on the lattice constant is shown in Fig. 2. From this figure, it is found that
the lattice parameter enhances with the increasing Zn content. This increment
may be related to the replace- ment of
Co2+ ion with smaller ionic radius (0.745 Å) [18] by Zn2+
ion with larger ionic radius (0.82 Å) [19]. During the crystallization
process, the unit cell expands to assemble
the substituted large Zn2+ ions. Thus, the addition of Zn2+ in the Cobalt ferrite is expected to
increase the lattice parameter. Similar trend has been reported in
Zn-Mg-Cu [20]; Zn-Mg [21] and Ni-Zn [22] and Cu-Zn [2] ferrites.
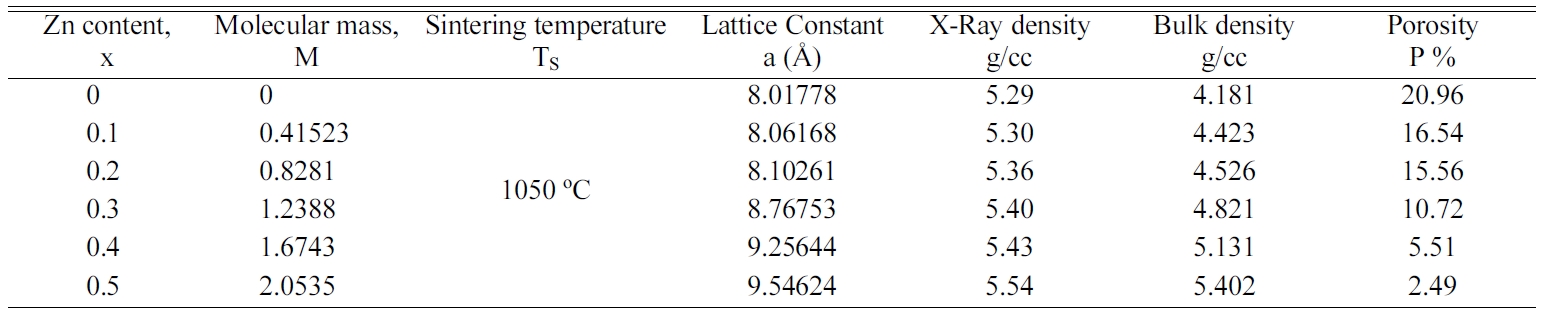
The effect of Zn substitution on lattice parameter, X-ray density,
Bulk density and porosity is shown in Table 1.
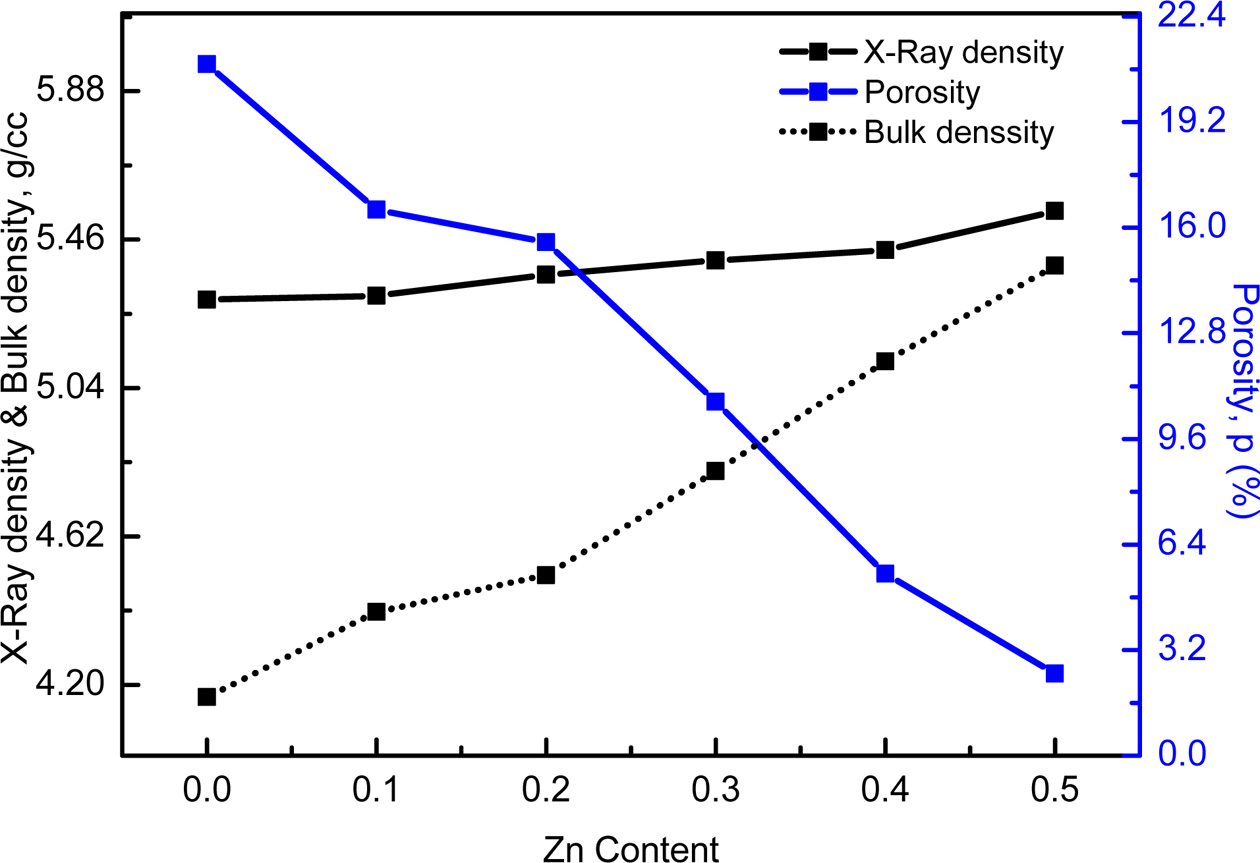
It is observed from Fig. 3 that the X-ray density, ρx
and bulk density, ρB increase increase with increasing Zn
content. The increase in X-ray and bulk density may be due to the increase in
molecular weight of the various compositions of Co-Zn ferrites.
Generally, the homo- geneous
distribution of any element in other element may increase the
density. This significant increase of the density can be explained by the
introduction of Zn ions through sintering and lattice diffusion. The lattice
diffusion increases the diffusion path length which in turn accelerates the
rate of cation inter-diffusion in the samples [23]. As a result, the density
increases with the introduction of Zn content. It is also observed from this
figure that the X-ray density is higher than the bulk density. This might be
evolved with the existence of pores on the macroscopic scale and vacancies in
the lattice on atomic scale during the sintering process or at the time of
sample preparation [13]. The smaller value of bulk density, ρB compared
to the X-ray density, ρx might be due to the presence of
inter-granular porosity. On the other hand, porosity exhibits completely
opposite trend as shown in Fig. 3. The porosity is found to decrease with the
increase in Zinc content as the samples become denser. Similar
results were observed in several ferrites [3, 13, 24, 25].
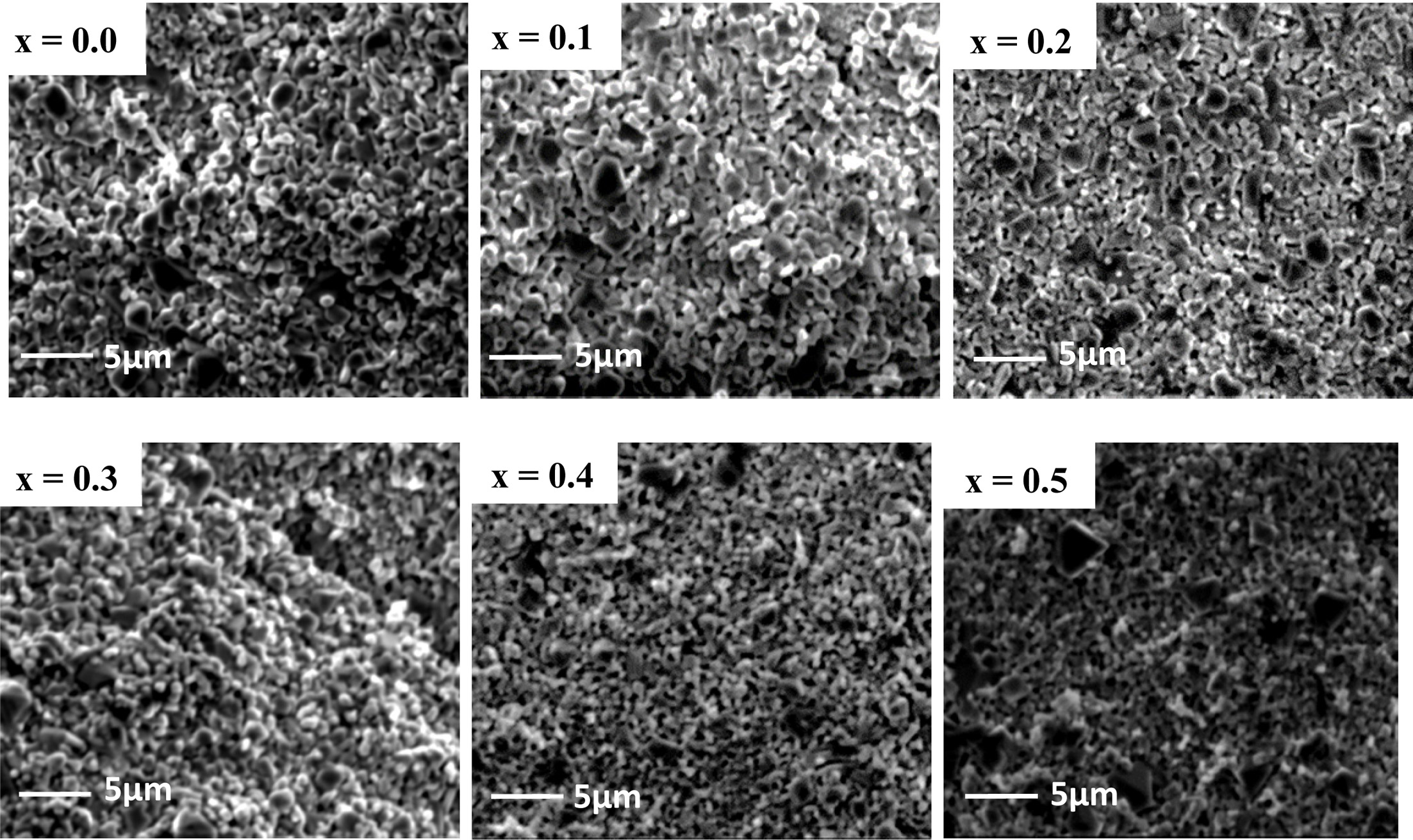
The microstructures of the investigated materials are
given in Fig. 4. The grain size remained almost same for the lower
Zinc substitution (x = 0 to 0.3) but for higher
Zn content (x = 0.4, 0.5), the grain size was found to decrease as
ZnO hinders the grain growth during sintering to stabilize the crystallization.
The grain growth amplifies the migration of the
pores to the grain boundary and hence, increases the sintered density. Grain
boundaries expand over the pores during the sintering which might produce the
denser material by reducing the pores volume [25]. The pores located at the
grain boundaries impede the domain wall move-
ment and impact on permeability and resistivity [26]. It has been
reported that Zn2+, Cd2+, Zn2+, Ti4+,
Nd2+ substitution do not favor the grain growth [27-29].
Dielectric
Properties
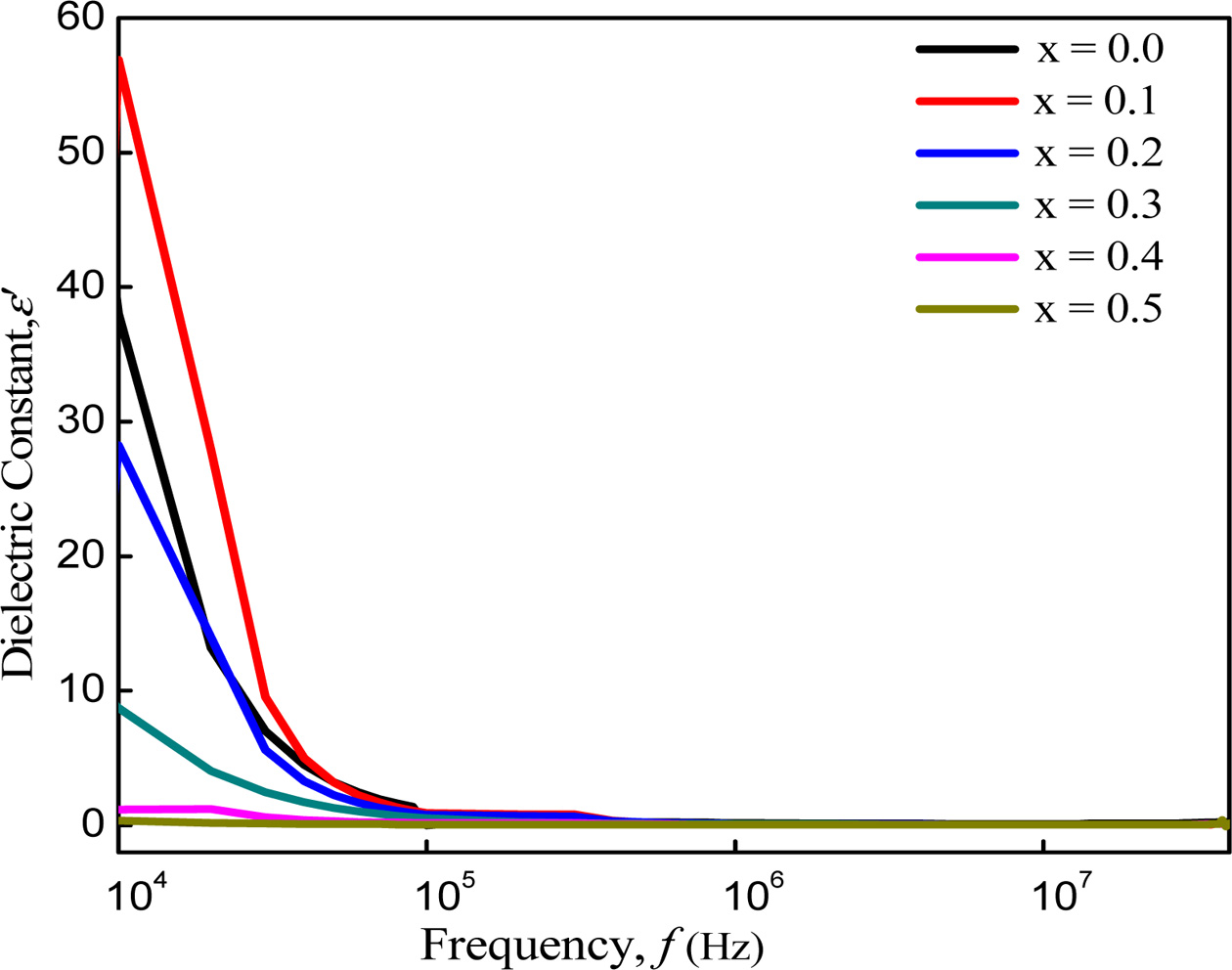
The variation of the real part (e ')
of dielectric constant with frequency from 10 kHz to 40 MHz at room
temperature are shown in Fig. 5. The dielectric constant (e ') decreases with increasing
frequency, which is rapid at lower frequencies and slower at higher
frequencies. At much higher frequency it is very small and becomes independent
of frequency. Similar dielectric behavior is observed in Mg-Cu-Zn ferrites
[30], Ni-Cu-Zn ferrites [31] and Cu-Zn ferrites [32]. All the samples have high
value of dielectric constant in the order of 103-105 at
low frequencies. Maxwell-Wagner interfacial type of polarization can describe
these higher values at lower frequency region [33, 34, 13] for the
inhomogeneous double layer dielectric structure. This is normal dielectric
behavior observed in most of the ferromagnetic materials in room tem- perature. Sintering temperature and time or
atmosphere including grain structure can influence the Fe2+ ions
concentration which is a characteristic property of a ferrite material. Fe2+
ions availability allows the electron to exchange between the +2 and +3
valance states of Fe ions and creates the local displacement of charges in the
direction of the applied electric field. This in turn causes the dielectric
polarization of the ferrites. The electron exchange was assumed to
increase with the increased concentration of Fe2+/Fe3+
ions pairs on B-site. However, as the frequency of the alternating field is
increased, the electron exchange between Fe2+ and Fe3+ ions
cannot follow the alternating field beyond a certain frequency and then the
dielectric constant becomes constant [28, 32].
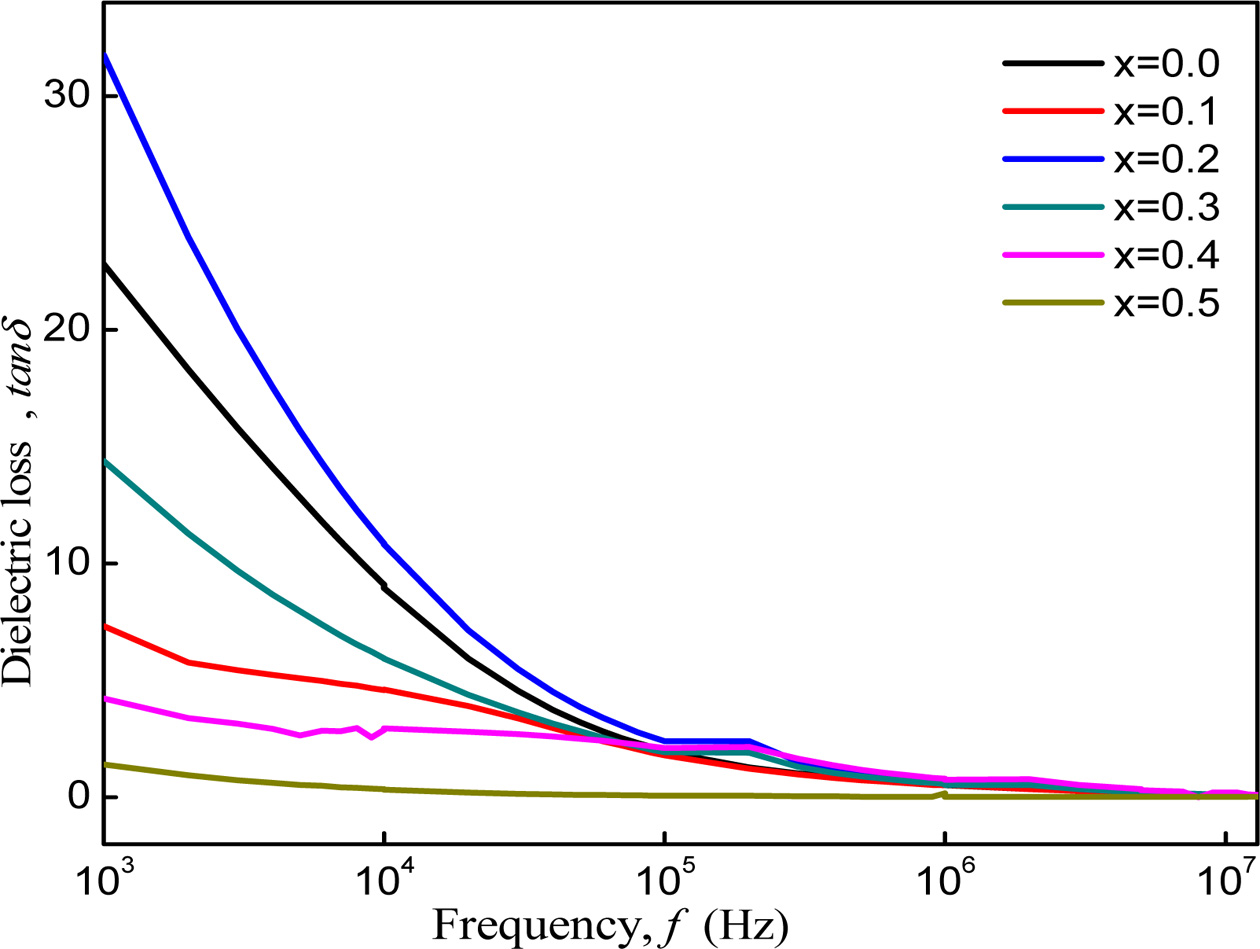
Fig. 6 shows that dielectric loss decreases rapidly with
increasing frequency and becomes constant at higher frequency range. In the
high frequency the conduction in between Fe2+ and Fe3+ ions
increases which corresponds to low resistivity and small energy is required for
electron exchange at the octahedral site results in lower value of dielectric
loss [32].
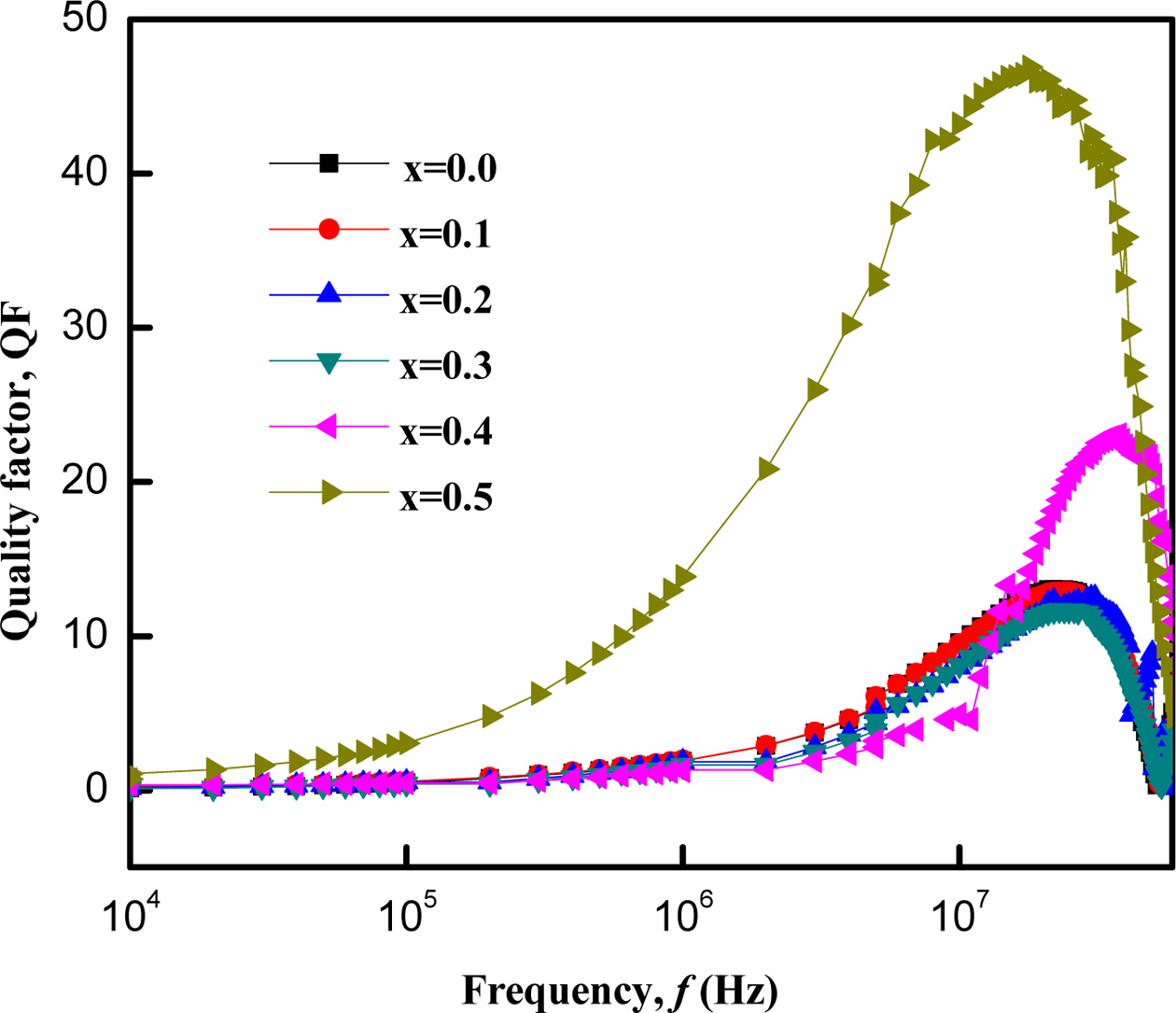
Fig. 7 shows the variation of Q factor with frequency as
log f for the samples. All the samples show a maximum at a certain
frequency. The appearance of a maximum can be described by the relaxation
formula, wt = 1,
where, w = 2pfmax
and relaxation time, t = 1/2p,
p is the hopping probability per unit time [35]. As stated by this
relaxation relation, a maximum can be appeared when the hopping frequency of
electron between Fe2+ and Fe3+ ions at the adjacent
B-sites and the frequency of applied AC field exactly match with each other
[28, 32]. It is also observed that fmax or the hopping probability
per unit time increases for the samples with Zn content. Therefore, the
concentration of the Fe2+ ions on the B-sites increases
in these samples which is also consistent with the resistivity
data.
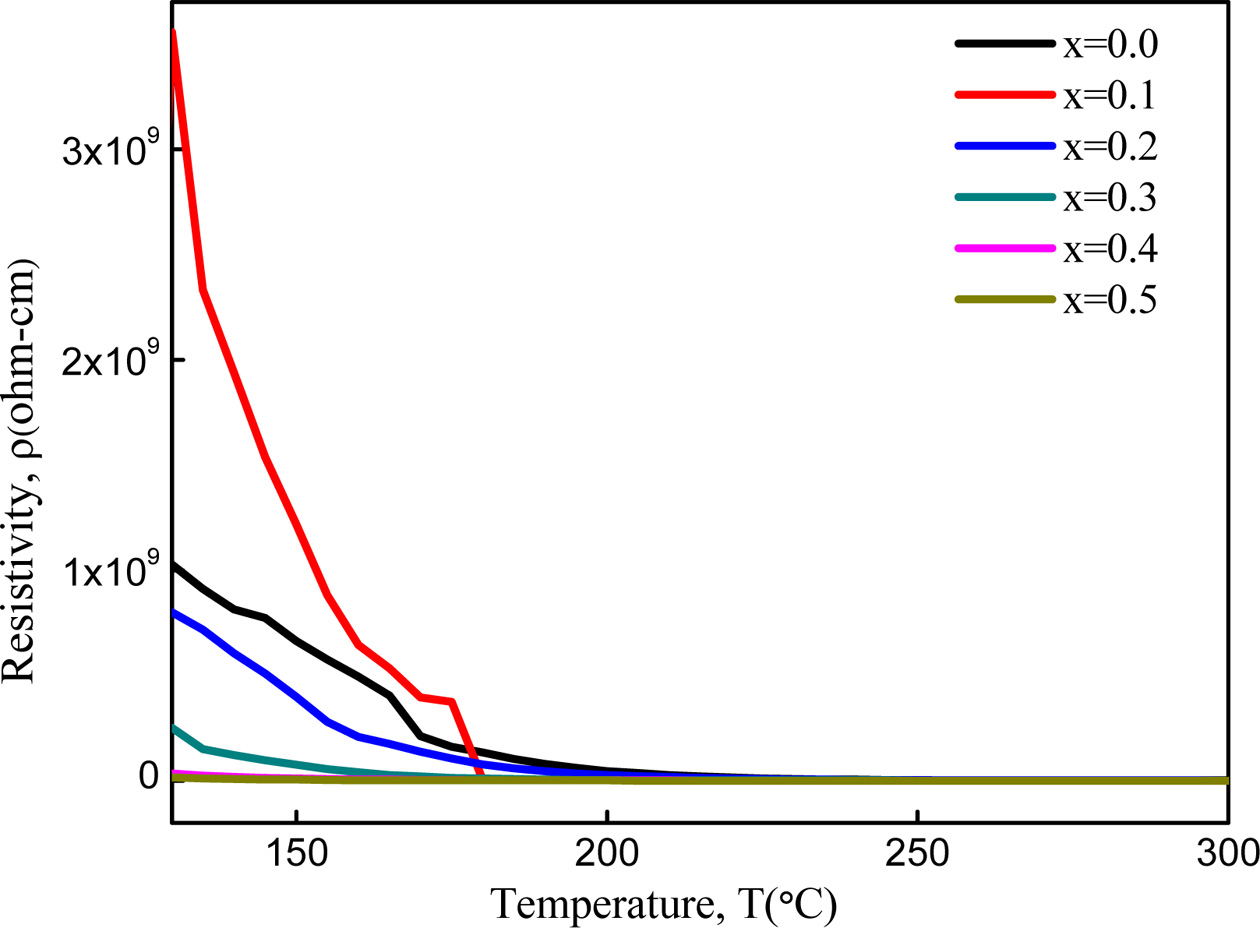
Fig. 8 shows that the DC resistivity decreases with the
increase in temperature for the samples which is the normal ferromagnetic
behavior. Generally, the hopping of electrons between
Fe2+ and Fe3+ ions at adjacent B-sites starts to get
thermally activated with the increase in temperature and produce local
displacement in the direction of the applied AC field. In the low tem- perature region,
the resistivity, r starts to decrease gradually because the thermal energy
breaks the localized dipoles and starts orienting themselves in the low temperature region. The orientation of these dipoles becomes more significant with
the increase of temperature, as
the exchange of charge carriers between the Fe3+ and Fe2+
ions on octahedral sites [34, 36] increases and this exchange decreases the resistivity of the samples. The intensified chaotic thermal oscillations at high temperature are responsible for this negligible
small resistivity [36].
It is observed that for x = 0.1, the dielectric
constant as well as the value of resistivity are greater than the pure Cobalt
ferrite. This sample may have higher dielectric properties and low eddy current
loss than the other samples.
Magnetic
Properties
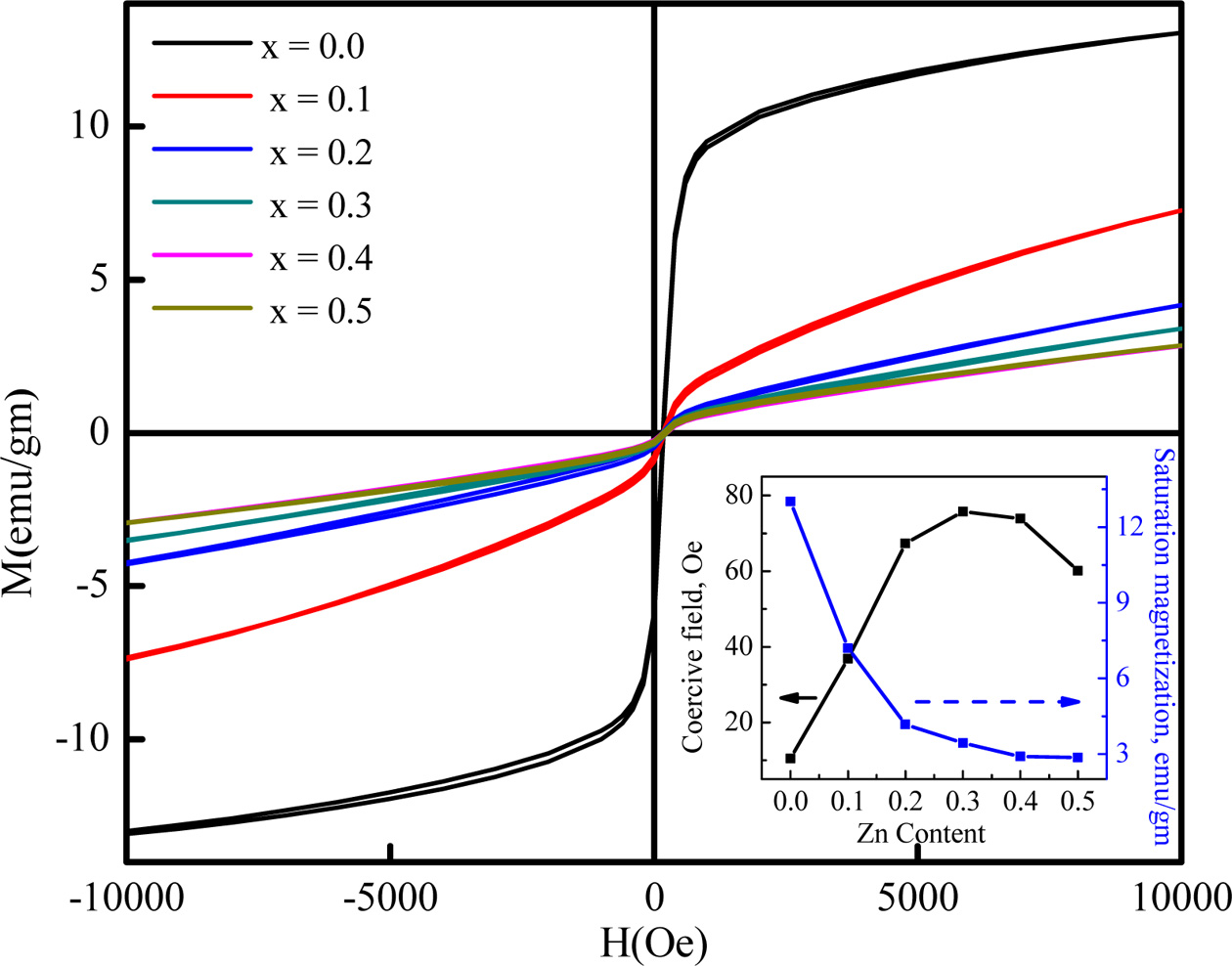
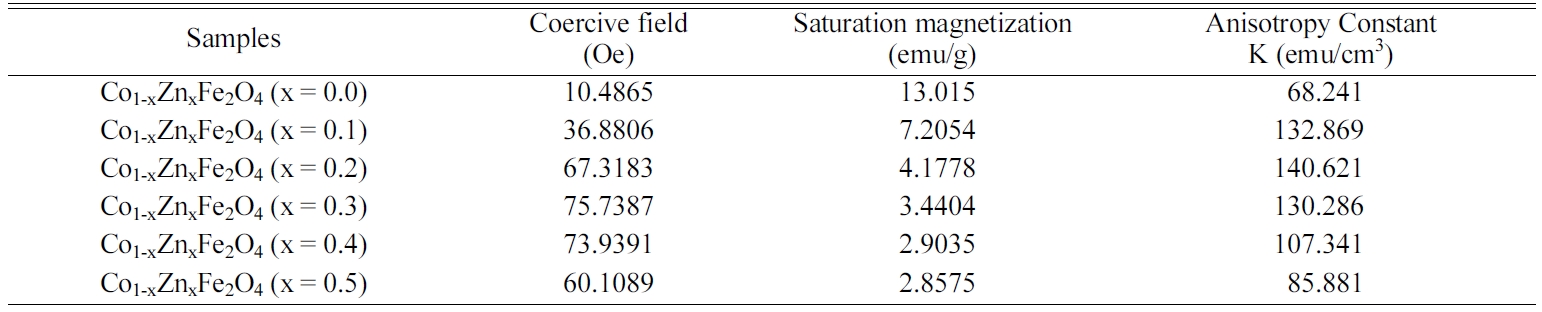
Figure 9 shows that the saturation magnetization decreases
with Zn content. Table 2 shows the variation of coercive
field, saturation magnetization and anisotropy constant
with increasing Zn content in Cobalt ferrites. According to
Néel's sublattice model, resulting magneti-
zation depends on the difference between B and A-site magnetization,
provided that the sites are collinear and antiparallel to each other. In Co-Zn
ferrite, Fe3+, Fe2+, Co2+ and Zn2+
elements have magnetic moment of 5 µB, 4 µB, 3 µB and
0 µB respectively which is coming from ‘d’
orbital electron distribution [25]. All the elements can
migrate to both A- and B-sites. The possible cation distribution
of CoFe2O4 can be presented as:
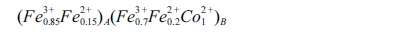
In case of CoFe2O4, most of the Fe3+
and a smaller part of Fe2+ take place on the A sites, and remaining
Fe3+, Fe2+, Co2+ goes to the B sites. For Co1-xZnxFe2O4,
the non-magnetic ion Zn2+ takes places to the
octahedral site along with the magnetic ion, Co2+.
Basically, the Zn2+ and Co2+ replace the Fe2+
and Fe3+ in octahedral sites, forcing a migration of
Fe2+ and Fe3+ to the tetrahedral sites [4].
The presence of large magnetic moments (caused by
the Fe3+ and Fe2+ ions) on the A-sites increases
the A-site magnetization which in turn leads to a decrease of
overall magnetization. The saturation magneti-
zation was found to decrease significantly from 13.015
emu/g to 2.85 emu/g with Zn substitution.
Here, from the inset of Fig. 9, the coercivity is noticed
to increase with the enhanced concentration of zinc ions while the saturation
magnetization behaves completely opposite which can be described by Browns
relations [25]. The high coercive force confirms that the cobalt ferrite
becomes hard from soft ferrite after Zn substitution. Anisotropy constant
represents the energy required to deflect the domains from the “easy” to
“hard” positions. The interaction between spin magnetic moment of
each atom and crystal lattice determine the easy and hard alignments of the
compounds. The change in anisotropy constant is similar to the change in
coercive field. It is observed from the change of anisotropy constant with
increasing Zn contents that cobalt ferrite behaved as “soft” magnet at the
lower concentration of Zn (x = 0.1,0.2). At x = 0.3-0.5,
the ferrite showed the characteristics of “hard” magnet.
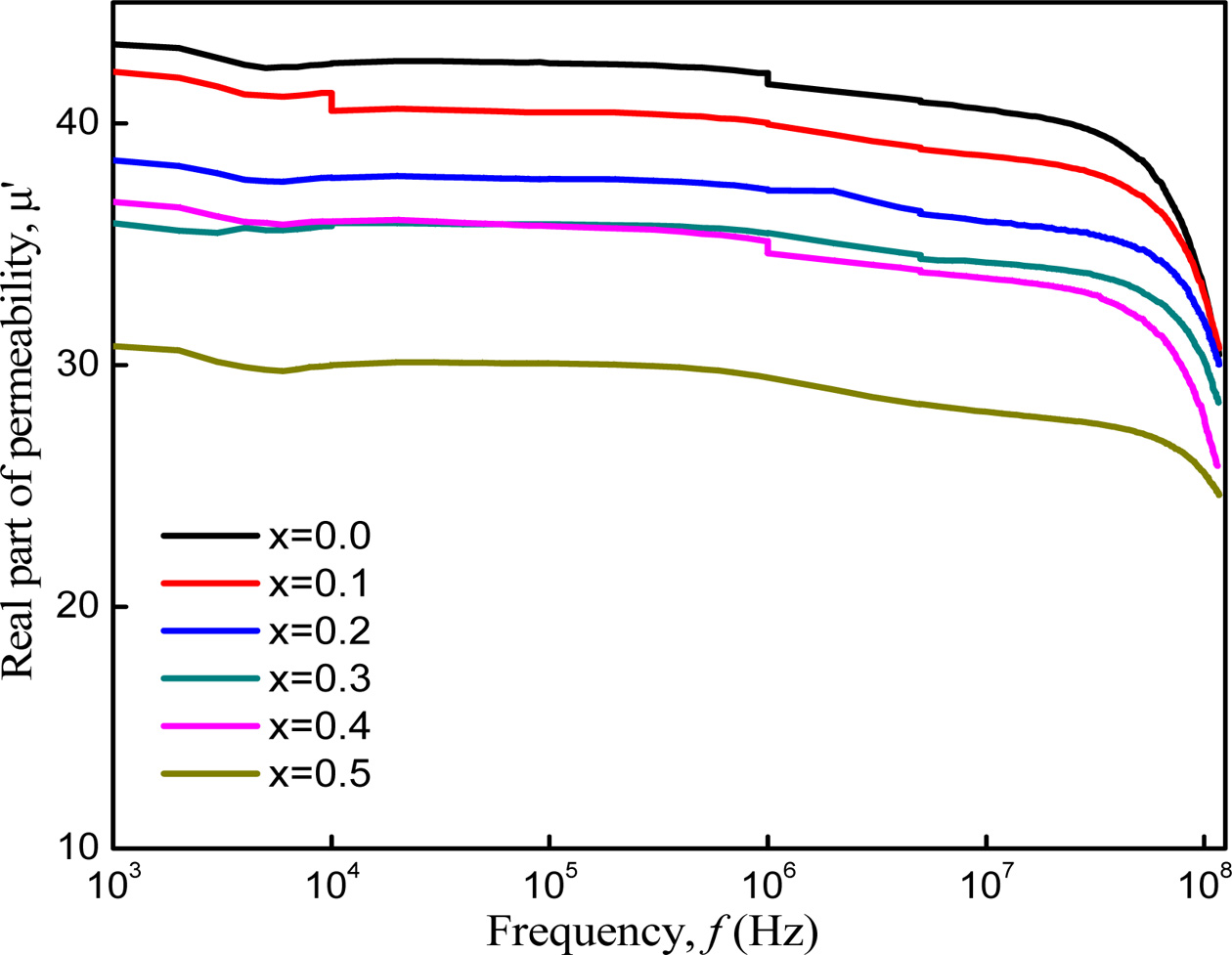
The complex permeability measurements on toroid shape
specimens have been carried out at room temperature for all the samples of
series Co1-xZnxFe2O4 (x = 0.0-0.5)
in the frequency range 1 kHz-100 MHz. The variation of μ' with frequency
is shown in Fig. 10. It is found that the value of initial permeability (μ')
shows flat profile up to a certain frequency (> 107 Hz)
indicating good low frequency stability and then it falls rapidly
to a very small value after a certain high frequency named as
Cut-off frequency. The permeability of polycrystalline ferrite is related to
spin rotation and domain wall motion. And Globus- Duplex model [28] says that
the permeability caused by wall motion is linearly dependent on the grain size.
From the micro- structure analysis of
this work, it is noticed that the grain sizes decrease with increasing Zinc
concentration and domain wall movement is influenced by grain size. Also, the
decrease of permeability is associated with the decrease in domain wall
movement. On the other hand, the saturation magnetization was found to be
decreased in our case, which decreases the number of domain walls within the
grain according to Globus relation [13, 37]. Therefore, the
permeability is expected to decrease with the addition of Zn
content. At high frequencies, the nonmagnetic impurities between intra-granular
pores influence both spin rotation and domain wall motion so that permeability
decreases at higher frequencies.
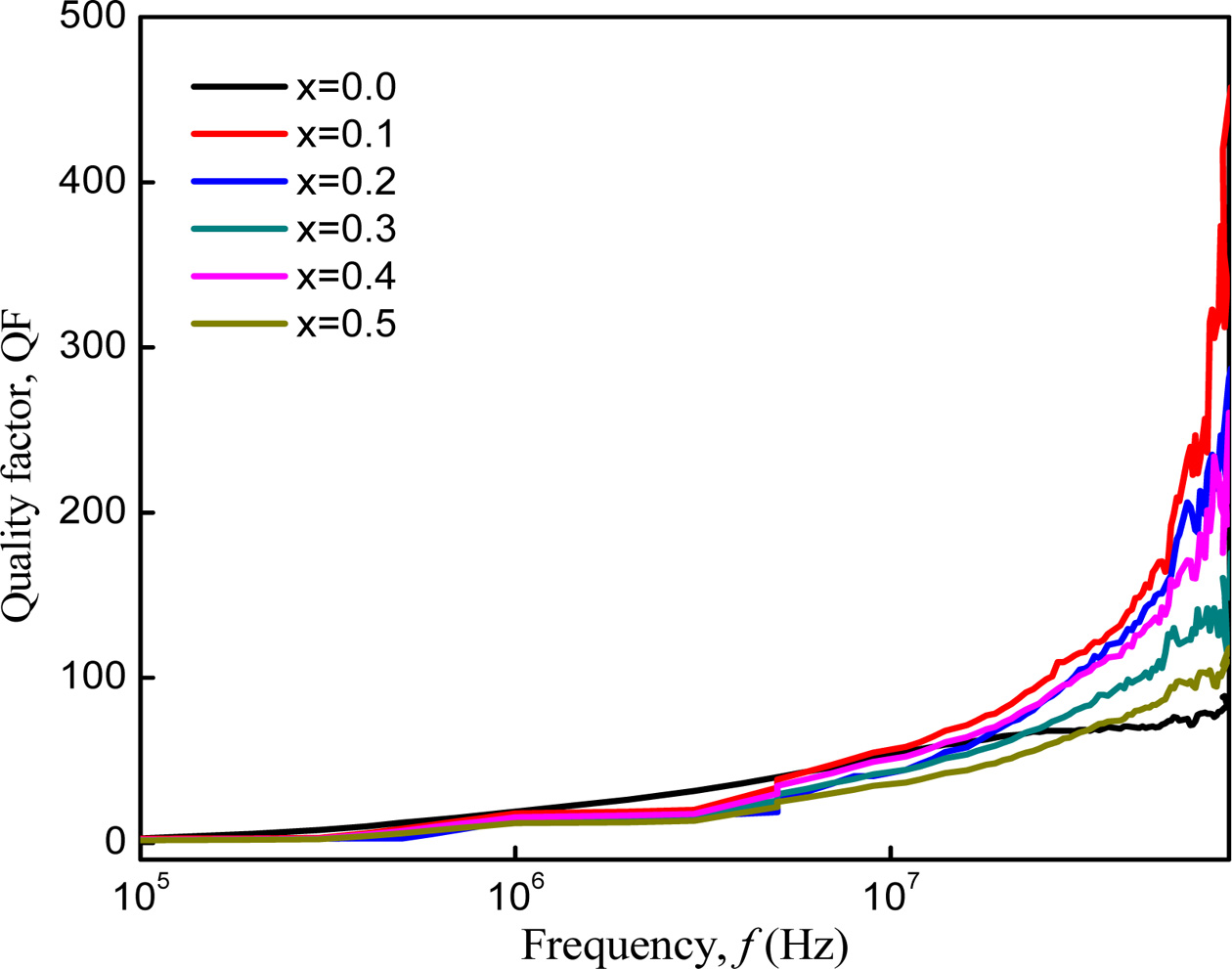
Fig. 11 shows the variation of Quality factor (QF) with
frequency of Co1-xZnxFe2O4 (x = 0.0-0.5)
that determines the efficiency of the magnetic materials. It increases with
increasing frequencies. The Q factor of Co-Zn ferrite samples increased for a
small amount of Zn2+ substitution and then decreased with addition
of Zn. Zn substituted sample has a higher value of QF than the unsubstituted
sample. This change of QF depends on magnetic loss and porosity of the samples [28]. Among the samples, Co-Zn ferrite with
x = 0.1 has the highest QF which indicates that this sample has the
lowest magnetic loss.
From the above discussion, it is claimed that the
polycrystalline Co-Zn ferrite is versatile ferrite, due to their high
resistivity and low eddy current losses. The adjustable
grain sizes and controllable magnetic properties make the
applicability of cobalt-zinc ferrite even more versatile. Cobalt-zinc ferrites
are suitable for magnetic recording applications such as audio and videotape
and high-density digital recording disks etc.
|
Fig. 1 (a) X-ray diffraction patterns for Co1-xZnxFe2O4 (x = 0.0-0.5) ferrites and (b) Phase shifting for the highest peak of (311). |
|
Fig. 2 Lattice parameter vs Zinc content of Co1-xZnxFe2O4 (x = 0.0-0.5) ferrites. |
|
Fig. 3 Variation of X-Ray density, bulk density and porosity of Co1-xZnxFe2O4 (x = 0.0-0.5). |
|
Fig. 4 Scanning Electron Microscope (SEM) images for Co1-xZnxFe2O4. |
|
Fig. 5 Variation of dielectric constant with increasing frequency of Co1-xZnxFe2O4 (x = 0.0-0.5). |
|
Fig. 6 Dielectric loss of Co1-xZnxFe2O4 (x = 0.0-0.5) as function of frequency. |
|
Fig. 7 Variation of quality factor, QF of Co1-xZnxFe2O4 (x = 0.0-0.5) with increasing frequency. |
|
Fig. 8 Variation of resistivity of Co1-xZnxFe2O4 (x = 0.0-0.5) as a function of temperature. |
|
Fig. 9 Hysteresis Loop with the inset of variation of coercive field and saturation magnetization of Co1-xZnxFe2O4 (x = 0.0-0.5) as function of Zinc content. |
|
Fig. 10 Variation of Permeability with increasing frequency of Co1-xZnxFe2O4 (x = 0.0-0.5). |
|
Fig. 11 Variation of quality factor, QF of Co1-xZnxFe2O4 (x = 0.0-0.5) with increasing frequency. |
|
Table 1 Lattice parameter, molecular mass, theoretical density, bulk density and porosity for the samples with the composition Co1-xZnxFe2O4 (x = 0.0-0.5) |
|
Table 2 Coercive field, Saturation magnetization and Anisotropy Constant for the samples with the composition Co1-xZnxFe2O4 (x=0.0-0.5) |
X-ray diffraction measurement confirmed the single-phase
cubic spinel structure of polycrystalline samples Co1-xZnxFe2O4
where x = 0.0-0.5, since no ambiguous reflections other than the
spinel structures were evidenced. The lattice parameter was observed to be
increased with Zn content substitution. From micro- structural study, it was observed that the
grain size remained almost the same with increasing Zn content. However, the
grain size decreased slightly for higher content of Zn. The dielectric constant
was found to decrease with increasing frequency, which was rapid at lower
frequencies and slower at higher frequencies. DC electrical resistivity
increased for 10% of Zn substitu- tion
then again decreased with Zn Content. The saturation
magnetization has been decreased and coercive field was increasing for up to
30% Zn content and then started to decrease with increasing Zn content.
Permea- bility decreased with Zn
content. From the Dielectric properties and Resistivity
measurement, it was observed that 10% of Zn
substitution in place of Cobalt increased the
dielectric properties and resistivity of the samples. Anisotropy constant is
enhanced for x = 0.1, 0.2 and then decreased with higher Zn content.
The authors acknowledge gratefully to Materials Science
Division, Atomic Energy Centre, Dhaka and Centre for Advanced Research of
Sciences, University of Dhaka, Dhaka, Bangladesh. Authors are sincerely
thankful to Nano and Advanced Materials Laboratory, Department of Physics,
University of Dhaka. The authors thank the Bose Centre for Advanced Study and
Research in Natural Sciences, University of Dhaka for partial support of this
work.
- 1. P.B. Belavi, G.N. Chavan, L.R. Naik, R. Somshekar, and R.K. Kotnala, Mat. Chem. And Phys. 132[1] (2012) 138-144.
-

- 2. K.H. Maria, S. Choudhury, M.A. Hakim, Int. Nano Lett. 42[3] (2013) 1-10.
-

- 3. Y. Kim, D. Kim, and C.S. Lee, Physica B 337[1-4] (2003) 42-51.
-

- 4. S.G.C. Fonseca, L.S. Neiva, M.A.R. Bonifácio, P.R.C. Santos, U.C. Silvad, and J.B.L. de Oliveira, Mater. Res. 21[3] (2018) e20170861.
-

- 5. A.V. Raut, R.S. Barkule, D.R. Shengule, and K.M. Jadhav, J. Magn. Magn. Mater. 358-359 (2014) 87-92.
-

- 6. S. Singhal, T. Namgyal, S. Bansal, and K. Chandra, J. Electromagn. Anal.Appl. 2[6] (2010) 376-381.
-

- 7. A. Khan, G.D. Al-Quaderi, M.A. Bhuiyan, K.H. Maria, S. Choudhury, K.M. Amjad Hossain, D.K. Saha, and S. Akhter, Biointerface Res. Appl. Chem. 10[3] (2020) 5665-5669.
-

- 8. S. Singhal, T. Namgyal, S. Bansal, and K. Chandra, J. Electromagnetic Analysis and Applications. 2[6] (2010) 376-381.
-

- 9. S. Tapdiya and A.K. Shrivastava, Int. J. Inno. Res. Sci. Eng. Tech. 5 (2016) 6681-6684.
- 10. M.T. Jamil, J. Ahmad, S.H. Bukhari, T. Sultan, M.Y. Akhter, H. Ahmad, and G. Murtaza, J. Ovonic Res. 13[1] (2017) 45-53.
- 11. R. Rani, G. Kumar, K.M. Batoo, and M. Singh, American J. Nanomaterials 1[1] (2013) 9-12.
-

- 12. S.T. Alone and K.M. Jadhav, Indian Academy of Sciences 70[1] (2008) 173-181.
-

- 13. I. N. Esha, Kazi Hanium Maria, Enayet Hossain, M. N. I. Khan, Md. Al-Amin, and F. T. Z. Toma, J. Ceram. Process. Res. 20[5] (2019) 530-539.
-

- 14. M.A. Ameer, and M. El-Hiti, J. Magn. Magn. Matter. 234[1] (2001) 118-125.
-

- 15. P. Puspitasari, Y. Yahya, N.A.M. Zabidi and N.A. Ahmad, J. Appl. Sci, 11[7] (2011) 1199-1205.
-

- 16. A. Poorbafrani, and E. Kiani, J. Magn. Magn. Mater. 416 (2016) 10-14.
-

- 17. A. Khan, M.A. Bhuiyan, G.D. Al-Quaderi, K.H. Maria, S. Choudhury, K.A. Hossain, S. Akther, and D.K. Saha, J. Bang. Aca. Sci, 37[1] (2013) 73-82.
-

- 18. R. Saravanan, in “Solid Oxide fuel cell materials” (Materials Research Forum LLC, 2018) p.134.
-

- 19. J. Smit, H.P.J. Wign, in “Ferrites” (John Willy & Sons, 1959) p.143.
- 20. S.M. Yunus, H. S. Shim, C.H. Lee, M.A. Asgar, F.U. Ahmad, and A.K.M. Zakaria, J. Magn. Magn. Mater. 232[3] (2001) 121-132.
-

- 21. B.P. Ladgaonkar, P.N. Vasambekar and A.S. Vaingankar, J. Magn. Magn. Mater. 210[3] (2000) 289-294.
-

- 22. M.M. Haque, K.H. Maria, S. Choudhury, M.A. Bhuiyan, and M.A. Hakim, J. Ceram. Process. Res. 14[1] (2013) 82-86.
- 23. T.K. Gupta, and R.L. Coble, J. Am. Ceram. Soc. 51[9] (1968) 521.
-

- 24. F.T.Z. Toma, I.N. Esha, Md. Al-Amin, M.N.I. Khan, and K.H. Maria, J. Ceram. Process. Res. 18[10] (2017) 701-710.
- 25. K.H. Maria, U.S. Akter, I.N. Esha, Md. S. Hossain, and M.N.I. Khan, J. Supercond. Nov. Magn. 33[7] (2020) 2133-2142.
-

- 26. T. Mariam, and S. Choudhury, J. Bangladesh Acad. Sci. 41[1] (2017) 85-93.
-

- 27. S.E. Jacobo, and P.G. Bercoff, Ceram. Int. 42[6] (2016) 7664-7668.
-

- 28. K.H. Maria, S. Choudhury, and M.A. Hakim, J. Bangladesh Acad. Sci. 34[1] (2010) 1-8.
-

- 29. S. Noor, M.A. Hakim, S.S. Sikder, S.M. Hoque, K.H. Maria, and P. Nordblad, J. Phys. Chem. Solids 73[2] (2012) 227-231.
-

- 30. Z. Yue, Z. Ji, L. Li, X. Wang and Z. Gui, Mater. Sci Eng B 86[1] (2001) 64-69.
-

- 31. Z. Yue, Z. Ji, Z. Gui and L. Li, J. Magn. Magn.Mat. 264[2-3] (2003) 258-263.
-

- 32. S.S. Bellad, and B.K. Choughule, J. Mater. Chem. Phys. 66[1] (2000) 58-63.
-

- 33. I.N. Esha, F.T.Z. Toma, Md. Al-Amin, M.N.I. Khan, and K.H. Maria, AIP Advances 8[12] (2018) 125207 (1-17).
-

- 34. F.G. Brockman, P.H. Dowliay and W.G. Stenneck, Phys. Rev. 77[1] (1950) 85-93.
-

- 35. M.A. Hakim, S.K. Nath, S.S. Sikder, and K.H. Maria, J. Phys. Chem. Solids 74[9] (2013) 1316-1321.
-

- 36. S.K. Gore, S.S. Jadhav, V. V. Jadhav, S.M. Patange, M. Naushad, R.S. Mane and K.H. Kim, Sci Rep. 7[1] (2017) 1-9.
-

- 37. S.K. Nath, K.H. Maria, S. Noor, S.S. Sikder, S. Manjura Hoque, and M.A. Hakim. J. Magn. Magn. Mater. 324[13] (2012) 2116-2120.
-

 This Article
This Article
-
2020; 21(4): 442-449
Published on Aug 30, 2020
- 10.36410/jcpr.2020.21.4.442
- Received on Jan 27, 2020
- Revised on Apr 24, 2020
- Accepted on May 4, 2020
 Services
Services
- Abstract
introduction
experimental details
results and discussion
conclusion
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Kazi Hanium Maria
-
Department of Physics, University of Dhaka, Dhaka-1000, Bangladesh
Tel : +880-2-01711987595
Fax: +880-2-8615583 - E-mail: kazimaria@du.ac.bd






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.