- Preparation and thermodynamic equilibrium analysis of neodymium disilicate via sol-precipitation and sintering
Shanjun Kea, Yanmin Wanga,*, Zhidong Pana,b,* and Heping Zenga
aSouth China University of Technology, Guangzhou 510640, China
bFoshan Oceano Ceramics Co. Ltd., Foshan 528138, China
Neodymium disilicate powders
were synthesized via a sol-precipitation method with different
precipitants (i.e., ammonia, ammonium bicarbonate and urea) and subsequent
sintering. The samples were characterized by X-ray diffraction. In addition, a
theoretical relationship between neodymium ion concentrations in different
precipitation equilibrium solutions was also investigated and analyzed based on
thermodynamic equilibrium calculation. The results show that the precipitant
type has an impact on the phase compositions of the precursors and the sintered
products. In the synthesis, neodymium ions can be completely precipitated as
amorphous neodymium hydroxide with ammonia as a precipitate at pH 8~9. For
ammonium bicarbonate as a precipitate, the precursor type is related to both
carbon concentration and pH value. The excessive hydroxide forms neodymium
hydroxycarbonate in a heated urea solution. The possible formation mechanism of
silicon sol in the presence of different precipitants was discussed.
Keywords: Precipitant, Precursor, Neodymium disilicate, Thermodynamis
Inorganic pigments are widely used in the field of ceramic
decoration [1-3]. As is known, the coloring ions of ceramic pigments are from
mainly transition metal elements. However, some ceramic pigments contain a
certain amount of heavy metal elements, which are strictly controlled due to
the corresponding environmental protection issues [4-7]. Due to special
structure and low toxicity, rare-earth ions are also widely used as colorants
in ceramic pigments [8, 9]. In addition, some functional ceramic
pigments are reported, such as effect pigments [10, 11],
luminescent pigments [12, 13], phosphorescent pigments [14, 15] and
near-infrared reflective pigments [16-19]. Moreover, recent work [20, 21]
revealed that neodymium disilicate (i.e., Nd2Si2O7)
powder can be applied as a functional ceramic pigment with allochroic effect
under various illuminants. Nd2Si2O7 powder has
a violet red color under an incandescent lamp, while presents a blue
color under a fluorescent lamp. The mechanism of color change
under different illuminations was discussed in detail in a previous work [22].
Solid-state reaction and sol-gel methods are the main preparation
routes for Nd2Si2O7 pigment [20-22]. However,
solid-state reaction usually requires harsh reaction con- ditions, such
as high temperatures and/or long treatment time [23,
24]. Although sol-gel method can achieve a low-temperature preparation, it
needs long-reaction time and precursors with severe agglomeration during
drying, leading to the formation of a poor dispersibility of the resultant
powder. In a previous study [25], Nd2Si2O7
powder was synthesized via a sol-precipitation method
with different precipitants. This sol-precipitation method is a
relatively simple way for the synthesis of Nd2Si2O7
pigment. However, different precipitants have a greater
impact on the phase composition of the precursor and
the final product. The relevant reasonable explanations
on the precipitation process of the precursor powder with
different precipitants are not reported so far.
As is well known, thermodynamic equilibrium analysis
is an effective method for describing the precipitation process
of ceramic materials. Many studies [26-28] reported that
the thermodynamic equilibrium calculation could
establish the equilibrium diagram to provide a theoretical guide for the
precipitation reaction. Li et al. [29] investigated the precipitation process
of silicate species in (NH4)2WO4-(NH4)2CO3-NH3-H2O
system by thermodynamic analysis. Their experimemtal results consisted with the
theoretical calculation.
In this paper, neodymium disilicate powders were synthesized
via a sol-precipitation method with different precipitants
(i.e., ammonia, ammonium bicarbonate and urea) and
subsequent sintering. The phase compositions of the
precursors and the sintered products with different precipitants were
discussed. In addition, the thermodynamic equilibrium parameters of precursors
in different precipitation equilibrium solutions were also calculated and
discussed based on the thermodynamic data and mass conservation.
The main ingredients include neodymium nitrate tetraethyl
orthosilicate (TEOS, C8H20O4Si, 98 wt.%,
Guangzhou Chemical Reagent Factory, China) and hexahydrate (Nd(NO3)3·6H2O,
99.5 wt.%, Ganzhou Ruihua Rare-earth Co. Ltd., China). Ammonia (NH4OH, 25
wt.%), ammonium bicarbonate (NH4HCO3, analytical
grade) and urea ((NH2)2CO, 99.0 wt.%) as precipitants were
purchased from Tianjin Fuchen Chemical Reagent Factory,
China. Polyethylene glycol (PEG-10000, analytical grade) was
used as a surfactant. Absolute ethanol (C2H5OH, 99.7%)
and deionized water were obtained from Guangzhou Qianghui Bose Instrument Co.
Ltd., China.
Nd(NO3)3·6H2O and TEOS
with a molar ratio of 1:1 were dissolved in 400 ml alcohol-water solution. 2.5
g PEG-10000 was firstly added into the mixture solution above, and then the
mixture was added in dropwise into the precipitant solution (2 mol/L NH4OH
or NH4HCO3) to keep the solution pH values 8-9 under
vigorous stirring at room temperature. After 12 h, the resultant suspension was
filtered and washed for three times with deionized water, and dried at 60
oC for 24 h. For (NH2)2CO as a precipitant, the
concentration ratio of (NH2)2CO and the total metal ions
was 15:1. The mixture solution was heated to 95 oC and kept at this temperature
for 6 h. The precipitate was filtered, washed and dried by
using the same procedure above. The dried precipitates were ground in an agate
mortar and calcined at 1,200 oC for 5 h, respectively.
The phase compositions of the precursor and sintered
product were determined by a model PW-1710 X-ray diffractometer
(XRD, Philips Co. Ltd., The Netherlands), using Cu Kα
radiation.
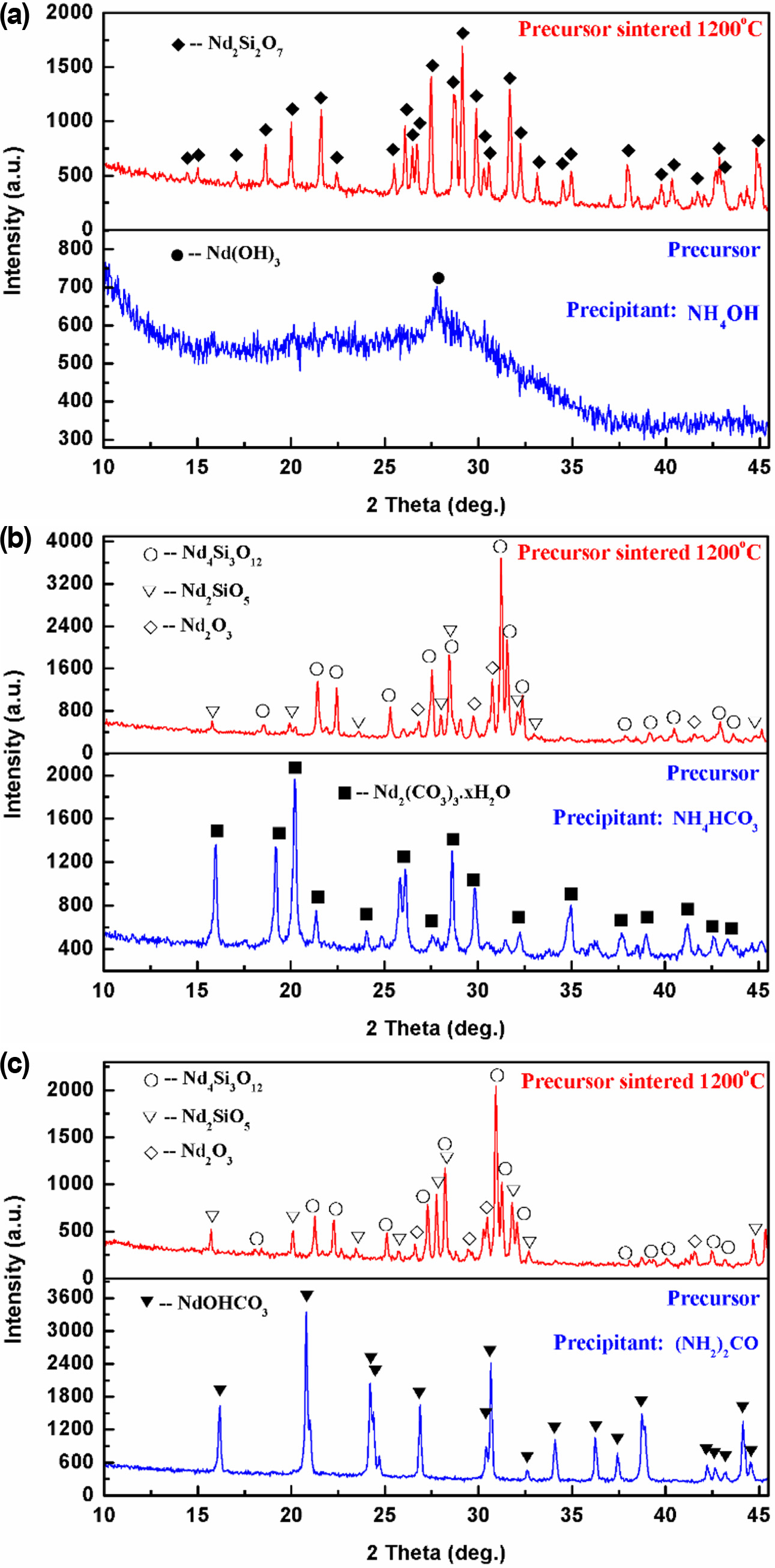
Fig. 1 shows the XRD patterns of the synthesized samples
with different precipitants. The precursor obtained with
NH4OH is amorphous Nd(OH)3 phase (JCPDS No. 83-2035).
When the sintering temperature reaches 1,200 oC, the tetragonal
Nd2Si2O7 (JCPDS No. 22-1177) is the main
crystalline phase for the sample synthesized with NH4OH (see Fig.
1(a)). For the precursor obtained with NH4HCO3, there are
some obvious diffraction peaks in the XRD pattern, which coincides with a
unknown phase of Nd2(CO3)3·xH2O. In
some previous studies [30, 31], the precursor obtained
with (NH4)2CO3 as a precipitant could be Nd2(CO3)3· 2.5H2O, Nd2(CO3)3·4H2O
or Nd2(CO3)3·8H2O. After the
precursor produced with NH4HCO3 is sintered at
1,200 oC, the main crystalline phases are Nd4Si3O12
(JCPDS No. 42-0171), Nd2SiO5 (JCPDS No. 40-0284) and Nd2O3
(JCPDS No. 43-1023) (see Fig. 1(b)). The XRD pattern for the precursor obtained
with (NH2)2CO is in agreement with that for NdOHCO3 (JCPDS
No. 27-1296). The main crystalline phases of the precipitate sintered at 1,200 oC
are also Nd4Si3O12, Nd2SiO5
and Nd2O3 (see Fig. 1(c)). After sintering, the phase compositions of the precursors prepared with
different precipitants are inconsistent, indicating a poor element (i.e., Si
and Nd) homogeneity of the precursors. In order to explain the result
above, the reaction processes of the systems with different precipitants should
be further analyzed based on thermodynamic equilibrium calculation.
For the prepared precursors, the neodymium element is
precipitated as different precipitates. Two variables such as temperature and
pressure are usually constants for the precipitation process in the solution.
Therefore, in this study, the diagram of the precipitated ion concentration and
pH value at different precipitation equilibrium solutions are drawn for further
discussion.
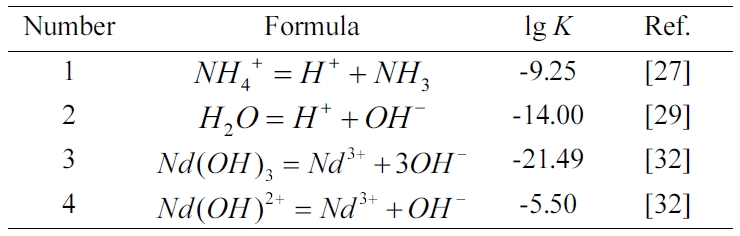
In the system of Nd3+-NH4OH-H2O
(NH4OH as a precipitant), the relevant reactions are shown in Table
1. Based on the principle of mass balance, the mathematical model of lg[Nd]T
vs pH value can be deduced, where [Nd]T is the
total concentration of Nd in equilibrium solution. The concentration is used instead
of the activity for the calculation. The total concentration of Nd can be
obtained by
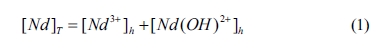
where [Nd 3+]h
and [Nd(OH)2+]h are the
concentration of Nd 3+ and Nd(OH)2+,
respectively.
The mathematical relationships of [Nd 3+]h
and [Nd(OH) 2+]h can be
expressed as follows:
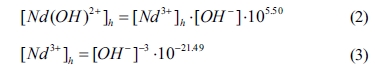
where [OH-] is the
concentration of OH- in the equilibrium solution. According
to Eqs. (2) and (3), Eq. (1) can be re-written as
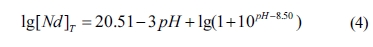
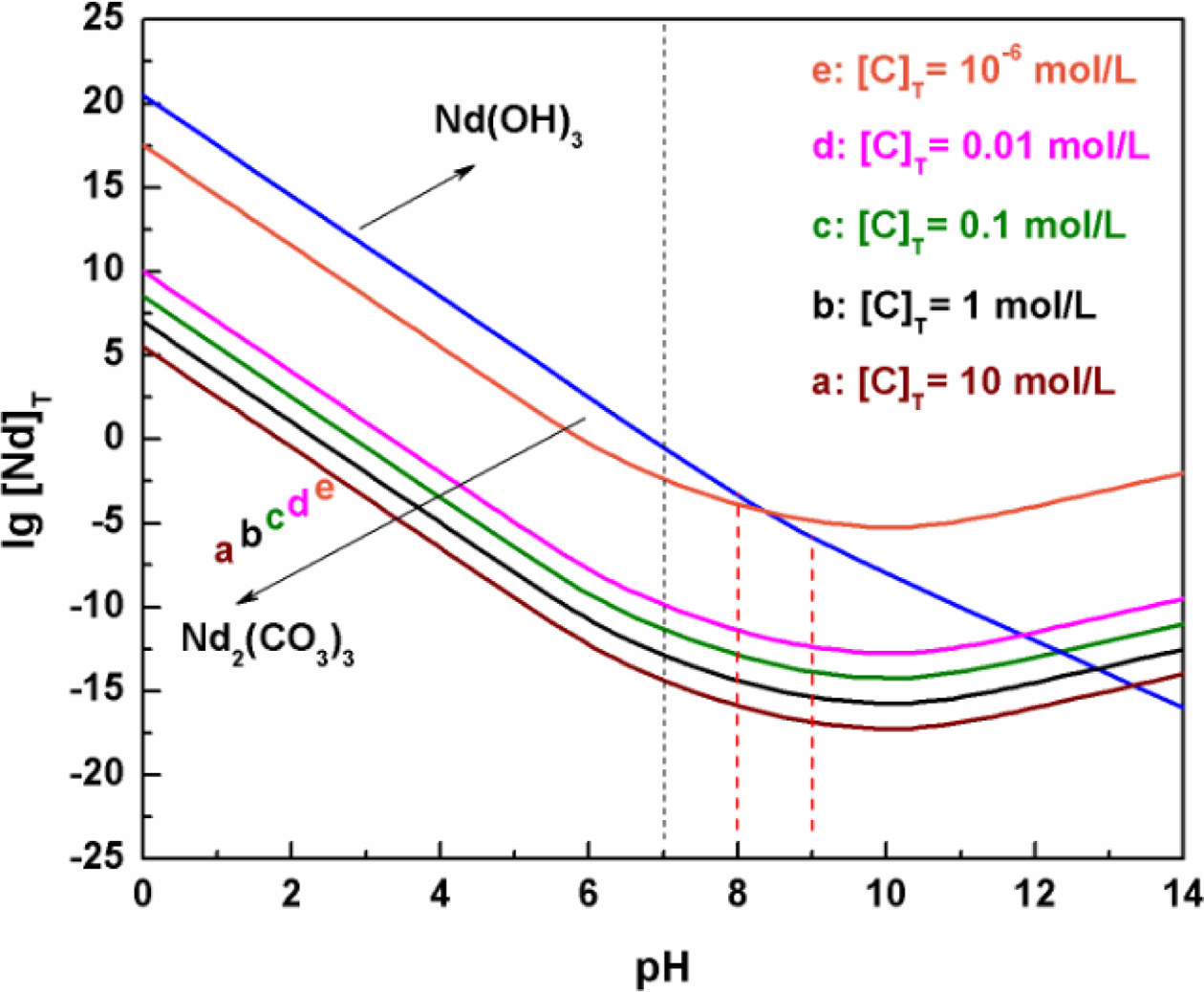
The diagram of lg[Nd]T vs pH
can be proposed according to Eq. (4), as shown in Fig. 2. Clearly, in the whole
pH value range, the concentration of Nd in the equilibrium
solution sharply decreases with the increase of pH value,
which is attributed to the formation of Nd(OH)3 (see Fig. 1(a)).
Moreover, a high pH value of equilibrium solution is beneficial for the
precipitation of Nd ions. When the pH value is 8~9, the concentration
of Nd ions is the range of 10-3~10-6
mol/L. In this experiment, the pH value of the equilibrium solution is 8.72
before the precursor is filtered, which can be defined as an almost complete
precipitation. Zhu et al. [33] found that Nd(OH)3 nanoparticles with
different morphologies can be synthesized using a microemulsion-precipitation
method with NH4OH as a precipitant in the presence of cetyltrimethyl
ammonium bromide. Arunachalam et al. [34] also reported that a facile chemical
precipitation method was used to prepare Nd(OH)3
nanopowder at an ambient temperature without the addition
of any surfactants.
In the system of Nd3+-NH4HCO3-H2O
(NH4HCO3 as a precipitant), the hydrolysis equation of NH4HCO3
can be expressed by
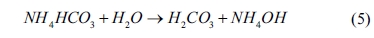
The other relevant reactions are listed in Table 2. Based
on the principle of mass balance, the mathematical model of lg[Nd]T
vs pH value is deduced, where [Nd]T is the
total concentration of Nd ions in the equilibrium solution. The concentration
is used instead of the activity for the calculation. The total concentration of
Nd ions can be expressed by
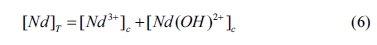
where [Nd 3+]c
and [Nd(OH)2+]c are the
concentration of Nd 3+ and Nd(OH)2+,
�respectively. The mathematical relationships of [Nd 3+]c
and [Nd(OH) 2+]c can be
obtained as follows:
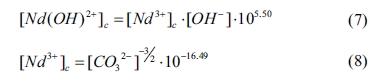
where [OH-] and [CO32-] are the
concentrations of OH- and CO32- in the
equilibrium solution, �respectively.
According to the mass balance of the system, the concentrations of OH-
and CO32- can be established by
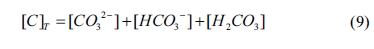
where [C]T
is the total concentration of C in the equilibrium solution. Moreover, [CO32-], [HCO3-] and [H2CO3]
are the concentrations of CO32-, HCO3- and
H2CO3, respectively. The mathematical
relationships of [HCO3-] and [H2CO3]
can be obtained as follows:
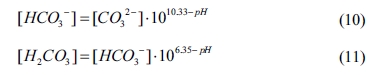
Based on Eqs. (10) and (11), Eq. (9) can be rearranged
by
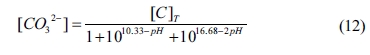
According to Eqs. (7), (8) and (12), Eq. (6) can be
deduced as
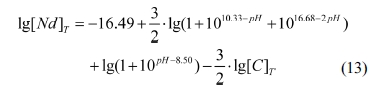
For the given values of [C]T at a
certain pH value, the diagram of lg[Nd]T vs pH
value can be obtained from Eq. (13) by a computer software named Origin 8.0.
The lg[Nd]T vs pH value
diagram of the Nd3+- NH4HCO3-H2O
system with different total carbon concentrations at equilibrium is shown in
Fig. 3. Clearly, the patterns of the lg[Nd]T vs
pH curves with different total carbon concentrations are similar. The
concentration of Nd ions sharply decreases with the increase of pH value in the
range of 7~10, which is attributed to the formation of Nd2(CO3)3.
A further increase of pH value results in the increase of Nd ion concentration
due to the partial dissolution of Nd2(CO3)3.
In fact, precipitates with a low solubility are preferentially
precipitated in the equilibration solution [29]. That is, the lower position of
the curve is beneficial to the formation of the precipitate in the lg[Nd]T
vs pH value diagram. According to the theoretical calculation, the
precipitate may be Nd2(CO3)3 or Nd(OH)3,
when NH4HCO3 is used as a precipitant. When the [C]T
value is 10-6
mol/L, the precipitate is Nd2(CO3)3 in the pH
value range of 7.0~8.3. The precipitate is Nd(OH)3 in the pH value
range of 8.3~14.0. Moreover, the pH value range of Nd2(CO3)3
presence becomes greater with the increase of [C]T
value. In this experiment, the [C]T value exceeds 0.01
mol/L. The precipitate is thus Nd2(CO3)3 for
the equilibrium solution in the pH value range of 8~9.
Due to the special structure of Nd element, neodymium
carbonate generally exists as hydrated carbonates.
Liu et al. [36] found that neodymium carbonate with an
orthorhombic system was synthesized using NH4HCO3 as a
precipitant, which contained a certain amount of
water. Zhu et al. [37] reported that Nd2(CO3)3· 8H2O with various
morphologics and sizes was synthesized using a
microemulsion-assisted solvothermal method in the
presence of Na2CO3. In addition, there also can be formed
poorly-ordered nanoparticulate precursors, as
termed amorphous amorphous neodymium carbonate [38].
In the system of Nd3+-CO(NH2)2-H2O
(CO(NH2)2 as a precipitant), the hydrolysis of CO(NH2)2
can be expressed as follows:
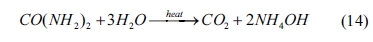
The hydrolysate is similar to that of Eq. (5). However,
the hydrolysis process of CO(NH2)2 requires auxiliary
heating. In this experiment, the hydrolysis temperature is kept at 95 oC.
At this temperature, the relevant equilibrium constants are difficult to
obtain. According to the XRD result in Fig. 1(c), the precipitate
obtained in the presence of (NH2)2CO is neodymium hydroxycarbonate
(i.e., NdOHCO3). The main reactions for the
formation of NdOHCO3 can be expressed as follows:
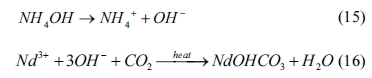
From Table 2 (see No. 3 and No.7), the equilibrium
constant of Nd2(CO3)3 is smaller than that of
Nd(OH)3, indicating that Nd2(CO3)3
is preferentially precipitated. However, in the hydrolysis solution of (NH2)2CO,
hydroxide concentration is much higher than that of carbon dioxide. The
excessive hydroxide may thus cause the formation of NdOHCO3
in the heated equilibrium solution. In fact, a ultrafine
NdOHCO3 powder was also synthesized using (NH2)2CO
as a precipitant by a hydrothermal method [39]. Some
studies showed reaction temperature and pressure both have
important effects on the formation of NdOHCO3. Tahara et al. [40]
synthesized hexagonal and orthorhombic Nd(CO3)OH via a
hydrothermal reaction at 220 oC and different pressures.
Dentritic NdOHCO3 nanostructures were prepared using a facile
hydrothermal approach [41].
For the prepared precursors, the silicon element is
aggregated in the form of silicon sol, which is the result of hydrolysis and
condensation of TEOS. The hydrolysis reaction of TEOS in abbreviated form can
be given as
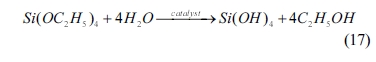
The condensation of silicon oxide can be expressed as
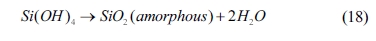
In the sol process of silicon alkoxide, generally, a homogeneous
catalyst is used to facilitate the hydrolysis reaction
since the reaction rate of hydrolysis is slow. Brinker et al. [42] proved that
all the hydroxyl groups of Si(OH)4 in hydrolysis reaction of TEOS
are derived from water. Iler et al. [43] also proposed the
mechanism for the basic hydrolysis of TEOS. The
hydrolysis process of TEOS can be affected by some parameters like pH
value, time and H2O/Si molar ratio as well [44-46]. Noted that in
general, the condensation reaction of silicon hydroxide is much faster than the
hydrolysis reaction of silicon alkoxide.
In alkaline solution, the hydroxyl anions attack Si
directly, leading to the abatement of Si-O bond and the acceleration
of -OC2H5 group cleavage [43]. In this work, three
reagents (i.e., NH4OH, NH4HCO3 or (NH2)2CO)
are selected as catalysts to promote the reaction of
hydrolysis of TEOS, respectively. When a catalyst is NH4OH,
the hydroxide ions formed by the ionization of ammonia water can effectively
attack Si, which is beneficial to the complete hydrolysis of TEOS. However, the
other reagents (i.e., NH4HCO3 and (NH2)2CO)
need to be hydrolyzed to form ammonia and then partially ionized, leading
to the incomplete hydrolysis reaction. Therefore, there is a
small amount of residual Nd2O3 that are not reacted due
to the lack of silicon element, when NH4HCO3 or (NH2)2CO
is a catalyst.
|
Fig. 1 XRD patterns of prepared samples with different precipitants, (a) NH4OH, (b) NH4HCO3 and (c) (NH2)2CO. |

|
Fig. 2 The lg[Nd]T vs pH value diagram of Nd3+-NH4OH-H2O system. |
|
Fig. 3 The lg[Nd]T vs pH value diagram of Nd3+- NH4HCO3-H2O system with different total carbon concentrations at equilibrium. |
Neodymium disilicate powders were prepared by a
sol-precipitation method with different precipitants (i.e., ammonia, ammonium
bicarbonate and urea) and subsequent sintering. The reaction processes of the
systems with different precipitants were analyzed based on thermodynamic
equilibrium calculation.
When NH4OH was used as a precipitate, the
precursor was amorphous Nd(OH)3. The precursor obtained
with NH4HCO3 was a unknown phase of Nd2(CO3)3·xH2O.
NdOHCO3 was the crystal phase of the precursor obtained in the
presence of (NH2)2CO. In the system of Nd3+-NH4OH-H2O,
a high pH value for the equilibrium solution was beneficial for the
precipitation of Nd ions. In the system of Nd3+-NH4HCO3-H2O,
the precursor type was related to both carbon concentration and pH value. A low
pH value was beneficial to Nd2(CO3)3 formation,
while a high pH value was beneficial to Nd(OH)3 formation. In the
system of Nd3+-CO(NH2)2-H2O, the
excessive hydroxide could form NdOHCO3 in the heated equilibrium
solution. Only a precursor with NH4OH could transform to a single Nd2Si2O7
phase after it was sintered at 1,200 oC for 5 h, which was due
to the incomplete hydrolysis of TEOS in the presence of NH4HCO3
or (NH2)2CO.
This work was supported by the China Postdoctoral Science
Foundation (No. 2019M650196) and the Major Scientific and Technological
Projects of Foshan (No. 2016AG101415).
- 1. P. Lunáková, M. Trojan, J. Luxová, and J. Trojan, Dyes Pigm. 96[1] (2013) 264-268.
-

- 2. K.-R. Pyon, K.-S. Han, and B.-H. Lee, J. Ceram. Process. Res. 12[3] (2011) 279-288.
- 3. N. Gorodylova, V. Kosinová, Z. Dohnalová, P. Bělina, and P. Šulcová, Dyes Pigments 98[3] (2013) 393-404.
-

- 4. B. Tanisan and S. Turan, J. Ceram. Process. Res. 12[4] (2011) 462-467.
- 5. S. Akdemir, E. Ozel, and E. Suvaci, Ceram. Inter. 37[3] (2011) 863-870.
-

- 6. J.-H. Lee, H.-J. Hwang, J.-W. Kwon, J.-H. Kim, K.-T. Hwang, and K.-S. Han, J. Ceram. Process. Res. 20[2] (2019) 127-132.
-

- 7. Y.-Z. Halefoglu and E. Kusvuran, J. Ceram. Process. Res. 11[1] (2010) 92-95.
- 8. G. George, L.-S. Kumari, V.-S. Vishnu, S. Ananthakumar, and M.-L.-P. Reddy, J. Solid State. Chem. 181[3] (2008) 487-492.
-

- 9. K.-J. Sreeram, S. Kumeresan, S. Radhika, V.-J. Sundar, C. Muralidharan, B.-U. Nair, and T. Ramasami, Dyes Pigm. 76[1] (2008) 243-248.
-

- 10. B.-B. Topuz, G. Gündüz, B. Mavis, and Ü. Çolak, Dyes Pigm. 96[1] (2013) 31-37.
-

- 11. P.-M.-T. Cavalcante, M. Dondi, G. Guarini, F.-M. Barros, and A. Benvindo da Luz, Dyes Pigm. 74[1] (2007) 1-8.
-

- 12. H. Sameie, R. Salimi, A.-A. Sabbagh Alvani, A.-A. Sarabi, F. Moztarzadeh, and M. Tahriri, Physica B: Condensed Matter 405[23] (2010) 4796-4800.
-

- 13. S. Kunimi and S. Fujihara, Dyes Pigm. 91[1] (2011) 49-54.
-

- 14. S.-Y. Kaya and B. Karasu, Ceram. Inter. 38[4] (2012) 2757-2766.
-

- 15. A. Tucks and H.-P. Beck, Dyes Pigm. 72[2] (2007) 163-177.
-

- 16. M. Paraman and S. Muthiah, Sol. Energ. Mat. Sol. C. 174 (2018) 530-537.
-

- 17. S. Jose, A. Jayaprakash, S. Laha, S. Natarajan, K.G. Nishanth, and M.L.P. Reddy, Dyes Pigm. 124 (2016) 120-129.
-

- 18. S. Sameera, P.-P. Rao, V. James, S. Divya, and A.K.V. Raj, Dyes Pigm. 104 (2014) 41-47.
-

- 19. Q. Gao, X.-M. Wu, Y.-M. Fan, and Q. Meng, Dyes Pigm. 146 (2017) 537-542.
-

- 20. S.-J. Ke, Y.-M. Wang, and Z.-D. Pan, Dyes Pigm. 108 (2014) 98-105.
-

- 21. S.-J. Ke, Y.-M. Wang, Z.-D. Pan, and H.-P. Zeng, J. Ceram. Process. Res. 20[3] (2019) 264-269.
-

- 22. S.-J. Ke, Z.-D. Pan, Y.-M. Wang, C.-Y. Ning, S.-L. Zheng, and J. Huang, Dyes Pigm. 145 (2017) 160-167.
-

- 23. A.-C. Tas and M. Akinc, J. Am. Ceram. Soc. 77[11] (1994) 2968-2970.
-

- 24. L.-S. Chi, H.-Y. Chen, S.-Q. Deng, H.-H. Zhuang, and J.-S. Huang, Chinese J. Struct. Chem. 16 (1997) 177-180.
- 25. S.-J. Ke, Y.-M. Wang, and Z.-D. Pan, Dyes Pigm. 118 (2015) 145-151.
-

- 26. Y.-Q. Fan, C.-F. Zhang, J. Zhan, and J.-H. Wu, Trans. Nonferrous Met. Soc. China 18[2] (2008) 454-458.
-

- 27. C. Xiao and L. Zeng, Hydrometallurgy 178 (2018) 283-286.
-

- 28. C. Xiao, L.-S. Xiao, C.-J. Gao, and L. Zeng, Sep. Purif. Technol. 156 (2015) 582-587.
-

- 29. X.-B. Li, L.-T. Shen, X.-Y. Tong, T.-G. Qi, G.-H. Liu, Q.-S. Zhou, and Z.-H. Peng, Trans. Nonferrous Met. Soc. China 28[11] (2018) 2342-2350.
-

- 30. S. Sanuki, K. Matsushita, M. Nishiwaki, and H. Majima, Metall. Mater. Trans. B. 31[1] (2000) 5-13.
-

- 31. S. Sanuki, S. Matsushita, Y. Morita, and H. Majima, Hydrometallurgy 57[3] (2000) 253-261.
-

- 32. R.-S.Tobias and A.-B. Garrett, J. Phys. Chem. 80[14] (1958) 3532-3537.
-

- 33. W.-Q. Zhu, J. Ma, L. Xu, W.-Z. Zhang, and Y.-S. Chen, Mater. Chem. Phys. 122[2-3] (2010) 362-367.
-

- 34. S. Arunachalam, B. Kirubasankar, E.-R. Nagarajan, D. Vellasamy, and S. Angaiah, Chem. Select. 3[45] (2018) 12719-12724.
-

- 35. K. Binran-Kisohen II, and K. Nakata, in “Handbook of Chemistry” (Maruzen, Tokyo, 1993),p.170.
- 36. S. Liu, R.-J. Ma, R.-Y. Jiang, and F.-C. Luo, J. Crystal Growth 203[3] (1999) 454-457.
-

- 37. W.-Q. Zhu, J. Ma, X.-P. Xing, L. Xu, and Y.-S. Chen, Mater. Res. Bull. 46[6] (2011) 830-834.
-

- 38. B. Vallina, J.-D. Rodriguez-Blanco, A.-P. Brown, J.-A. Blanco, and L.-G. Benning, Nanoscale 7[28] (2015) 12166-12179.
-

- 39. Z.-Y. Xu, Y.-J. Zhang, Z.-Y. Fang, X.-B. Yin, and W. Zhu, Mater. Res. Bull. 45[1] (2010) 74-79.
-

- 40. T. Tahara, I. Nakai, R. Miyawaki, and S. Matsubara, Z Kristallogr 222 (2007) 326-334.
- 41. X.-F. Shang, W.-C. Lu, B.-H. Yue, L.-M. Zhang, J.-P. Ni, Y. Iv, and Y.-L. Feng, Crystal Growth Design 9[3] (2009) 1415-1420.
-

- 42. C.-J. Brinker, J. Non-Crystall. Solids 100[1-3] (1988) 31-50.
-

- 43. R.-K. Iler, in “The Chemistry of Silica” (Wiley, New York, 1979), p. 209.
- 44. S.-L. Chen, P. Dong, G.-H. Yang, and J.-J. Yang, Ind. Eng. Chem. Res. 35[12] (1996) 4487-4493.
-

- 45. E.-R. Pohl and F.-D. Osterholtz, in “Molecular characterisation of composite interfaces” (Plenum, New York, 1985), p. 157.
-

- 46. S.-H. Kim, B.-Y.-H. Liu, and M.-R. Zachariah, Langmuir 20[7] (2004) 2523-2526.
-

 This Article
This Article
-
2020; 21(3): 386-391
Published on Jun 30, 2020
- 10.36410/jcpr.2020.21.3.386
- Received on Feb 24, 2020
- Revised on Mar 10, 2020
- Accepted on Mar 16, 2020
 Services
Services
- Abstract
introduction
experimental
results and discussion
conclusions
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Yanmin Wang a, Zhidong Pan a,b
-
aSouth China University of Technology, Guangzhou 510640, China
bFoshan Oceano Ceramics Co. Ltd., Foshan 528138, China
Tel : +86 20 87114883
Fax: +86 20 87110273 - E-mail: wangym@scut.edu.cn, wangym@scut.edu.cn






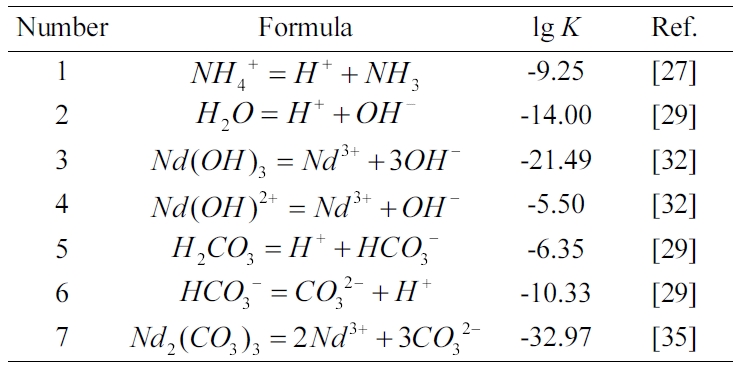
 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.