- Evaluation of the crack formation of feldspathic ceramic reinforced with bor chemicals
Mustafa Hayati Atalaa,*, Esma Başak Gül Aygünb and Arife Doğanc
aIstanbul Medeniyet University, Faculty of Dentistry, Department of Prosthodontics, İstanbul, Turkey
bCukurova University, Faculty of Dentistry, Department of Prosthodontics, Adana, Turkey
cGazi University, Faculty of Dentistry, Department of Prosthodontics, Ankara, Turkey
The purpose of the current
study was to improve the mechanical strength and reduce the micro-cracks on the
microstructure of the dentin ceramic through addition of various boron
compounds (borax, boric acid). Following addition of borax and boric acid (1,
2, 3% of the weight percent) to the traditional feldspathic porcelain; crack
lengths, flexural strength and fracture toughness were analyzed. The data
analyses were performed by using one-way ANOVA (p<0.05). Differences between
groups were determined by Tukey HSD. The results of the present study suggested
that the crack lengths were decreased and the fracture toughness were increased
in all boric acid added groups (p<0.05). The group containing 1% boric acid
had significantly decreased the biaxial flexural strength value compared to the
control group (p<0.05). The Vicker’s hardness value of the group containing
1% borax was found to be significantly lower (p<0.05). The present study
demonstrated that various proportions of borax and boric acid addition in
dentin ceramic had reduced the formation of cracks. The current study could be
a good starting point on reinforcement of dental ceramics with a possible
outcome that will reduce the failures of dental restorations.
Keywords: boric acid, borax, feldspathic ceramic, mechanical features
The function, phonation and aesthetic deficiencies
resulting from damage or loss of teeth and surrounding tissues are restored
with appropriate prosthetic restorations. For nearly a hundred years, dentists
have been searching for the ideal restoration material. Although in recent
years, amalgam, composites and restorative cements have been used successfully,
they are not suitable for multi-faceted restorations.
Currently, dental ceramics are widely used in restorative dentistry.
They are used as monolithic restorations, porcelain inlays, onlays and metal
veneering materials due to the material quality and their aesthetic
appearance. Moreover, ceramic materials are chemically stable, have
long-lasting color stability, excellent biocompatibility and have an acceptable
wear resistance.
Dental ceramics are different from other restorative
materials, including metal and acrylic resin, by their chemical, physical,
mechanical and thermal properties. Even though conventional dental ceramics are
resistant to heat, they are fragile. Therefore, they have limited strength and
fracture toughness. Sudden changes in temperature and/or force can result in
fractures [1].
The ceramic used in dentistry is a not fully fused glass
derivative formed by sintering process. The sintering process can be defined as
a complex sequence of high temperature reactions that occur above the softening
point of the porcelain and lead to the partial melting of the glassy matrix by
combining the powder particles [2]. However, during the cooling of the sintered
dental ceramic to room temperature, micro-cracks occur
on the ceramic surface due to the volumetric
contraction of the material. Due to the shortcomings that occur during the
preparation and modeling of the ceramic as well as the faults that occur during
the sintering and cooling in the oven, these micro-cracks may spread to even
the deeper layers. The number, depth, width and even direction of these micro
cracks play an active role on the mechanical strength of the material
[3].Generally, these cracks or defect regions act as zones where stress is
concentrated. Previous cracks or subsequent cracks may result in larger cracks
and their propagation in the presence of stress. Such an expansion of cracks
causes catastrophic failure in material [4]. Fracture toughness is the
mechanical resistance of the brittle material to crack formation and the
destructive propagation of cracks under stress [5]. Therefore,
improving the fracture toughness of porcelain is an utmost
important criterion for extending the clinical performance of porcelain
restorations by improving low fracture strength in the mouth [1]. Recently,
there has been a focus on reinforcing ceramic materials by introducing
particles, whiskers, or fibers into matrix structure to improve the mechanical
properties and prevent crack propagation.
Boric oxide is a glassy solid, does not exist in nature
and is never used in the free state. The value of the boron mines is generally
measured by the amount of B2O3 (boric oxide) and the ones
with a high content of B2O3 are considered to be more
valuable. Borates (Boric oxide) plays an important structural function as a
network former, which is similar to that of silica. Borates with its flux and
binding properties can be used as an initiator for glass formation in that they
reduce the glass viscosity, decrease surface tension and crack
propagation. Moreover, they help to reduce thermal expansion,
and, hence, cause an increase in thermal shock resistance. They increase the
mechanical strength and scratch resistance of ceramic structure and provide
resistance to chemicals. Substituting boric oxide for a portion of the silica
content is a sure means of reducing the melting temperature of the glaze
without causing a devitrivication. Although small amounts of boric oxide
improve the mechanical properties of glaze, larger quantities
have adverse effects on crazing resistance [6-8].
Boric acid and sodium tetraborates are used for the
inserting boric oxide into ceramic structure. Boric acid is an inorganic binder
that is widely used in ceramic industry. Boric acid contains 56.3% boric oxide
[6, 7]. Borax is the most produced refined product after boric acid, which is
used as a strong melting agent in glaze. It reduces the viscosity and surface
tension and creates resistance to scratches, cracks and surface staining. Since
borax and boric acid are resistant to heat, they are used for making heat
resistant materials. Borax contains 36.52% of boric oxide [6-8]. Boric acid and
borax are widely used in glass, porcelain, and ware industry, especially in the
production of special glasses, where certain properties, including heat
resistance, surface hardness and durability, are desired [8].
The aim of the current study is to evaluate the
differences in the mechanical resistance of feldspathic ceramics with the
addition of borax and boric acid in varying amounts because they are so widely
used in glass and porcelain industry.
In this study, different proportions of borax and boric
acid were added into the feldspathic porcelain to examine the crack length,
hardness, flexural strength and fracture toughness. A feldspathic
porcelain [Ceramco 3 (Dentsply Degudent GmbH, USA)] dentin powder,
which is a Type 1 dental ceramic (DIN EN ISO 6872 standard) containing
opacifier, color pigment, 80-95% sodium potassium aluminasilicate and 0-20% tin
oxide, was used as a test material.
Boric acid and borax compounds and dentin porcelain powder
were dispersed in ethyl alcohol in a concentration of 1, 2 and 3%. “Ceramco 3”
commercial dentin porcelain powder by weight was homogenized by sonication for
half an hour in an ultrasonic bath and stirred for 4 hours with a magnetic
stirrer (Heidolph MR Hei-Standart, Nuremberg, Germany). The alcohol was
evaporated off and the samples were dried under vacuum for 24 hours.
Powder mixtures were prepared in 6 groups. Boron acid and
boron free dentin porcelain powder were used as a control group. In each group,
20 disc samples were prepared by using metal molds
(thickness 1.2 ± 0.2 mm; diameter
15 mm), where the sizes were determined by the standards for the flexure
test of Type 1 ceramics. Ten of these samples were used to determine the
biaxial flexural strength, and the rest of the samples were used to detect
Vickers' stiffness and crack size.
The samples were prepared by a baking process, where the
samples were first kept at 650 oC for 4 min with a
temperature rise of 50-70 oC per minute. When the temperature reached
910 oC, baking process was finished
within 2 minutes. The final surface conditioning of the
samples was performed using the diamond burs with a grain size of
15-20 µm. A total of 10 test specimens were prepared for 7 groups with a
thickness of 1.2 ± 0.2 mm and a diameter of 15 mm. After
final surface treatment, the samples were cleaned for 3 minutes in ultrasonic
bath (Euronda; Erosonic Energy, Italy) that contained distilled water.
Biaxial
Flexural Strength Test
For the biaxial flexural strength test, a testing machine
(Instron 5565A, Instron UK, England) with the lower
part containing 3-stainless steel balls (diameter of
2.5 mm) with an angle of 120° between them, was placed on a table
(diameter of 10 mm) and the piston head with a pin having a diameter of
1.4 mm was used. The load was applied vertically to the center of the
sample at a speed of 1 mm /min. A thin and soft film layer was placed
between the sample and the piston head to obtain an even
distribution of the applied forces.
The force at the time the sample got fractured was recorded
in Newton by the computer and then converted to MPa.
Vickers
Hardness Test and Fracture Toughness
After the samples were embedded in acrylic molds, their
surfaces were polished for 30 seconds on a sanding machine (Phoenix Beta,
Buehler, USA) at a speed of 300 rpm by pouring 0.3 µm grain size of
Al2O3 solution on a velvet cloth under cooling water.
Vickers micro hardness tester (Shimadzu HMV-2000, Germany)
was used to measure the Vickers hardness value (VHN)
after a 19.6 N load. Following the hardness tests,
acceptable crack models were examined with SEM (LEO, EVO
40, Cambridge-England). The samples were plated with
gold-palladium (Au-Pd) to determine crack lengths in the coating apparatus
(BAL_TEC; SCD50, Liechtenstein). Chemical analysis of the sintered
samples was done by EDS (Bruker, 125 eV, Minden-Germany) connected to SEM
unit.
Acceptable crack models were created with a 19.6 N
load with Vickers micro hardness tester (Shimadzu HMV-2000, Germany). It was
noted that the cracks were removed from the corners of the vickers indentation,
but not from the branch. Moreover, the average of the cracks was at least twice
the diagonal length. Reindentations were made for unacceptable crack models.
Crack lengths were determined by SEM device (LEO, EVO 40, Cambridge-England).
For each samples, traces of cracks that did not branch out
of the four corners of the Vickers indentation were measured. The crack length
was measured from the center of indentation in accordance with the literature.
The length of the fissures extending from all four corners were determined by
means of arithmetic mean and the crack length was determined by means of
arithmetic mean of the length of the cracks stretching from the four corners[5,
9]. Then, for the 10 samples in each group, the fracture toughness value was
calculated with the formula found by Anstis et al. [10] and it was expressed in
MPa.m0.5.
In this study, due to the fact that boric acid and borax
additions were as low as 1, 2 and 3% by weight and that the value of the
elastic modulus could not be found exactly, the calculation of fracture
toughness was performed by assuming the constant was 0.88 instead of using E /
H. [11].
Kıc = 0.016 (E/H)0,5 (P/c1,5)
(KIC: Fracture toughness value (MPa.m0.5);
E: Elastic Modulus; H: Hardness; P: Applied Force (N); c: Crack Length (m).
Crystallographic analyzes of the prepared samples were
analyzed using computer controlled X-ray diffrac- tometer (RigakuRadB-DMAX-II, Woodlands-USA).
IBM SPSS for Windows version 22 software was used for
statistical evaluation. The quantitative data definition for the variables were
presented as arithmetic mean (mean) ± standard deviation (SD).
Shapiro Wilk normality test was used to determine the normal distribution of
quantitative data (p>0.05). One-way analysis of
variance was used for intergroup comparison and Tukey
HDS test was used to determine the group that caused the difference.
Statistical significance was accepted as p < 0.05.
Biaxial
Flexural Strength Test
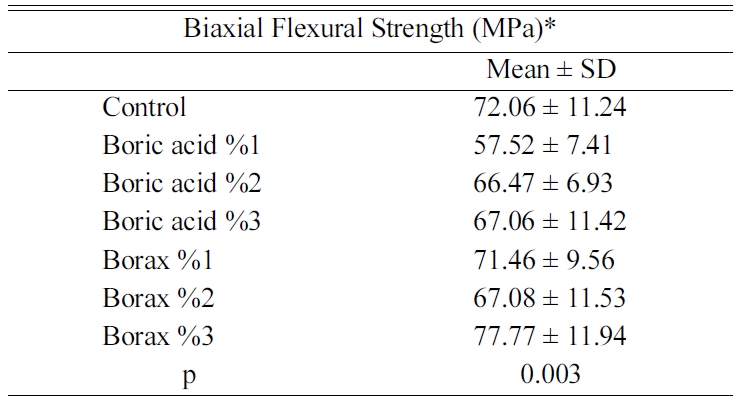
Biaxial flexural strength test results were presented in Table
1. The results demonstrated a significant difference between the
groups (p = 0.003; p < 0.01). The biaxial flexural
strength values of the group boric acid 1% was found to be significantly lower
than the control group (p = 0.034;
p < 0.05), the borax 1% (p = 0.048;
p < 0.05) and the borax 3%
(p = 0.001; p < 0.01). The mean values of biaxial
flexural strength of the other groups were similar to each other
(p > 0.05).
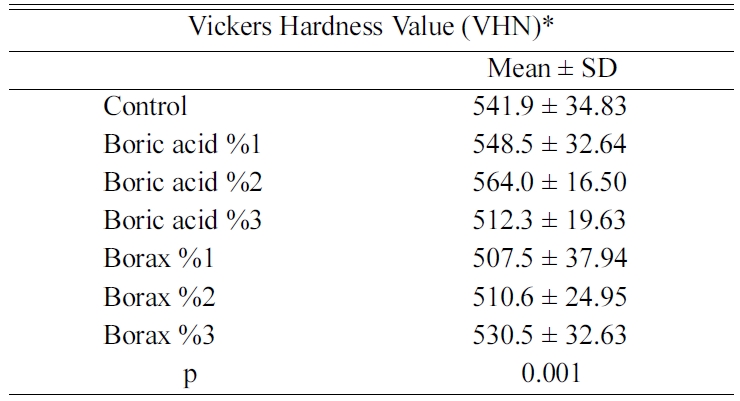
Hardness
Test
The mean and standard deviation values of the hardness
test results are given in Table 2. The Vickers hardness
values of the groups were significantly different
(p = 0.001; p < 0.01). The mean Vickers hardness value
of the group boric acid 2% was significantly higher than the groups of boric
acid 3% (p = 0.004; p < 0.01), borax 1%
(p = 0.001; p < 0.01) and borax 2% (p = 0.003; p < 0.01).
Moreover, the mean Vickers hardness value of the 1% of boric acid group was
significantly different than borax 1% group (p = 0.042;
p < 0.05) The Vickers hardness value was not significantly
different between the other groups (p > 0.05).
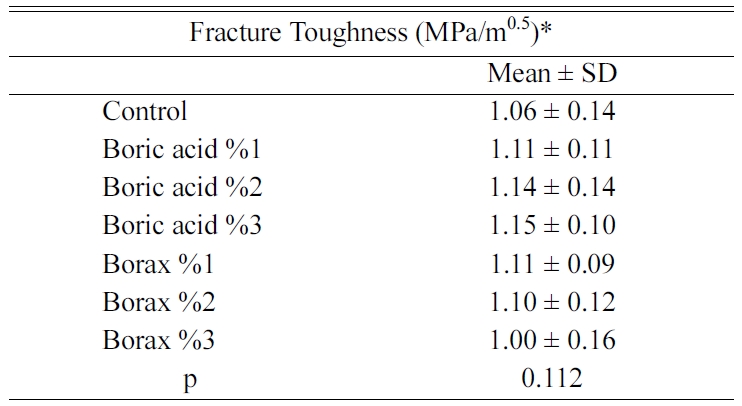
Fracture
Toughness
Fracture toughness values were determined by using crack
length values that were monitored by SEM. Mean and
standard deviation values of fracture toughness are given
in Table 3. SEM images obtained from the groups are also presented in Figure 1.
Although, 1%, 2% and 3% boric acid addition and 1% and 2%
borax addition had increased fracture toughness values, the mean fracture
toughness between the groups were similar to each other (p = 0.112;
p > 0.05).
XRD
Analysis
When XRD patterns of boric acid added groups and control
groups in Figure 2 as well as borax added groups and control groups in Figure 3
were examined, it was observed that the main phase was silica. The results
demonstrated that boric acid and borax added to the feldpastic matrix enters
the structure from the decreased intensity of 2q
peaks of silica at 27° and the increased intensity of 2q peaks of boron compounds at 55°.
Since the position and shape of the XRD peaks of the feldpastic phase were
similar, addition of boric acid and borax did not change the crystal structure
of the main phase.
Besides the excellent esthetic features, low tensile and
shear strength of ceramic restorations makes them fragile
during chewing. The main reason for the failures is the
formation of cracks and their propagation [12]. Feldspathic porcelain is the
most commonly used ceramic in dentistry. However, containing large
amounts of glassy phase reduces its
durability [13, 14]. Therefore, ceramic restorations are subjected
to firing in the laboratory. During the cooling of the restorations to the room
temperature, volumetric contractions in the material caused the formation of
micro cracks on the surface of the porcelain. These micro cracks can
propagate even deeper. In addition, the final conditioning of the
restoration surface also adversely affects the micro cracks. As a result, the
negative impact of the layer containing the micro-cracks further changes the
mechanical properties of the ceramic material [3, 15].
Boron compounds are used to strengthen the mechanical
properties of ceramics. Boron oxide may undergo normal glass form, but it is
generally used as a binder and network former. Borate plays a significant role
in glazing technology and is the second most important constituent after the
silica. It has been reported that strength to the mechanical resistance and
scratch formation increases with the amount of borate in the content of ceramic
glazures [6].
Boron oxide (B2O3) acts as a glass
modifying agent by reducing the viscosity and softening temperature of the
silica network, such as alkali metal ions. Boron oxide has its own network
structure, which enters between the three-dimensional silica
network structures. Such silica tetrahedrans is partially degraded by
the boron-oxygen (-Si-O-B-) interaction occured between oxides in silica
tetrahedral and boron compounds. This allows coefficient of softening
temperature and thermal expansion of silica network structure to decrease
compared with pure silica [16].
Boric acid and borax are inorganic binders which are
widely used in ceramic industry [7, 17]. It is stated in the literature that
they provide resistance to scratches, cracks and surface staining by decreasing
viscosity and surface tension [7, 17, 18]. In previous studies, the amount of
boric acid used ranged between 1% and 3% by weight [7, 18].
Structures with a low coefficient of thermal expansion
have higher thermal shock resistance due to less stress [19]. Therefore, dental
ceramics have low thermal shock resistance, since they have high expansion
coefficient. In the current study, the addition of boric acid and borax, which
has its own network structure, was expected to reduce thermal expansion
resistance and decrease the formation of cracks by lowering the thermal
expansion coefficient in the silica structure of feldspathic porcelain.
It was determined that the crack lengths were generally
reduced by 1, 2 and 3% boric acid addition, respectively.
The crack lengths demonstrated a tendency to reduce
with the addition of 1% and 2% borax, but the
difference was not statistically significant. Moreover, the
addition of 3% borax was found to increase the crack length. Since each group
was treated with similar final surface conditioning process, it could mimic the
difference between the groups. However, glaze application
in future studies can provide better understanding.
One of the factors affecting the mechanical behavior of
ceramics against forces is the presence of crystal structures such as alumina,
leucite and zirconium oxide [20-22]. These crystals arrest the progression of
cracks within the structure, since it is difficult to penetrate into the
crystal [1]. Because dental ceramics contain a high proportion of glassy
phases, cracks can easily progress further. However, alumina particles, which
are added to the ceramic structure, are more difficult to break than glass, a
higher amount of energy is required for the formation of fracture. Hence, it
makes the crack pro- gression
more difficult. Therefore, alumina-glass composi- tion is twice as endure as the
glass phase [22-24].
Hammouda and Beyari [25] added yttrium, which is a
partially stable zirconium, to the conventional dental porcelain by 3, 5 and 7%
by mass and reported that 3 and 5% addition elevated the flexural resistance
but decreased the hardness and increased its radiopacity. Some researchers have
added wollastonite, which is an industrial mineral and defined as natural
calcium metasilicate, and reported that the addition of 2% wollastonite to the
porcelain powder increases the flexural resistance of the dental alumina or
porcelain [26]. Moreover, Medeiros et al. [27] performed
reinforce- ment studies
with porcelain and reported that addition of Gadolinium Aluminum Perovskit
(GdAlO3 / Al2O3) fibers increased the
resistance of porcelain. Bayca [17] reported that addition of ulexite, is a
boron mineral, to ceramic body decreased the sintering temperature of the
tiles, however there is an increase in the bending strength of the ceramic
tiles.
Boric acid and borax have a similar structure with silica
and they are used as a dissolver in ceramic industry. Therefore, the aim of the
present study was to investigate the effects of the addition of crystalline
boric acid and borax compounds on the mechanical properties of feldspathic
porcelain.
The International Organization for Standardization (ISO
6872) proposed and assigned the biaxial flexural strength examination with a
three-point bending test to measure the flexural resistance (flexing endurance)
of dental ceramics [28]. The three point bending test is susceptible to cracks
and superficial pits formed on the sample surface and edges as the samples are
prepared. The formation of these cracks, defects and pits on the surface
significantly affect the results that will be analyzed [9, 29, 30]. In
addition, the fact that the dimensions and volumes of the samples are very
different from those of dental restorations and the risk of porosity formation
during the preparation of samples are also disadvantages of this method [31].
For these reasons, in the present study, biaxial flexuaral strength test was
preferred to determine the flexural resistances of the disc-shaped samples in
accordance with the 6872 standard prepared by ISO for durability testing.
Feldspathic porcelain is the most widely used dental
ceramic, but its flexural resistance is only between 60 and 70 MPa;
therefore, it requires a metal infrastructure to be strengthened [32]. Many
studies have been conducted to investigate the mechanical properties of
feldspathic porcelain [33-35]. In our study, piston test was used on three balls
and in parallel with the literature, the flexural resistance of conventional
felds- pathic Ceramco 3 porcelain used
for the control group was established to be 72.06 ± 11.24 MPa.
It is deter- mined that all values
found in the test result are above 50 MPa, which is the lowest value according
to ISO 6872 “Dental Ceramics” standard, and it is compatible with dental
ceramic standard.
The atomic bonds in the ceramic crystal are both covalent
and ionic and have no free electrons. These strong atomic bonds provide
stability, hardness and resistance to chemicals. However, they adversely affect
other conditions, including low thermal conductivity and increased fragility
[36].
In our study, it was observed that 2% and 3% boric acid
additions did not cause a significant change in flexural resistance
(p = 0.003; p < 0.01). The flexural resistance of the
feldspathic porcelain group with 1% boric acid was found to be
57.52 ± 7.41 MPa and was significantly lower than the control
group (p = 0.034; p < 0.05). The results of the current
study showed that the addition of 1% boric acid did not reduce the hardness of
the structure. It is thought that the silica tetrahedrals in the
three-dimensional network structure may have reduced the amount of Si-O
binding. As a result, the atomic bonds in the ceramic crystal may stay
stronger.
Although flexural–strength was similar between the control
and borax added groups (p = 0.003; p < 0.01), a
numerical increase was observed with the addition of 3% borax compared to the
control group. The addition of 3% borax may have entered the complex with its own
cage structure by greatly reducing the Si-O connec- tions and covalent bonds in
the three-dimensional network composition of the silica tetrahedrals. While it
decreases the stability and hardness of the material, borax slightly increases
flexural resistance due to its unique lattice structure. The hardness of the
materials are directly related to the crystal structures and bond strength
between atoms. As the bond strength increases, the hardness
of the minerals increases[37].
The hardness value of the restorative materials used in
the mouth is important. If their hardness is higher than that of dental enamel,
they can cause damage to the natural teeth. Therefore, the hardness value of
the material should be close to the enamel. On the other hand, if the hardness
value of the ceramic material is higher than that of the enamel, then the
abrasion effect is reduced with the glaze layer[37,38].In the light of these
findings, in the present study, Vickers hardness test method was used
considering the fragile and sensitive structure of dental ceramics. Moreover,
cracks were determined by Vickers hardness measuring device.
In this study, the hardness value of Ceramco 3 feldspathic
porcelain and the hardness value of boric acid and borax added feldspathic
porcelain samples were compared. The hardness value of 1% and 2% of borax added
groups were significantly lower than the control group (p = 0.042;
p < 0.05). The decrease in the 3% borax added group was not
statistically significant (p > 0.05). The addition of 1, 2, and 3%
borax through its cage structure reduced Si-O connections in the three-dimensional
network structure of silica tetrahedrals. However, it
is believed that this application reduced the strength of the atomic
connections in the ceramic crystal and decreased the hardness. In addition, the
addition of 1, 2, and 3% of borax did not have any effect to
increase the abrasiveness of feldspathic porcelain.
On the other hand, the addition of 1 and 2% of boric acid
showed no significant difference in the hardness value of the control group. It
is thought that it did not decrease the stability and hardness of the structure
by reducing the Si-O connections that affect the stability of
the ceramic structure. However, its addition improves the amount.
It can be claimed that the addition of 3% boric acid greatly reduced the Si-O
connections in the three-dimensional network of the silica tetrahedrals.
Moreover, its addition also reduced the softening temperature,
the expansion coefficient, and atomic bonds while
decreasing the intermittent space, thus reducing the hardness.
The indentation fracture toughness technique is relatively
simple to apply; it only requires a smoothly polished surface for the sample to
be measured. When the literature is examined, it is seen that this technique is
generally used to determine the fracture toughness of ceramic materials
[39-42]. Therefore, in the current study, indentation fracture toughness test
method was used to evaluate fracture toughness.
Hammouda and Beyari [25] evaluated the fracture toughness
of conventional dental porcelain, which was treated with
3, 5 and 7% of yttrium stabilized tetragonal zirconium
polycrystalline (Y-TZP). Their results showed that the
fracture toughness value of the conventional feldspathic
porcelain was 0.538 ± 0.049 MPa.m0.5. However,
the fracture toughness values of 3, 5 and 7% yttrium partially stable zirconium
addition groups were 1.424 ± 0,064 MPa.m0,5,
0.782 ± 0.106 MPa.m0,5, 0.489 ± 0.069 MPa.m0,5
respectively. These results suggest that increased particle
size reduces the fracture toughness when other microstructural variables are
assumed constant.
Yoshimura et al. [43] evaluated the fracture toughness of
high heat alumina core ceramics, feldspathic porcelain, hot press
leucite based glass ceramics, glass ceramics and glass infiltrating alumina
ceramics reinforced with hot press lithium disilicate according to Astm e399
standards. The fracture toughness values were 0.67 ± 0.08 MPa.m0,5,
0.84 ± 0.07 MPa.m0,5, 0.96 ± 0.03 MPa.m0,5,
1.81 ± 0.18 MPa.m0,5 and
2.91 ± 0,20 MPa.m0,5, respec- tively. Researchers have stated that the
materials they used may have different mechanical properties and that the
highest fracture toughness value of glass infiltrating alumina ceramic may be
related to the high elastic modulus and fracture surface energy.
In this study, the fracture toughness value of the control
group was 1.06 ± 0.14 MPa.m0.5, while the fracture
toughness values of 1, 2, and 3% boric acid added groups were
1.11 ± 0.11 MPa.m0.5, 1.14 ± 0.14 MPa.m0.5,
1.15 ± 0.10 MPa.m0.5, respectively.
Although there was a quantitative difference between the groups, it was not
statistically significant. With the addition of boric acid, the Si-O connection
amount of the silica tetrahedrals in the three-dimensional network structure
could be reduced as well as the softening temperature and thermal expansion
coefficient of the porcelain may also be decreased. Thus, the distance between
the particles has diminished and the crack propagation may have been more
difficult. Therefore, it is thought that the fracture toughness of the
structure may be elevated. In addition, boric acid has a less complex
structure, which has a better penetration into the silica structure and higher
ability to fill the gaps in the structure. As a result, elevated fracture
toughness may be resulted in increase of the numerical value.
Similar effects were achieved with 1 and 2% Borax
additions. While the fracture toughness of the ceramic structure is increased,
the addition of 3% borax may significantly reduce the connections
and covalent bonds in the Si-O network which affect the
stability of the ceramic structure and damaged it. As a result, the fracture toughness
value was reduced. Moreover, with the wider lattice structure of borax,
interactions within the structure have been reduced with respect to particle
sizes. As a result, the fracture toughness value may be decreased as the
percentage by weight in the structure increases.
On the other hand, the fracture toughness values of
feldspathic porcelain with 1, 2 and 3% borax added groups were
1.11 ± 0.09 MPa.m0.5,
1.11 ± 0.09 MPa.m0,5 respectively.
It was determined that the addition of borax in the ratio of 1% and 2% in
fracture toughness value was 1.00 ± 0.16 MPa.m0,5,
but this increase was not statistically significant. However, the addition of
3% borax was found to cause a relative decrease in fracture toughness.
Several techniques have been proposed in the literature to
examine the formation and length of cracks in dental materials. These are SEM,
light microscope, a convergent laser scanning microscope and transillumi- nation (posterior light imaging) technique
[44-46]. Among them, SEM analysis is an effective and widely accepted method
for the examination of properties, such as surface topography, dispenser and
connections [47]. Therefore, in the present study, SEM technique was used to
determine the crack length. Moreover, chemical analyses of samples were
evaluated with EDS detector connected to SEM unit.
It has been observed that using Vickers micro hardness
device, traces were formed within cracks that did not progressively advanced
through porosities. The image of these traces is consistent with the literature
[5]. In addition, images of boric acid and borax added groups were uniform and
homogeneous that were consistent with the image of the
unadulterated feldspathic porcelain group. These results
suggested that boric acid and borax additions did not damage the feldspathic
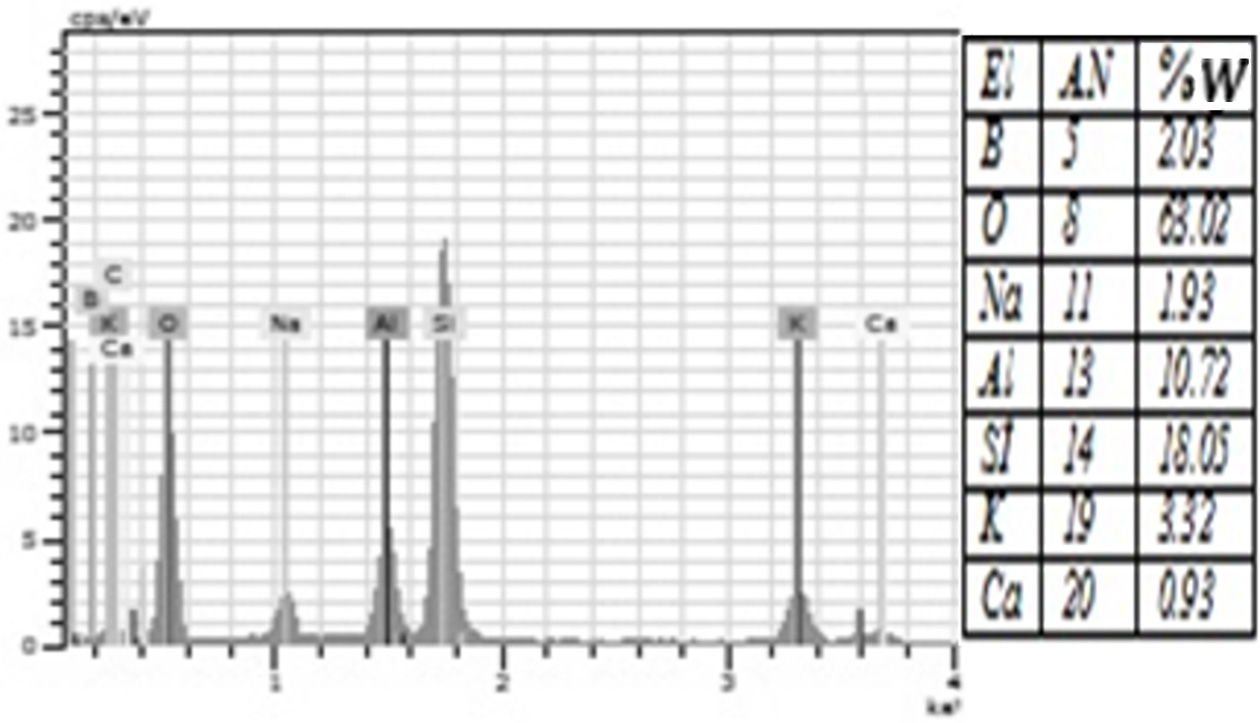
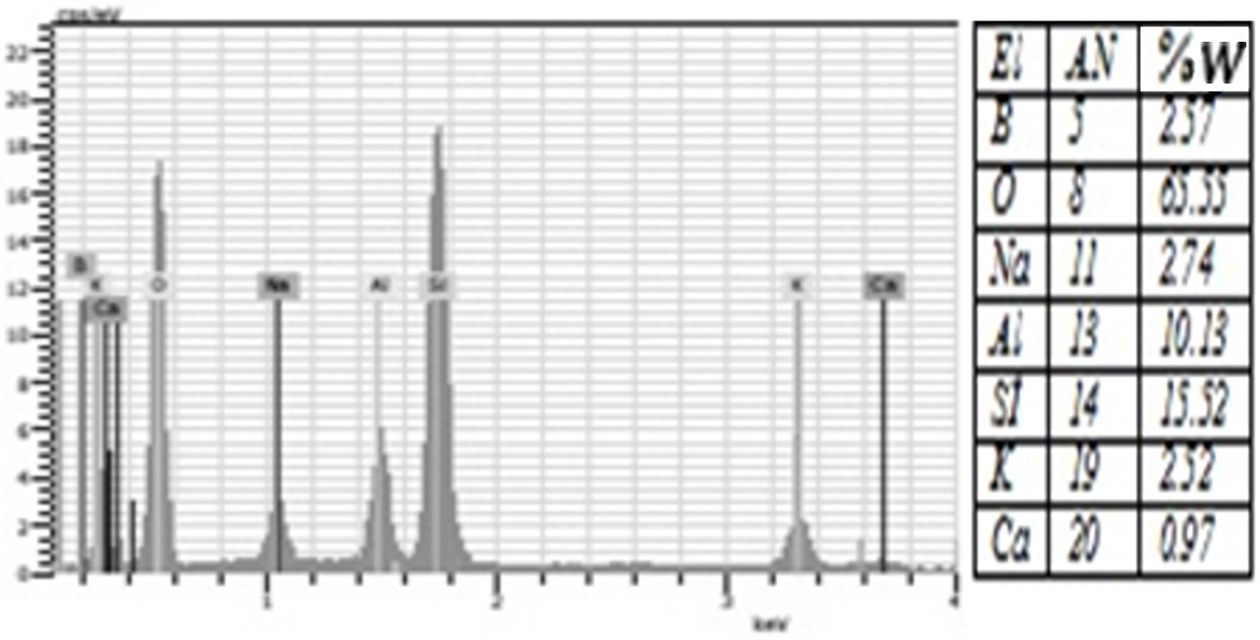
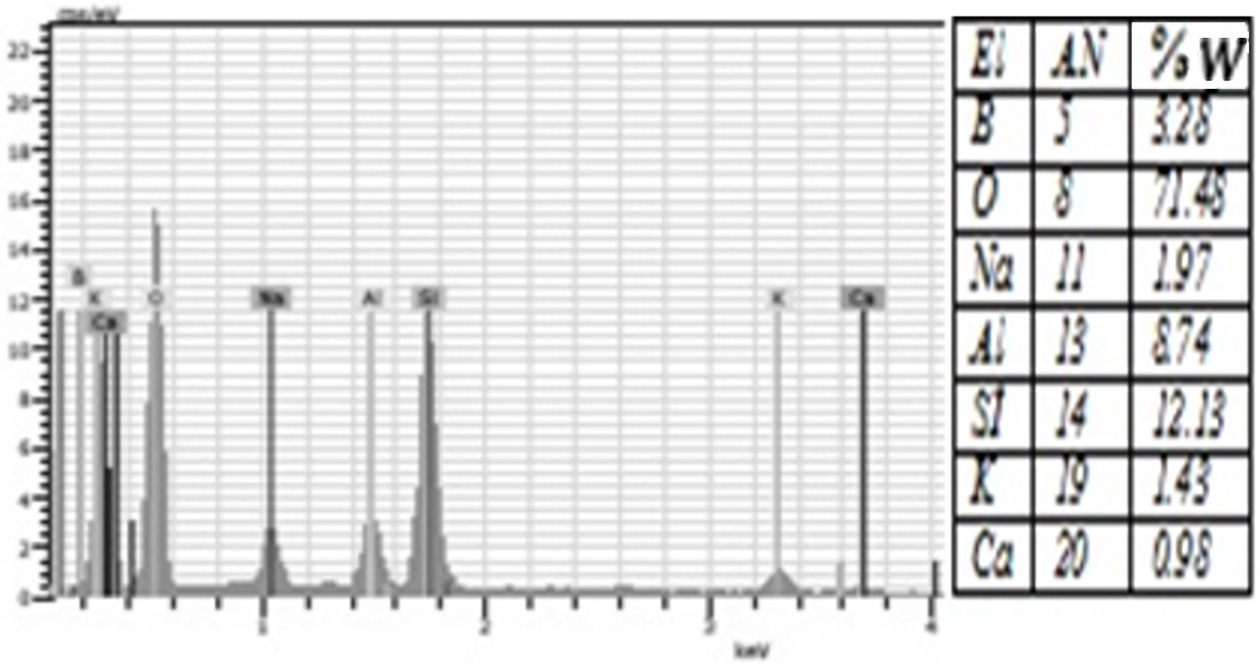
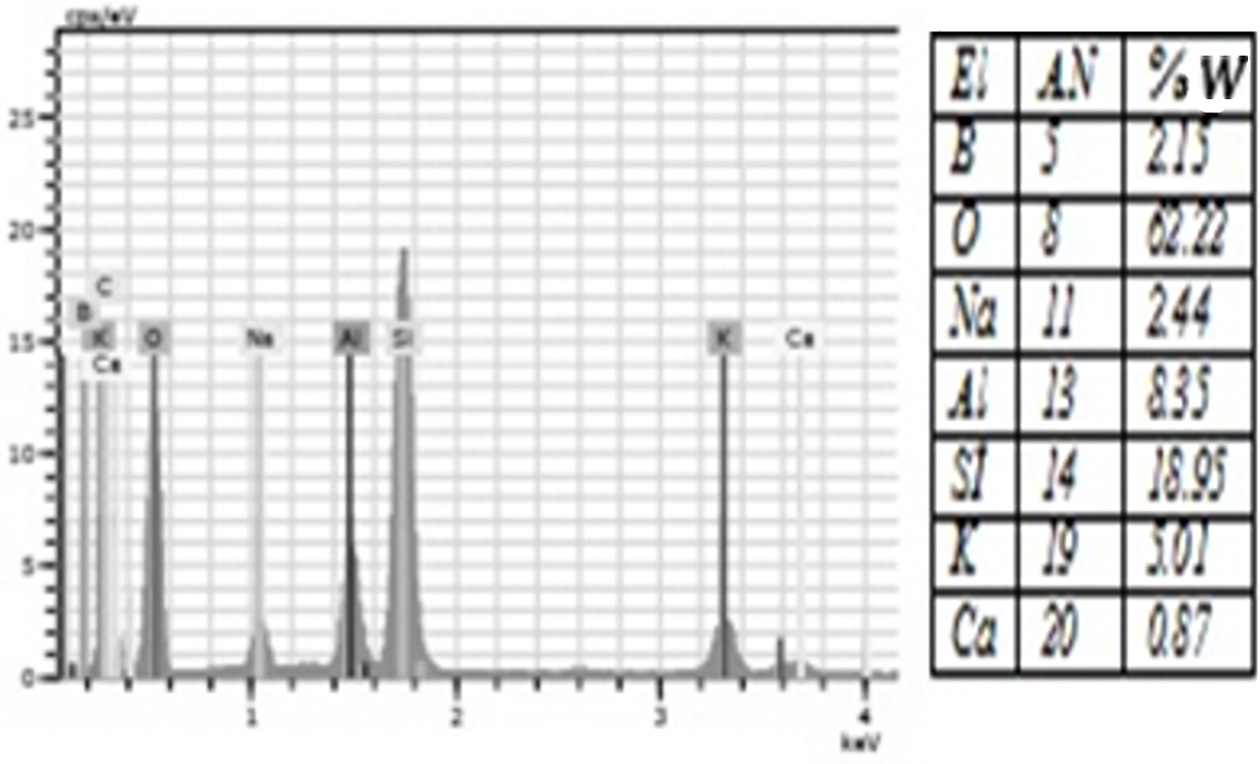
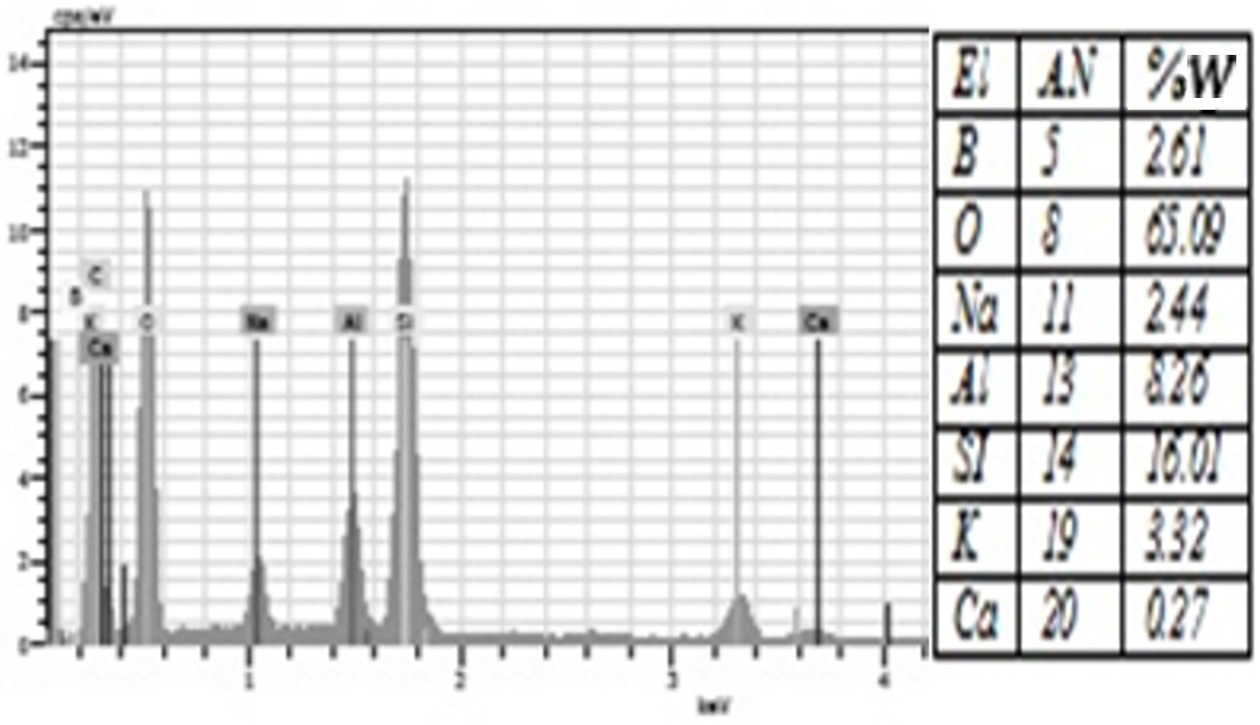
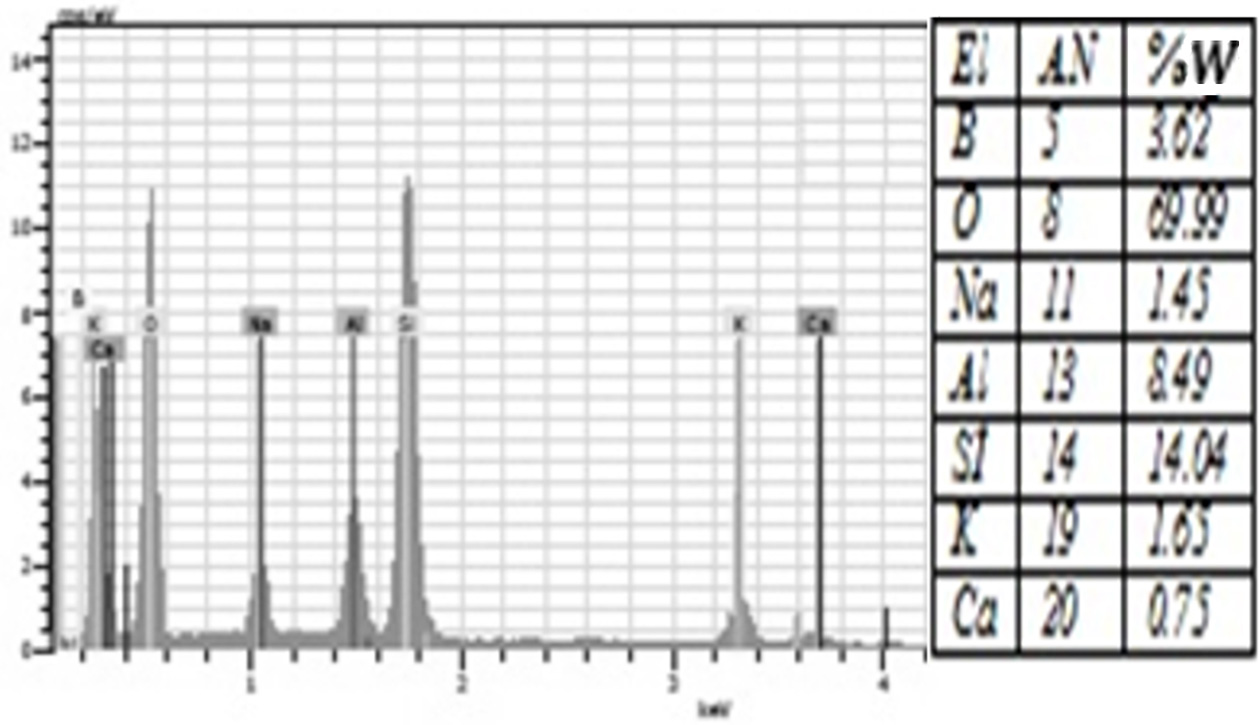
porcelain structure. Moreover, EDS analysis showed that all groups were rich in
silicon and oxygen elements (Figure 4-10). The detection of boron in
borax and boric acid added groups following the analyses of
individual elements suggested the involvement of borax and boric acid in the
porcelain structure.
X-ray diffraction method (XRD) is widely used to determine
different crystal structures and knitting parameters in the materials. In the
present study, XRD analyzes were performed to investigate the effect of boron
compounds on ceramic structure. When the XRD graphs were obtained from sintered
unadulterated feldspathic porcelain group, the boric acid and borax added
groups were examined. It was observed that the silica was
the main phase. These findings are compatible with the
structure of the feldspathic porcelain and also support the result of EDS
analysis. XRD patterns of borax and boric acid added groups were different from
the control group. The entrance of boric acid and borax to the Feldpastic
matrix was observed from decreased intensity of the 2q
peak of silica at 27° and from the 2q
peaks of boric acid or borax at 55°. As the positions and shapes of XRD peaks
of the Feldpastic phase did not change, it was concluded that addition of borax
and boric acid did not change the crystal structure of the main phase. The SEM
images of the sintered porcelain samples obtained from the control, borax and
boric acid groups support that the crystal structure did not change. In
addition, XRD results are consistent with EDS analysis results.
Fig. 5 Fig. 6 Fig. 7 Fig. 8 Fig. 9
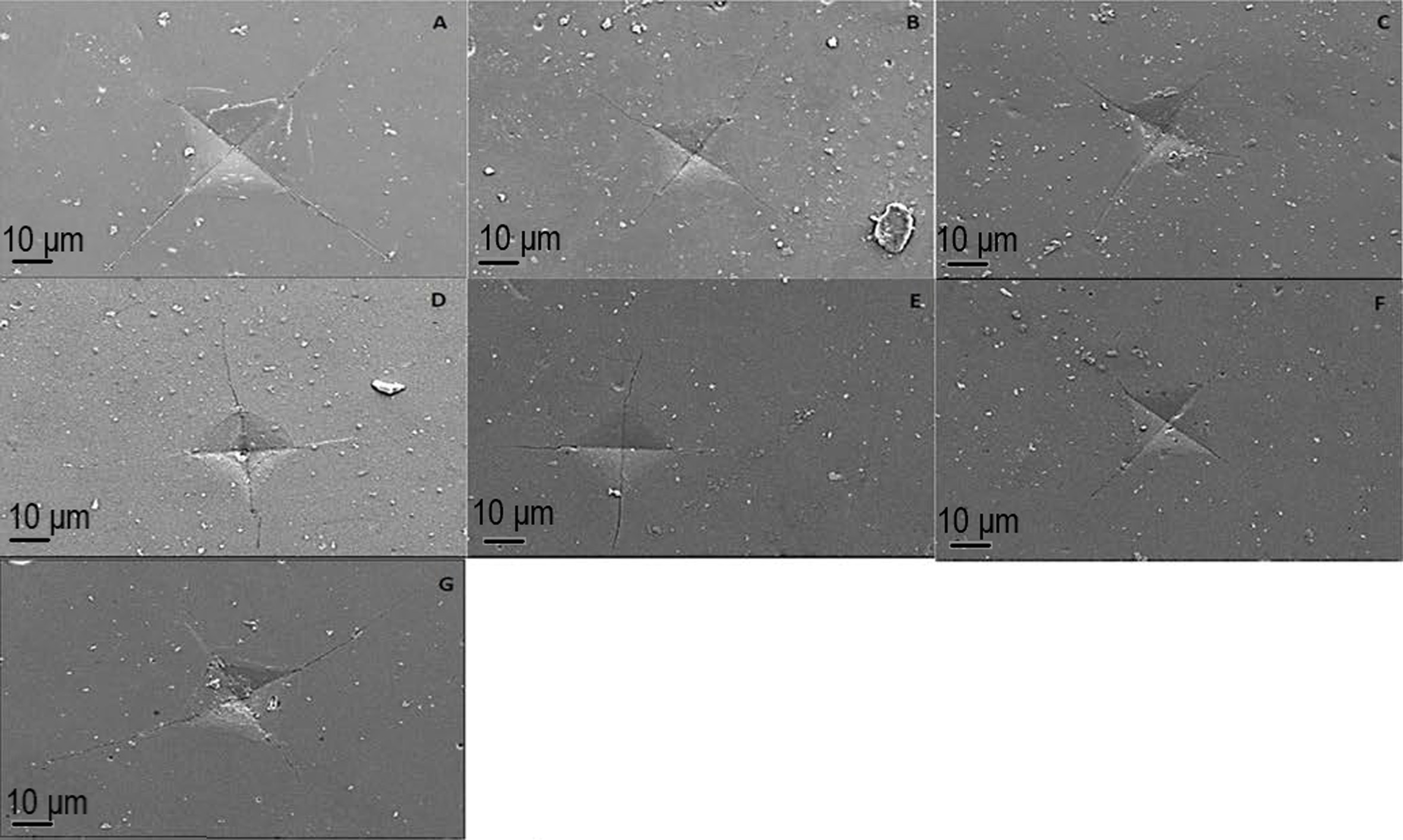
|
Fig. 1 SEM images of crack formation on sintered samples. (A) SEM Image (×1000) of crack formation in control group at (B) SEM Image (×1000) of crack formation in %1 boric acid added group (C) SEM Image (×1000) of crack formation in %2 boric acid added group, (D) SEM Image (×1000) of crack formation in %3 boric acid added group, (E) SEM Image (×1000) of crack formation in %1 borax added group, (F) SEM Image (×1000) of crack formation in %2 borax added group, (G) SEM Image (×1000) of crack formation in %3 borax added group. |
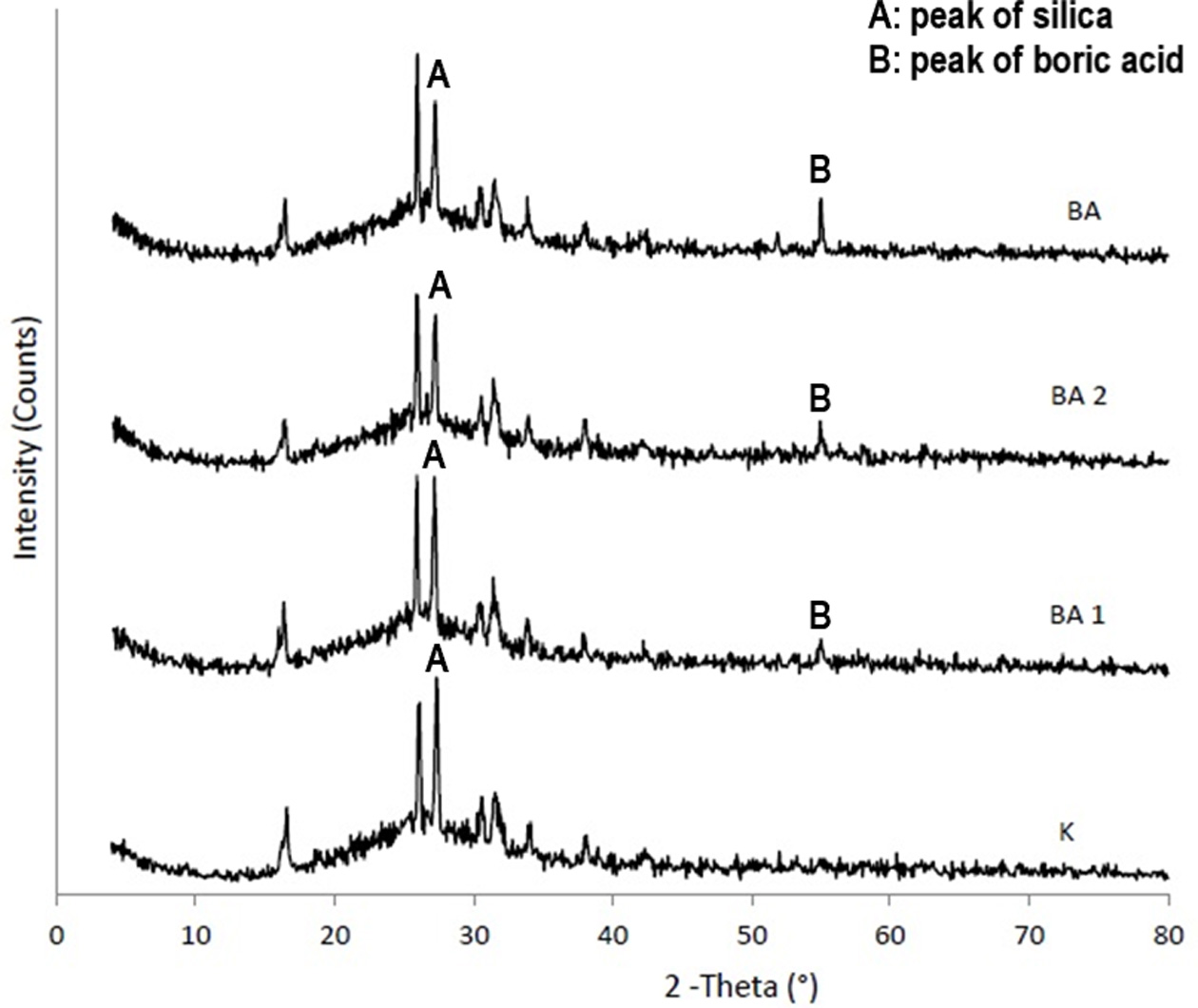
|
Fig. 2 Comparative XRD results of boric acid added groups compared with control group. |
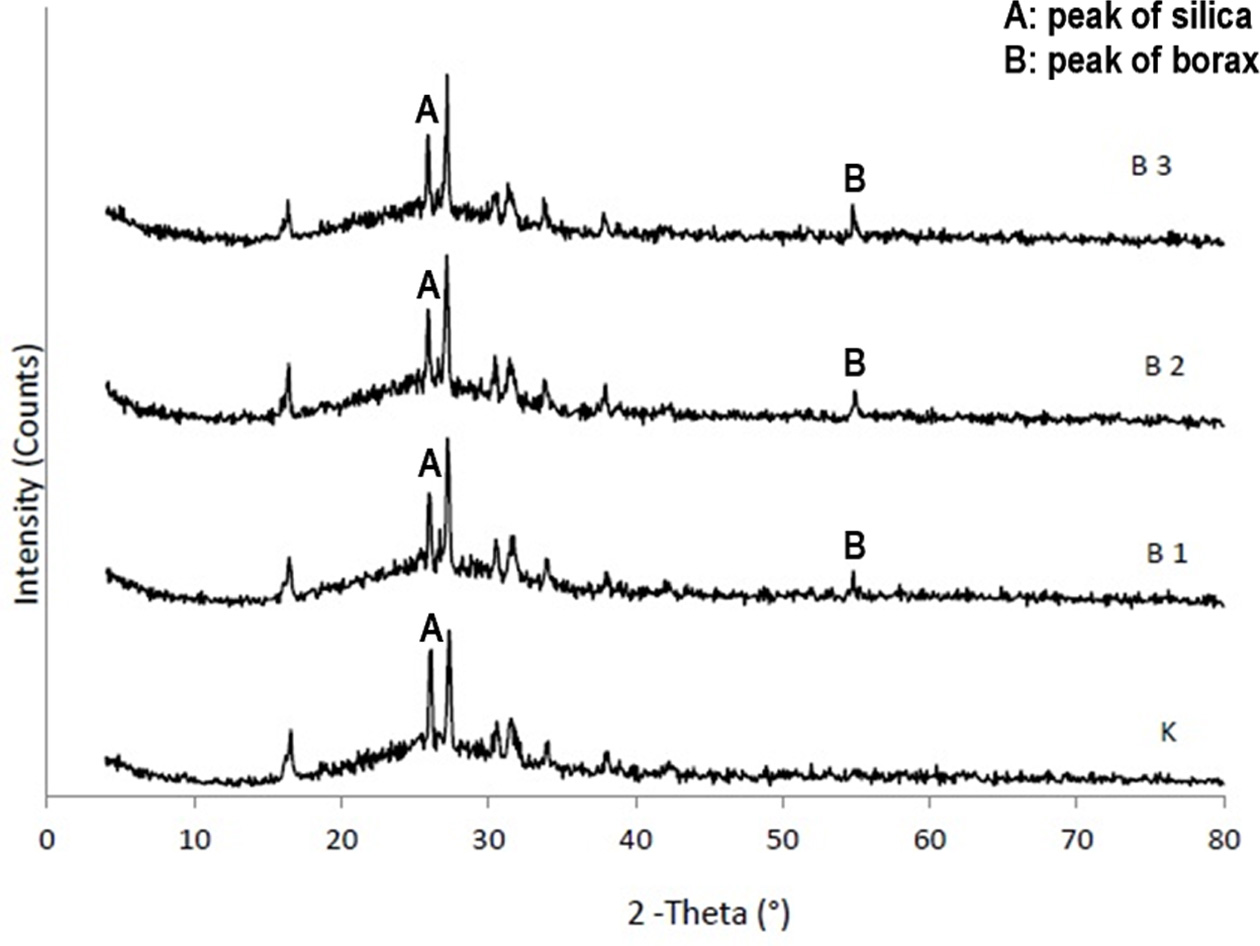
|
Fig. 3 Comparative XRD results of borax added groups compared with control group. |
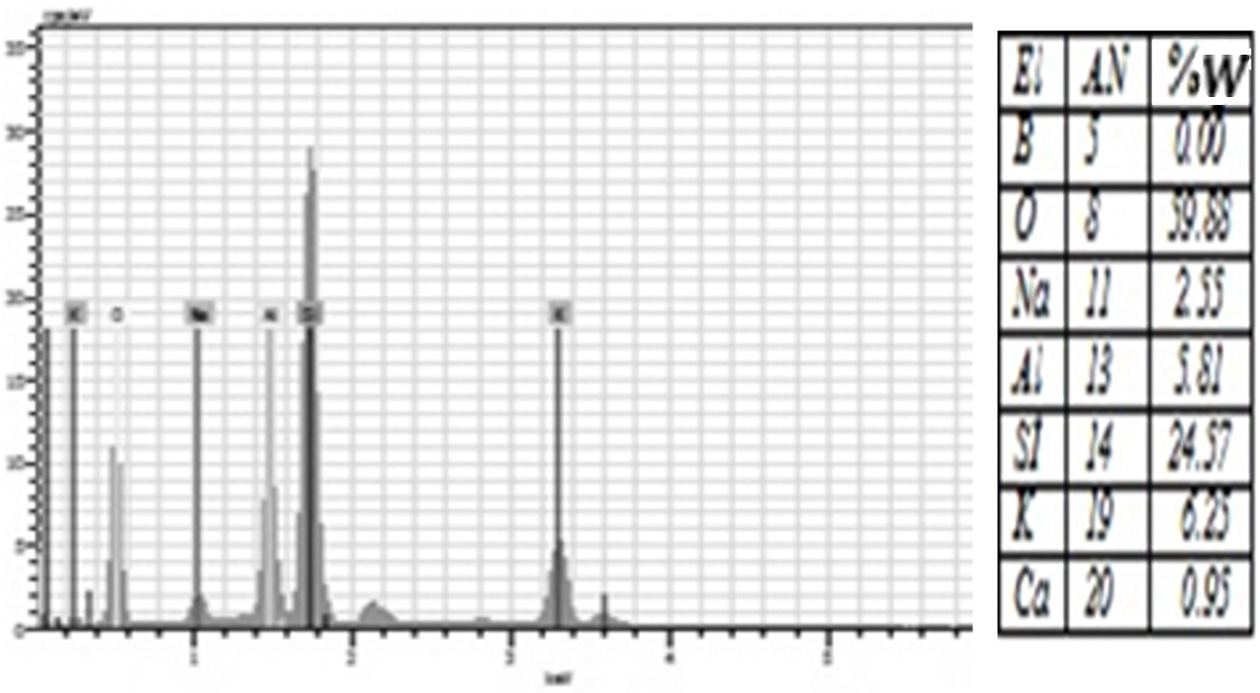
|
Fig. 4 The amount of elements contained in the control group according to EDS analysis. |
|
Fig. 5 The amount of elements contained in the %1 Boric Acid group according to EDS analysis. |
|
Fig. 6 The amount of elements contained in the %2 Boric Acid group according to EDS analysis. |
|
Fig. 7 The amount of elements contained in the %3 Boric Acid group according to EDS analysis. |
|
Fig. 8 The amount of elements contained in the %1 Borax added group according to EDS analysis. |
|
Fig. 9 The amount of elements contained in the %2 Borax added group according to EDS analysis. |
|
Fig. 10 The amount of elements contained in the %3 Borax added group according to EDS analysis. |
Within the limits of current study, reinforcement of
feldspathic ceramic with small quantities of boric acid or borax resulted in an
increased fracture toughness value compared to the control group. This may
indicate that boron products also have fluxing effect on dental ceramics. On
the other hand, biaxial flexural strength of feldspathic ceramic decreased with
the addition of varied amounts boric acid and borax but was not significant
compared to the control group. It is considered that the
current study could be a good starting point for future studies on
reinforcement with boron compounds of feldspathic dental ceramic
and innovation of different high technology dental ceramics
with strengthening properties.
This study was supported by İnönü University Scientific
Research Project Department (Project no: 2013/185).
- 1. K.J. Anusavice, C. Shen, and H.R. Rawls, in “Phillips' science of dental materials” (Elsevier Health Sciences, 2013) p.418-474.
- 2. I.L. Denry, Crit. Rev. Oral Bio. Med. 7 (1996) 134-43.
-

- 3. K. Anusavice and B. Hojjatie, J. Dent. Res. 70 (1991) 1009-1013.
-

- 4. M. Albakry, M. Guazzato, and M.V. Swain, J. Biomed. Mater. Res., Part B 71 (2004) 99-107.
-

- 5. S.S. Scherrer, I.L. Denry, and H.A. Wiskott, Dent. Mater. 14 (1998) 246-255.
-

- 6. S. Stefanov, Glass Tech. 41 (2000) 193-196.
- 7. Ö. Cengiz and A. Kara, AKU-J. Sci. Eng. 9 (2009) 29-35.
- 8. S. Cook, in “Ceramic engineering and science proceedings” (Wiley Online Library, 2002) p.47-55.
-

- 9. H. Yilmaz, C. Aydin, and B.E. Gul, J. Prosth. Dent. 98 (2007) 120-128.
-

- 10. G. Anstis, P. Chantikul, B.R. Lawn, and D. Marshall, J. Amer. Cer. Soc. 64 (1981) 533-538.
-

- 11. P. Chantikul, G. Anstis, B.R. Lawn, and D. Marshall, J. Amer. Cer. Soc. 64 (1981) 539-543.
-

- 12. M.A. Rosenblum and A. Schulman, J. Amer. Dent. Assoc. 128 (1997) 297-307.
-

- 13. R. Seghi, T. Daher, and A. Caputo, Dent. Mater. 6 (1990) 181-184.
-

- 14. L.K. Weinstein and A.B. Weinstein, US Patent No. 3,052,982 (1962).
- 15. R. Cook, B. Lawn, T. Dabbs, and P. Chantikul, J. Amer. Cer. Soc. 64 (1981) C-121-C-122.
-

- 16. M. Kah, in “Comparison of Surface Treatments on the Strength of Dental Porcelain”. (The University of Sydney, 1992) p.11.
- 17. S.U. Bayca, J. Ceram. Process. Res. 10 (2009) 162-166.
- 18. T. Aydın and A. Kara, AKU-J. Sci. Eng. 9 (2009) 53-60.
- 19. R.E. Loehman, in “Characterization of ceramics” (Momentum Press, 2010) p.169-187.
- 20. A.S.C. Nathan, R. Tah, and M.K. Balasubramanium, J. Oral Biol. Craniofac. Res. 8 (2018) 221-224.
-

- 21. R. Tokunaga, H. Takahashi, N. Iwasaki, M. Kobayashi, K. Tonami, and N. Kurosaki, Dent. Mater. J. 27 (2008) 347-355.
-

- 22. H. Fischer and R. Marx. J. Dent. Res. 80 (2001) 336-339.
-

- 23. W.J. O'Brien, in “Dental materials and their selection” (Quintessence Publishing Company, 2002) p.212-229.
- 24. A. Shenoy and N. Shenoy, J. Conserv. Dent. 13 (2010) 195.
-

- 25. I.M. Hamouda and M.M. Beyari, Int. J. Sci. Re.s Know. 1 (2013) 404.
-

- 26. N.V. Asar, T. Korkmaz, and E.B. Gül, Mater. Des. 31 (2010) 2540-2545.
-

- 27. I.S. Medeiros, L.A. Luz, H.N. Yoshimura, P.F. Cesar, and A.C. Hernandes, J. Mech. Beh. Biomed. Mater. 2 (2009) 471-477.
-

- 28. ISO, No. 6872 (2008) p.11-21.
- 29. B. Ersu, M. Yenigül, and I. Tulunoğlu, J. Hacettepe Univ. Fac. Dent. 31 (2007) 71-78.
- 30. A. Miller, J. Long, B. Miller, and J. Cole, J. Prosth. Dent. 68 (1992) 38-41.
-

- 31. J.R. Kelly, Dent. Mater. 11 (1995) 103-110.
-

- 32. J.W. McLean, Oper. Dent. 16 (1991) 149-156.
- 33. R.A. Giordano, L. Pelletier, S. Campbell, and R. Pober, J. Prosth. Dent. 73 (1995) 411-418.
-

- 34. M.A. Kılıçarslan and A. Zaimoğlu, J. Istanbul Univ. Fac. Dent. 32 (1998) 28-33.
- 35. R.R. Seghi, J.A. Sorensen, R. Seghi, and J. Sorensen, Int. J. Prosth. (1995) 8.
- 36. A. Zaimoğlu, G. Can, E. Ersoy, and L. Aksu, in “Diş hekimliğinde maddeler bilgisi” (AÜ Publisher, 1993) p.515.
- 37. J.D. Hudson, G.R. Goldstein, and M. Georgescu, J. Prosth. Dent. 74 (1995) 647-654.
-

- 38. D. Jagger and A. Harrison, J. Prosth. Dent. 72 (1994) 320-323.
-

- 39. A.S. Rizkalla and D.W. Jones, Dent. Mater. 20 (2004) 198-206.
-

- 40. J. Gong, Z. Zhao, and Z. Guan, J. Euro. Cer. Soc. 21 (2001) 941-946.
-

- 41. G. Elssner, H. Hoven, G. Kiessler, and P. Wellner, in “Ceramics and ceramic composites: materialographic preparation” (Elsevier, 1999) p.151-153.
-

- 42. M. Guazzato, M. Albakry, S.P. Ringer, and M.V. Swain, Dent. Mater. 20 (2004) 441-448.
-

- 43. H.N. Yoshimura, C.C. Gonzaga, P.F. Cesar, and W.G. Miranda Jr, Ceram. Int. 38 (2012) 4715-4722.
-

- 44. M.K. Etman. J. Prosthodontics 18 (2009) 550-559.
-

- 45. N. Beck, F. Graef, O. Gerstbrein, and M. Karl, J. Prosth. Dent. 104 (2010) 301-305.
-

- 46. I. Denry, J. Mackert Jr, J. Holloway, and S. Rosenstiel, J. Dent. Res. 75 (1996) 1928-1935.
-

- 47. D. Xie, W. Brantley, B. Culbertson, and G. Wang, Dent. Mater. 16 (2000) 129-138.
-

 This Article
This Article
-
2020; 21(4): 407-415
Published on Aug 30, 2020
- 10.36410/jcpr.2020.21.4.407
- Received on Nov 25, 2019
- Revised on Mar 11, 2020
- Accepted on Mar 16, 2020
 Services
Services
- Abstract
introduction
experimental
results and discussion
conclusions
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Mustafa Hayati Atalaa
-
Istanbul Medeniyet University, Faculty of Dentistry, Department of Prosthodontics, İstanbul, Turkey
Tel : +90-216-280-3803
Fax: +90-216-602-28-05 - E-mail: hayatiatala@gmail.com






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.