- Influences of characteristics of the alkaline activator on the compressive strength and microstructure of the fly ash-based geopolymer pastes
Qingbo Tiana,*, Daquan Suna, Zeyu Gua and Zhijie Lvb
aSchool of Materials Science and Engineering, Shandong Jianzhu University, Jinan, China
bSchool of Mechanical and Electrical Engineering, Shandong Jianzhu University, Jinan, China
The effects of Na2O
content, water to binder (W/B) ratio, and the modulus (Ms) of
alkaline activator solution on a fly ash-based geopolymer paste (FGP) were
investigated. The microstructure of the pastes was observed and characterised
by Scanning electron microscope and X-ray diffraction. The results showed that
the increase of Na2O content and Ms resulted in an
increase in compressive strength: an increase in the W/B ratios led to a
decreased compressive strength. It was found that the high compressive strength
was achieved as Ms of the alkaline activator was 1.5, and the
content of this activator was 8.0-12.0% by the proportion of Na2O to
fly ash. The compressive strength of these pastes was between 26.5-39.6 MPa at
28 days when they were cured at 85 ºC for 1 h followed by curing under standard
conditions. OH- concentration, determined by Na2O content,
Ms, and water content, seemed to be a crucial parameter
influencing the compressive strength. There was an optimal OH- concentration range, in which the appropriate
strength of geopolymer paste would be achieved. An increased alkaline content
promoted the reaction between the mineral phases of the fly ash with the
alkaline activator and the formation of an additional sodium aluminosilicate
hydrate (N-A-S-H) gel.
Keywords: Fly ash-based geopolymer pastes (FGP), Geopolymers, Alkaline activation, Compressive strength, OH- concentration
Geopolymers are synthesised using an aluminosilicate
raw material and an alkaline activator solution (AAS) [1-6]. Compared to
ordinary Portland cement (OPC), geopolymer materials are known for their
excellent properties, such as comparable or better mechanical properties [3],
thermal and chemical stability [4], and impact resistance [7], and are
considered as potential alternatives to OPC [8]. In particular, waste materials
that are not currently reused in other industrial sectors can be used as
precursors to geopolymer materials. Therefore, geopolymers have
received increasing attention among those
trying to reduce the consumption of natural raw
materials and their environmental impact.
Fly ash is a by-product derived from the combustion of
coal powder in thermal power plants. It becomes an important material for
geopolymeric precursor due to its containing suitable components of amorphous
silica and alumina [4, 5]. Therefore, fly ash-based geopolymer
products have attracted much interest and studies have been undertaken
therewith in recent years [9-18]. In the fabrication of geopolymers, a strong
alkaline medium is necessary to increase the dissolution of silica and alumina
in precursor materials, in which, alkaline solutions such
as sodium hydroxide, and sodium silicate, are most commonly adopted.
Fernández-Jiménez and Palomo [13] investigated
the effects of NaOH content on the properties thereof: at NaOH contents of 5,
6, 7, 8, and 10% (by mass of binders),
compressive strengths of 9 MPa, 21
MPa, 30 MPa, 31 MPa, and 50 MPa at 28 days ensued, respectively. Palomo et al. [14] also observed that the
concentration of the activator affected the compressive strength and stiffness
of fly ash-based geopolymers (other results also confirm this [15-17]):
however, some authors reported that the ratios of SiO2/Na2O
or Al2O3/Na2O exerted greater influences on
the strength than NaOH content alone [10, 13, 17]. Palomo et al.
[14] investigated an alkaline activated fly ash mortar: the compressive
strength reached almost 70 MPa at SiO2/Al2O3
and Al2O3/Na2O ratios of 3.95 and 2.62. As the
SiO2/Al2O3 and Al2O3/Na2O
ratios were changed to 3.87 and 3.13, the strength decreased to 54.5 MPa. Ryu
et al. [18] also examined the effects of SiO2/Al2O3
and Al2O3/Na2O ratios in fly ash-based
alkaline activated pastes. At SiO2/Al2O3 ratio
of 4.13, the compressive strength was reduced with the increase of Al2O3/Na2O
ratio.
Besides, the water content also played crucial roles in
the reactivity and strength development of the FGPs [2, 13, 19-23]. As the W/B
ratio was 0.47, the compressive strength was 50 MPa. When the ratio decreased
to 0.42, 0.31 and 0.26, the strength increased to 63.5 MPa,
82.5 MPa and 96.0 MPa, respectively [3], indicating that the rises of water
content greatly decreased the strength. Fang et al. [23] observed that a large
portion of water was entrapped in discontinuous gel pores, this might be the
cause to impair the strength of the paste. In addition, the effects of the curing
processes on the properties of FGP has been generally recognised.
Palomo et al. [14] studied a FGP cured at 65 ºC and 85 ºC
respectively for 2-24 h. The compressive strength was 30
MPa in the binder cured at 65 ºC for 5 h; while as cured at
85 ºC for only 2 h, the compressive strength could arrived at 31.6 MPa. As
the curing time attained 24 h, the strength of 68.7 MPa would reached in
the binders cured at 85 ºC. In another FGP, 28-day compressive strength
was 24.4 MPa in the binder cured for 24 h at 40 ºC; it
increased by 96.7%, 104.1% and 145.8% as the curing temperature rose to
70 ºC, 80 ºC and 100 ºC, respectively
[16]. However, it was found that there was a crucial
curing temperature and time in the geopoly-
merisation of FGPs and the optimal curing
processes varied with different FGP systems [15, 24, 25]. In fact, all parameters,
including the concentration of the activator,
the SiO2/Na2O or Al2O3/Na2O
ratio, the water content, and pH, and the curing processes, seem to play roles
in the synthesis of FGPs. However, the influences thereof are complex and it is
difficult to predict which one is more important [13, 17, 21, 26]. In addition,
the optimum conditions are different in fly ash based geopolymers due to the
differences in fly ash resource and its
characteristics [5, 20, 27-30]. Here, a fly ash with high iron content was used
as the precursor to fabricate
geopolymer pastes. The roles of the contents of the alkaline activator, the ratios of SiO2/Al2O3,
Al2O3/Na2O, and W/B, especially the effects of
OH-
concentration, on the properties and microstructure of FGPs are
discussed.
Precursor
material properties
The fly ash used in the study was supplied from Guizhou
Alumina Corporation, Guiyang, China. The chemical composition of the fly ash,
obtained by X-ray fluorescence (XRF) on a ZSX Primus II X-ray spectro- meter, is summarised in Table
1. The reactive amounts in fly ash, which were estimated by a combined
dissolution of fly ash in NaOH solution by following extraction with HCl
solution [31], are also listed in Table 1. The fly ash was found to have 47.8%
amorphous material, comprising 23.7% SiO2, 13.2% Al2O3,
8.1% Fe2O3, 1.4% CaO, and 1.4% other elements.
Preparation
of the AAS
Analytical sodium hydroxide pellets (99 wt %) were
dissolved in distilled water, and then mixed with glass-water solution (30.4%
SiO2, 9.6% Na2O, and 60.0% H2O by mass). Three
different moduli (Ms) of SiO2/Na2O
(mol) were adopted to explore the effect of Ms on the
geopolymer pastes. The mix proportions of sodium hydroxide and water-glass
solution for the AAS are summarised in Table 2.
Preparation
of the FGPs
The pastes were prepared by stirring the fly ash and AAS
together. The equivalent sodium oxide content in the AAS was set to 6, 8, 10,
and 12% by mass of the fly ash binder, respectively. The appropriate amount of
water was added to adjust the W/B ratios to 0.38, 0.49, and 0.57. After being
stirred thoroughly, the pastes were cast in 40 mm × 40 mm × 160
mm metal moulds, vibratory compacted, wrapped in thin plastic film and cured
for 24 h at 80 ºC, then cured under standard curing conditions. The curing
temperature of 80 ºC was selected as it provided the required energy
guaranteeing the reaction between the fly ash and activating solution [30,32].
The mix proportions of AAS, extra water, the molar ratios of SiO2/Al2O3,
Al2O3/Na2O, and the OH- concentration for preparing
FGPs are summarised in Table 3.
Characteristic
material properties
The compressive strength of FGPs was determined using
a SANS machine (MTS CDT 1305-2) at a maximum load of
300 kN. The average of three measurements was calculated. The images of
the FGPs were captured by scanning electron microscopy (SEM:
Hitachi SU8010, Japan) method on a fractured section and a thin
film of Au was sputter-coated onto the specimen surface before
observation. The mineral phases of fly ash and alkaline activated binders were
identified by X-ray diffraction (XRD: Bruker D8 ADVANCE, Germany) on
powdered samples.
|
Table 2 Mix proportions of sodium hydroxide and water-glass for the AAS and its properties |
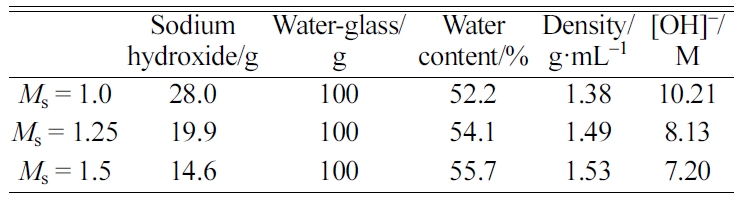
|
Table 3 Mix proportions for fly ash-based binder pastes and their characteristics. |
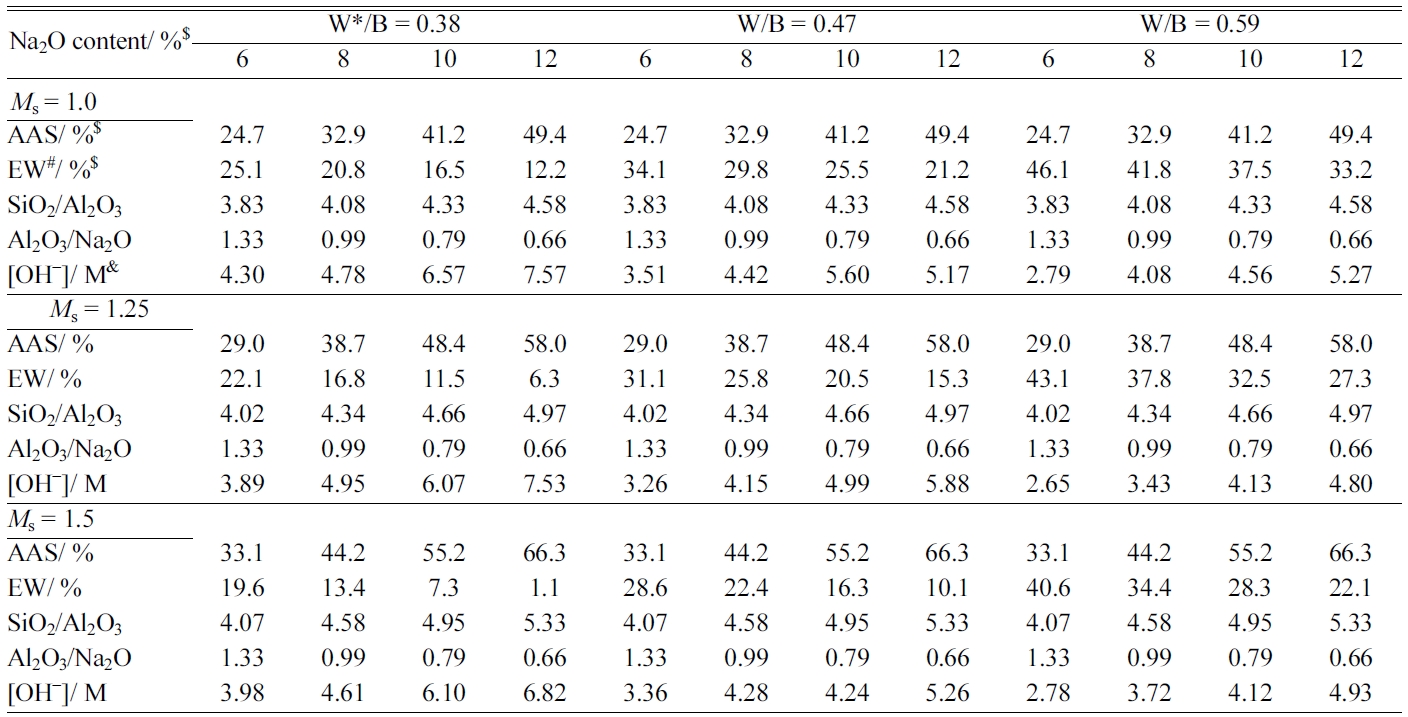
Note: $, percentage relative to fly ash; #, Extra water; *, Water includes water from water-glass and extra water; &, OH− concentration was measured by acid-base titration. |
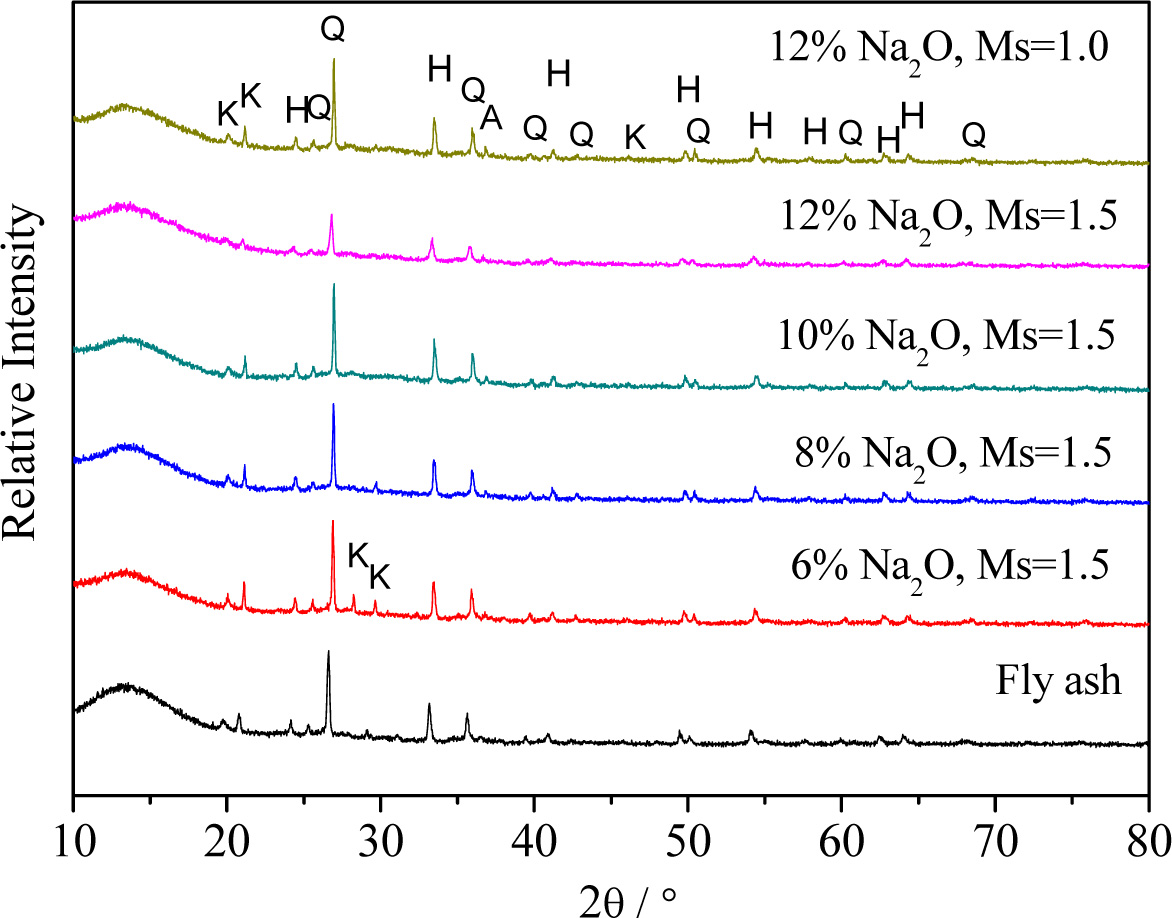
Fig. 1 shows XRD patterns of fly ash and geopolymer
pastes developing at various Na2O contents and values of Ms.
The fly ash shows different mineral peaks related to the presence of quartz (Q,
SiO2), hematite (H, Fe2O3), and kyanite (K, Al2SiO5)
mineral phases. Besides, the broad diffuse hump at about 10-20º indicates
that it contains a certain amount of amorphous material
[33]. After alkaline activation, the mineral phases in the pastes are not
significantly altered from those found in the fly ash. The predominant phases
in the fly ash are also found in all pastes, however, as the Na2O
content increased, the diffraction peaks of quartz, hematite, and kyanite
gradually decreased in amplitude, indicating that the mineral phases present in
the fly ash were reacting with AAS. In particular, diminished peak intensities
in the XRD spectra are seen at a Na2O content of 12% and Ms
= 1.5. Meanwhile, an albite phase (A, NaAlSi3O8) is
gradually formed in the alkaline activated pastes. This albite phase is
associated with the strong Na-Al-Si hydrated bonds which can be considered to
play major effects to the strength of such geopolymers [34]. In addition, a
slight shift in the peak for the binder was observed, implying that a new amorphous phase formed [19]. When alkaline
activator solution was added, Na+ was accommodated within the cavities of -Si-O-Al- networks as a charge
balancer to form sodium aluminosilicate gel (N-A-S-H). The formation of the gel also contributed to the development of
compressive strength [35-38].
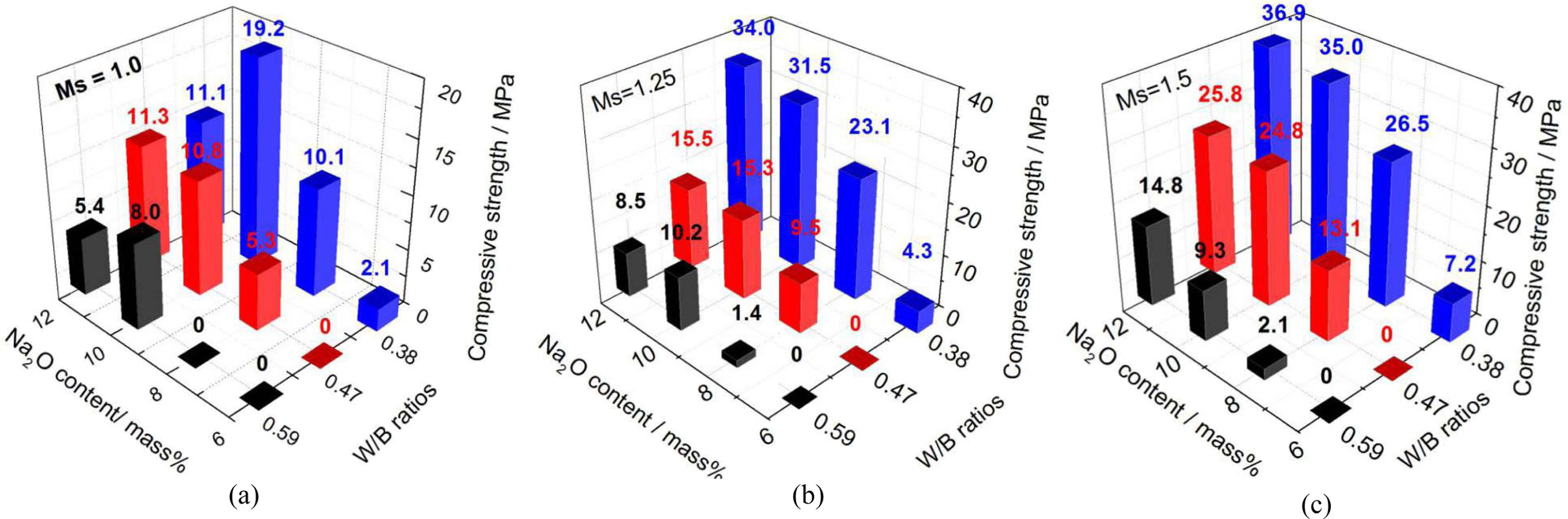
The effects of Na2O percentage to binder, W/B
ratios, and Ms on the compressive strength of FGPs are shown
in Fig. 2. As Ms is 1.0 (Fig. 2(a)), the strength at 28 days
is negligible in pastes with 6.0% Na2O content (2.1 MPa) at a W/B
ratio of 0.38. As the Na2O content was increased, the strength
gradually developed at 28 days for the pastes tested here. Compressive
strengths of 10.1 MPa and 19.2 MPa in the paste samples with Na2O
contents of 8% and 10% were achieved (increased 3.8 and 8.1
times, respectively, compared with that at 6% Na2O
content). When the W/B ratios were increased to 0.47 and
0.59, a similar trend was observed. The compressive strength of pastes was low
at low Na2O content, and increased therewith;
however, the difference was also observed in that the
strength decreased with increasing W/B ratios at the same Na2O
content. At a Na2O content of 8%, the strength decreased to
5.3 MPa and to almost zero from 10.1 MPa, when the W/B ratio was increased
to 0.47 and 0.59 from 0.38. As the Na2O content was 10%, the
compressive strength of the paste was decreased by 43.4% and 58.3% when the W/B
ratio was increased to 0.47 and 0.59, respectively.
The alkaline activation of fly ashes leads to the
formation of an alkaline aluminosilicate of amorphous nature[39]. Alkali
activator solutions play an important role to dissolve Si and Al oxides in fly
ash. In a low alkaline activator solution, the silica and alumina are filtered
less [40] and a weak chemical reaction will occur [13, 26, 28, 39, 41, 42]. It
is undoubtedly that a low compressive strength would be attained in a
low-alkalinity solution. With the increase in Na2O content, the
higher reactivity of the fly ash would be expected [21]. Thus, a
higher compressive strength was anticipated. It has been
reported that there is a threshold alkaline activator
content [7, 15, 20, 41]. Bakharev [15] reported that the
strength developed rapidly at 8% Na2O concentration in a geopolymer material. Cho
et al. [16] observed that the strength at 28 days increased as the Na2O
content increased from 4.0% to 6.0%; however, when Na2O content
further increased beyond 6.0%, the rate of strength
development decreased. Steveson et al. [35] found that the optimum compressive
strength of geopolymer materials could be achieved when the
Na2O content in AAS is between 7% and 9% by mass of fly ash. From
our experimental results, an 8%-10% Na2O content in the fly ash was
a critical value range able to activate the fly ash efficiently. As the Na2O
content was less than 8%, regardless of any adjustment of Ms
and W/B ratio, the compressive strength of the pastes did not exceed 10 MPa
(Figs. 2(a)-(c)).
As the Na2O content was further increased to
12%, no obvious increase in the strength, and even a decrease
therein, was observed: this may have been caused by free OH- present in the
alkali-activated matrix [27] (as also observed elsewhere [26, 35, 41]). Table 3
also lists the OH-
concentration changes of the AAS at various W/B ratios, Na2O
contents, and Ms. The W/B ratios, Na2O contents,
and Ms exerted significant influences on the OH- concentration.
With the increase in W/B ratios, the water content increases and dilutes OH- at the same Ms
and Na2O content so the OH- concentration
decreased accordingly. On the contrary, the OH- concentration
increased with increasing Na2O content. The influences of Ms
on the OH-
concentration are mutual: on one hand, more NaOH was needed to obtain an AAS
with lower Ms (Table 2), thus, with a larger Ms
in the AAS, a lower NaOH content resulted, causing the decrease in OH- concentration. On the other
hand, as water-glass was mixed with NaOH, more NaOH was generated through
the hydrolysis of sodium silicate, further increasing the OH- concentration [13, 18, 43]
(Table 3).
From the results (Fig. 2), the W/B ratios played an
important role as described elsewhere [12-14, 42]. Palomo et al. [14] note that
the roled of the added water must be taken into account when investigating the
properties of a geopolymer material. Firstly, enough water was necessary to
ensure the workability of the mixtures [11], or a high strength was
difficult to achieve: however, when excess water was added, there was
sufficient mobility of ions in the mixtures for the solid to dissolve, causing
a low level of unreacted material and a low strength [9]. This is attributed to
the increase of porosity in the binder matrix [35].
At Ms of 1.25 or 1.5, similar trends in
the compressive strength of the pastes along with the Na2O
content and W/B ratio were observed (Figs. 2(b) and 2(c)). That is: the
compressive strength increased with increasing Na2O content
(relative to fly ash) and decreased with increasing
W/B ratio. The compressive strength increased at larger Ms
at the same Na2O content and W/B ratio. At Ms
of 1.25 and 1.5, the compressive strength increased to
31.5 MPa and 35.0 MPa from 19.2 MPa, respectively, with 10% Na2O
content and a W/B ratio of 0.38; while, the strength
increased by 27.6% and 46.4%, respectively, with 8%
Na2O content and a W/B ratio of 0.38. The maximum strength of 36.9
MPa was achieved in that geopolymer paste with 12% Na2O content at Ms
= 1.5.
In fact, the changes in Ms and Na2O
content also changed the SiO2/Al2O3 and Al2O3/Na2O
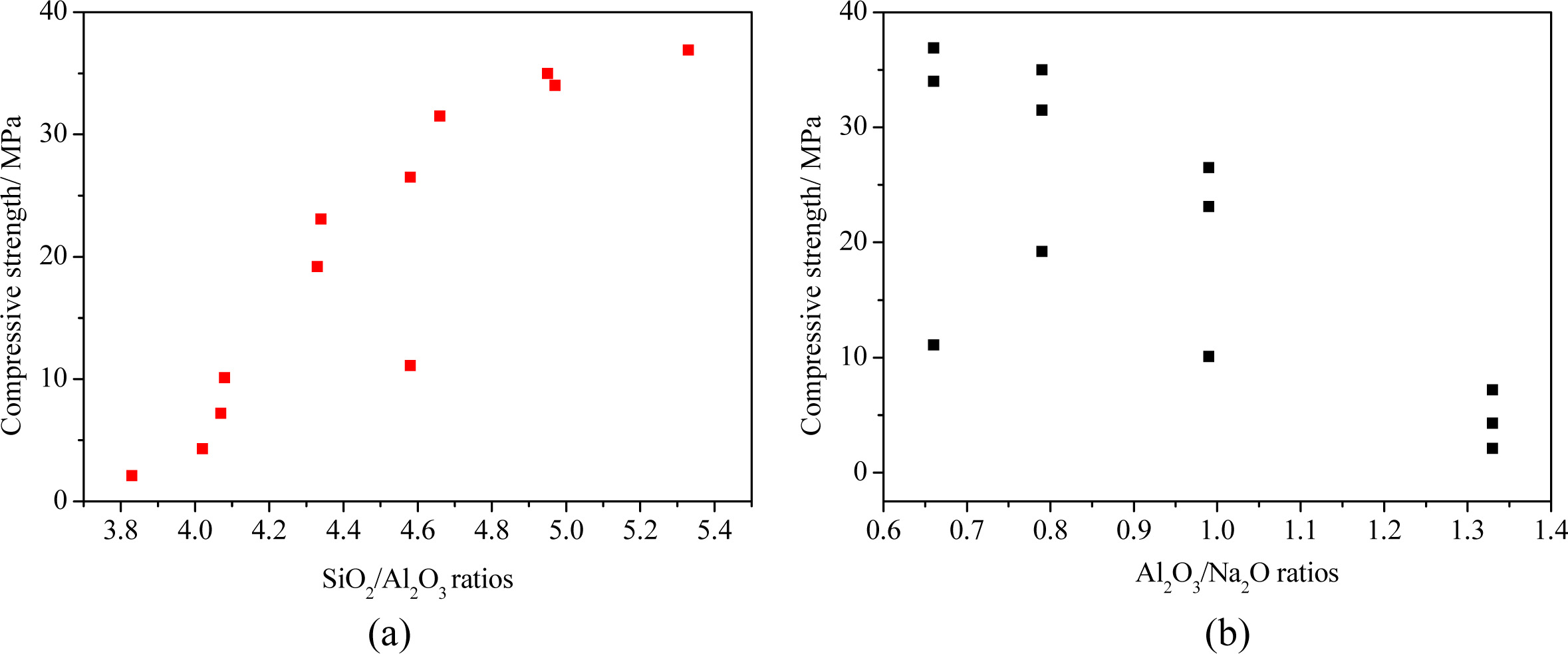
ratios, which are important parameters in geopolymer synthesis [18]. Fig. 3
plots the results of the effects of the SiO2/Al2O3
and Al2O3/Na2O ratios on the compressive
strength of pastes at a W/B ratio of 0.38. The strength increased as the SiO2/Al2O3
ratio was increased from 3.83 to 5.33 (Fig. 3(a)): because the
active SiO2 content was relatively low (23.7%)
in the fly ash used in the present study, the larger Ms, led
to the higher SiO2 content in the AAS and the binder matrix, thus
increasing the compressive strength of fly ash-based geopolymers. The strength
values were scattered with the change in Al2O3/Na2O
ratio, however, a high strength could be attained only with an Al2O3/Na2O
ratio of less than 1.0 (Fig. 3(b)). According to these results, the strength at
28 days is improved by more than 1.6 times at SiO2/Al2O3
= 4.58, compared to that at SiO2/Al2O3 = 4.08
at the same Al2O3/Na2O ratio of 0.99; while, a
strength greater than 30 MPa at 28 days could be achieved with an Al2O3/Na2O
ratio of less than 0.80. These indicated the roles of SiO2/Al2O3
and Al2O3/Na2O ratios to strength development in the geopolymers.
The SiO2/Al2O3 and Al2O3/Na2O
ratios cannot reflect the role of water on the compressive strength, as they
were kept constant as the W/B ratio was changed (Table 3). The OH- concentration, which
reflected the comprehensive effects of parameters such as the Na2O
content, W/B ratio, and Ms, represented parameter that could
be used to characterise the compressive strength of FGA. From Table 3, it can
be seen that OH-
concentration are influenced by the W/B ratio, Na2O content, and Ms.
The variation in OH-
concentration, which influences the chemical reaction of AAS with fly ash,
would affect the final strength of these geopolymers as also demonstrated by
our results. Considering the mutual effects of W/B ratio and Na2O
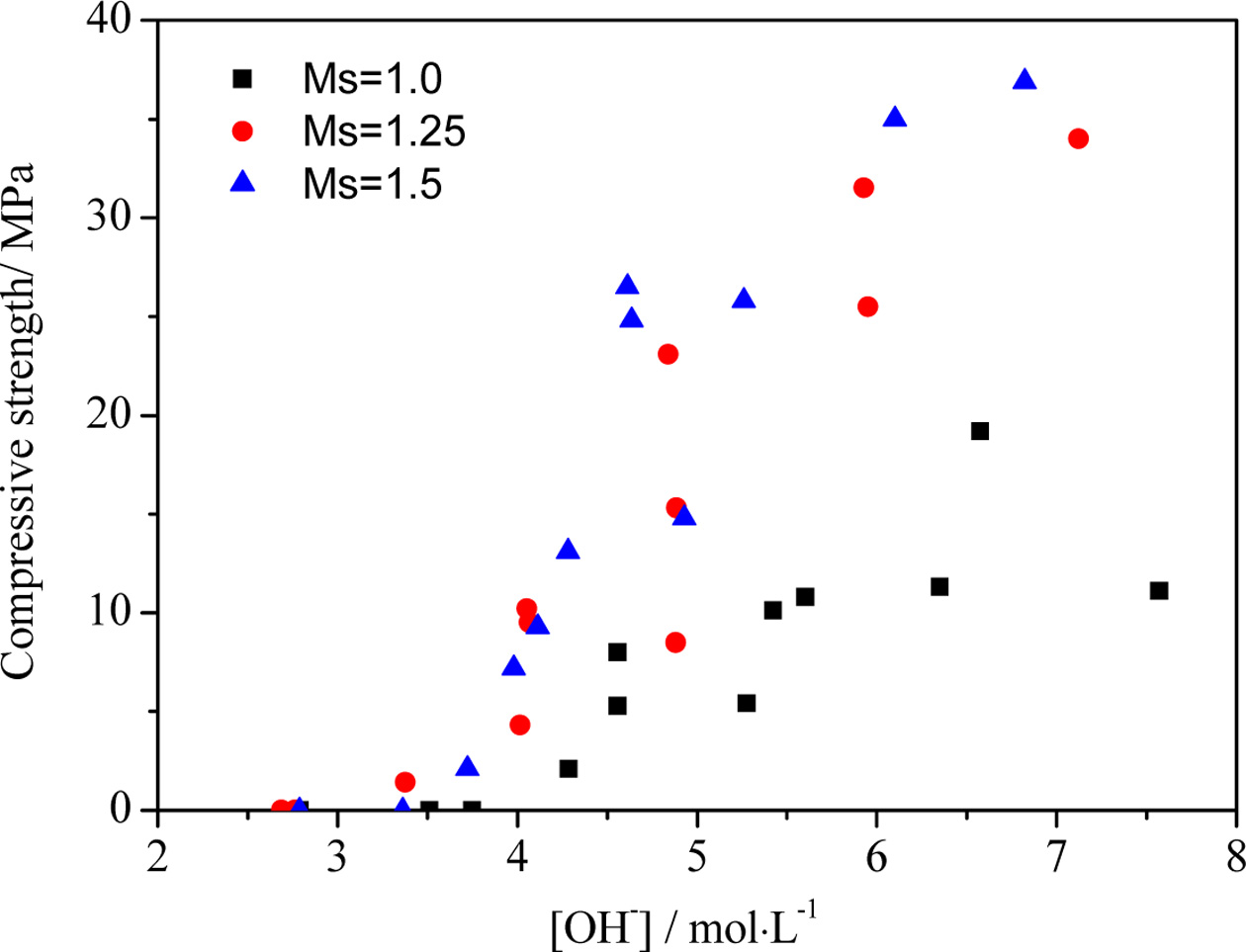
content, the influences of OH-
concentration on the compressive strength were as shown in Fig. 4. Regardless
of the effects of Ms, the compressive strength could not be
detected as the OH-
concentration was initially below its critical threshold value: it then
generally increased at greater OH-
concentrations, and finally decreased at an excessive OH- concentration. The critical
values of OH-
concentration inducing the turning point in the compressive strength were
similar, although the strength varied with Ms. When the OH- concentration was less than
4.5 mol/L, it could not induce an efficient reaction, thus a
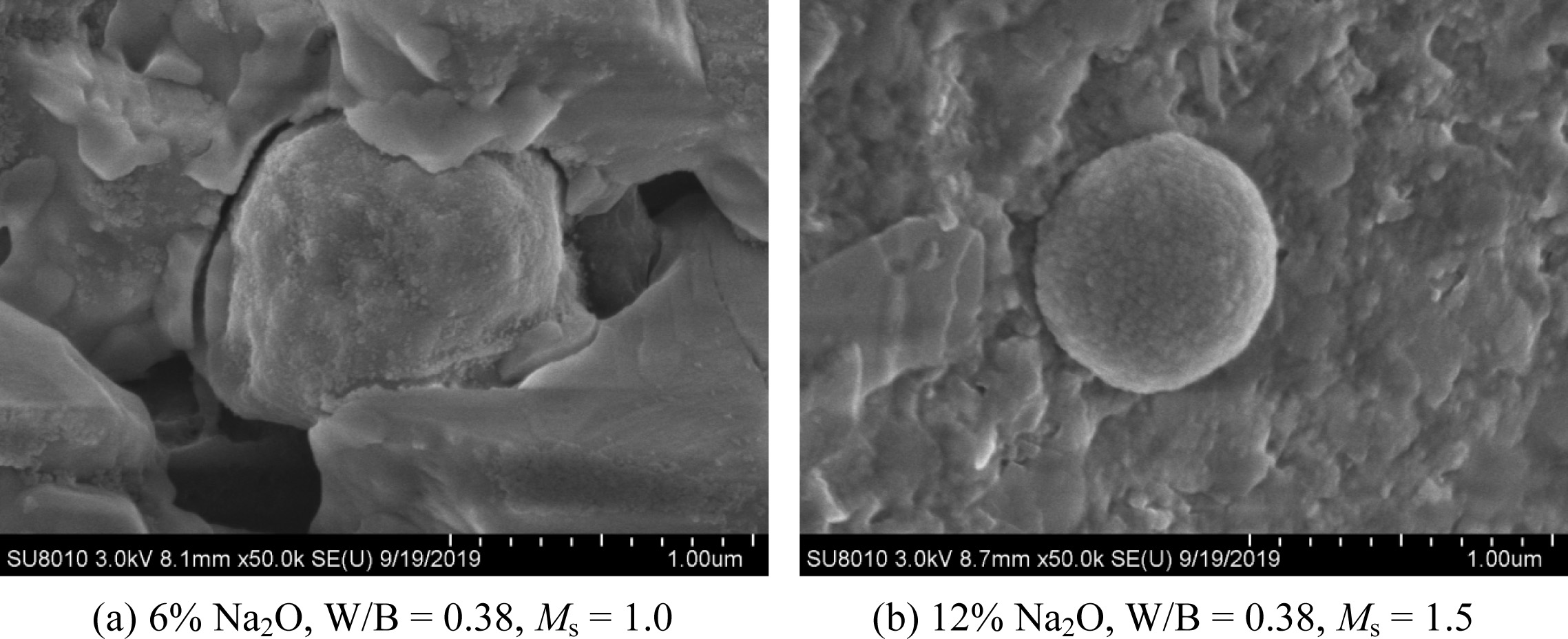
low-strength product ensued. From the SEM image of the sample
with [OH-] of 4.30, the unreacted fly ash particles were
visible (Fig. 5). In addition, the presence of voids and cracks along the
surface of fly ash particle was found, which may have impaired the compressive
strength (Fig. 5(a)). At an OH-
concentration greater than 7.0 mol/L, a high strength could not be reached due
to the free OH-
in the pastes, causing efflorescence from the binder [44].
An OH-
concentration of between 4.5-7.0 mol/L was able to
induce the chemical reaction so as to produce a high strength product using the
fly ash system adopted here [13, 18, 43]. From the microstructure as imaged
under an SEM (Fig. 5(b)), the material was less void and more well
development, compared with that seen in Fig. 5(a). This also
indicated the higher degree of polymerisation and the presence of N-A-S-H gel
[33], as evinced by XRD results. The compressive strength of geopolymer pastes
at Ms = 1.5 was higher than that at Ms
values of 1.25 and 1.0 at the same OH-
concentration: this may have been caused by the higher SiO2/Al2O3
ratio (Table 3).
|
Fig. 1 XRD patterns of fly ash and geopolymer pastes at various conditions. |
|
Fig. 2 Influences of Na2O content, W/B ratio and Ms on compressive strength of FGAs (a) Ms = 1.0, (b) Ms = 1.25 and (c) Ms = 1.5. |
|
Fig. 3 Influence of SiO2/Al2O3 and Al2O3/Na2O ratios on the compressive strength at W/B=0.38. |
|
Fig. 4 Effects of [OH−] in AAS on the compressive strength of pastes. |
|
Fig. 5 SEM image of paste with different [OH−] concentrations of (a) 4.30 and (b) 6.82. |
The influences of variables, such as Na2O
content, W/B ratio, and Ms of alkaline activator solution, on
the compressive strength of a FGA were investigated. There was a critical value
of the OH-
concentration of the alkaline activator required for optimal compressive
strength development, as influenced by the W/B ratio, Ms, and
Na2O content. At an OH-
concentration of less than 4.5 mol/L, the compressive strength was less than 20
MPa due to the low OH-
concentration having induced an low reaction of fly ash with
alkaline activator: however, at OH-
concentrations greater than 7.0 mol/L, free OH-
was present in the binder, and the compressive strength reached
a plateau or decreased. Only at an OH- concentration of
between 4.5-7.0 mol/L, could the compressive strength have reached between
20.0-36.0 MPa. The ratios of SiO2/Al2O3
and Al2O3/Na2O affected the compressive strength. According to the
results, the increase in SiO2/Al2O3 values
over the range of 3.83-5.33 promoted the increase of compressive strength; whilst, it was possible to achieve a high strength only when the Al2O3/Na2O
ratio was less than 1.0. As the
alkali content was increased to 12%
(relative to the mass to fly ash), the reaction of the mineral phases in the fly ash with the alkali
activator was strengthened, which
induced the formation of N-A-S-H gel, thus increasing the compressive strength.
This research was financially supported through the
National Natural Science Foundation of China (Grant No. 51375281).
- 1. M. Asadi, R. Naghizadeh, A. Nemati, K. Arzani, and R. Nassiri, J. Ceram. Process. Res. 13[4] (2012) 425-428.
- 2. T. Luukkonen, Z. Abdollahnejad, J. Yliniemi, P. Kinnunen, and M. Illikainen, Cem. Concr. Res. 103 (2018) 21-34.
-

- 3. K. Neupane, Mech. Mater. 103 (2016) 110-122.
-

- 4. J. Feng, R. Zhang, L. Gong, Y. Li, W. Cao, and X. Cheng, Mater. Des. 65 (2015) 529-533.
-

- 5. Y. Kim and T. Chae, J. Ceram. Process. Res. 19[5] (2018) 378-382.
- 6. P. Duxson, J.L. Provis, G.C. Lukey, and J.S.J. van Deventer, Cem. Concr. Res. 37 (2007) 1590-1597.
-

- 7. M. Khandelwal, P.G. Ranjith, Z. Pan, and J.G. Sanjayan, Arabian J. Geosciences 6[7] (2013) 2383-2389.
-

- 8. H.A. Abdel-Gawwad, and S.A. Abo-El-Enein, HBRC Journal 12[1] (2016) 13-24.
-

- 9. W. Zhou, C. Yan, P. Duan, Y. Liu, Z. Zhang, X. Qiu, and D. Li, Mater. Des. 95 (2016) 63-74.
-

- 10. M. C. Bignozzi, S. Manzi, M. E. Natali, W. D.A. Rickard, and A. van Riessen, Constr. Build. Mater. 69 (2014) 262-270.
-

- 11. J. Temuujin, A. van Riessen, and K.J.D. MacKenzie, Constr. Build. Mater. 24 (2010) 1906-1910.
-

- 12. S. Lee, A. van Riessen, and C.M. Chon, Materials 9[7] (2016) 598-608.
-

- 13. A. Fernández-Jiménez and A. Palomo, Cem. Concr. Res. 35 (2005) 1984-1992.
-

- 14. A. Palomo, M.W. Grutzeck, and M.T. Blanco, Cem. Concr. Res. 29 (1999) 1323-1329.
-

- 15. T. Bakharev, Cem. Concr. Res. 35 (2005) 1224-1232.
-

- 16. Y.K. Cho, S.W. Yoo, S.H. Jung, K.M. Lee, and S.J. Kwon, Constr. Build. Mater. 145 (2017) 253-260.
-

- 17. A.S. de Vargas, D.C.C. Dal Molin, A.C.F. Vilela, F.J. da Silva, B. Pavãoe, and H. Veit, Cement Concr. Compos. 33[6] (2011) 653-660.
-

- 18. G.S. Ryu, Y.B. Lee, K.T. Koh, and Y.S. Chung, Constr. Build. Mater. 47 (2013) 409-418.
-

- 19. S. Kumar, R. Kumar, T.C. Alex, A. Bandopadhyay, and S.P. Mehrotra, Adv. Appl. Ceram. 106[3] (2007) 120-127.
-

- 20. S. Kumar, R. Kumar, and S.P. Mehrotra, J. Mater. Sci. 45[3] (2010) 607-615.
-

- 21. M. Palacios, M.M. Alonso, C. Varga, and F. Puertas, Cement Concr. Compos. 95 (2019) 277-284.
-

- 22. H. R. Gavali, A. Bras, P. Faria, and R.V. Ralegaonkar, Constr. Build. Mater. 215 (2019) 180-191.
-

- 23. Y. Fang, and O. Kayali, Constr. Build. Mater. 39 (2013) 89-94.
-

- 24. F. Winnefeld, A. Leemann, M. Lucuk, P. Svoboda, and M. Neuroth, Constr. Build. Mater. 24 (2010) 1086-1093.
-

- 25. D. Hardjito, S.E. Wallah, D.M.J. Sumajouw, and B.V. Rangan, ACI Mater. J. 101 (2004) 467-472.
- 26. M. Soutsos, A.P. Boyle, R. Vinai, A. Hadjierakleous, and S.J. Barnett, Constr. Build. Mater. 110 (2016) 355-368.
-

- 27. Y.K. Cho, S.H. Jung, and Y.C. Choi, Constr. Build. Mater. 204 (2019) 255-264.
-

- 28. L. Assi, S. Ghahari, E. Deaver, D. Leaphart, and P. Ziehl, Constr. Build. Mater. 123 (2016) 806-813.
-

- 29. J.S. Tenepalli and D. Neeraja, J. Build. Eng. 19 (2018) 42-48.
-

- 30. G. Görhan and G. Kürklü, Composites: Part B 58 (2014) 371-377.
-

- 31. K.U.A. Sanalkumar, M. Lahoti, and E. Yang, Constr. Build. Mater. 225 (2019) 283-291.
-

- 32. J.G.S. van Jaarsveld, J.S.J. van Deventer, and G.C. Lukey, Chem. Eng. J. 89[1-3] (2002) 63-73.
-

- 33. A. Mehta and R. Siddique, Constr. Build. Mater. 141 (2017) 325-334.
-

- 34. A. Mehta, R. Siddique, B. Singh, S.Aggoun, G. Lagod, and D. Barnat-Hunek, Constr. Build. Mater. 150 (2017) 817-824.
-

- 35. M. Steveson and K. Sagoe-Crentsil, J. Mater. Sci. 40[16] (2005) 4247-4259.
-

- 36. C.E. White, J.L. Provis, A. Llobet, T. Proffen, and J.S.J. van Deventer, J. Am. Ceram. Soc. 94[10] (2011) 3532-3539.
-

- 37. P.V. Krivenko, and G.Y. Kovalchuk, J. Mater. Sci. 42[9] (2007) 2944-2952.
-

- 38. A. Palomo, A. Fernández-Jiménez, G. Kovalchuk, L.M. Ordoñez, and M.C. Naranjo, J. Mater. Sci. 42[9] (2007) 2958-2966.
-

- 39. F. Puertas, S. Martı́nez-Ramı́rez, S. Alonso, and T. Vázquez, Cem. Concr. Res. 30 (2000) 1625-1632.
-

- 40. P. Chindaprasirt, C. Jaturapitakkul, W. Chalee, and U. Rattanasak, Waste Manage. 29[2] (2009) 539-543.
-

- 41. X. Guo, H. Shi, and W. A. Dick, Cement Concr. Compos. 32 (2010) 142-147.
-

- 42. Z. Xie and Y. Xi, Cem. Concr. Res. 31 (2001) 1245-1249.
-

- 43. J.W. Phair, J.S.J. Van Deventer, and J.D. Smith, Appl. Geochem. 19[3] (2004) 423-434.
-

- 44. Z. Zuhua, Y. Xiao, Z. Huajun, and C. Yue, Appl. Clay Sci. 43[2] (2009) 218-223.
-

 This Article
This Article
-
2020; 21(3): 358-364
Published on Jun 30, 2020
- 10.36410/jcpr.2020.21.3.358
- Received on Oct 16, 2019
- Revised on Apr 23, 2020
- Accepted on May 4, 2020
 Services
Services
- Abstract
introduction
materials and methods
results and discussion
conclusion
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Qingbo Tian
-
School of Materials Science and Engineering, Shandong Jianzhu University, Jinan, China
Tel : +86 131 53128675
Fax: +86 531 86367282 - E-mail: tqb11@sdjzu.edu.cn







 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.