- Effect of the ratio of eggshell and rice husk as starting materials on the direct synthesis of bioactive wollastonite by solid state thermal method
Sazia Sultana, Md. Maksudur Rahman, Zenefar Yeasmin, Samina Ahmed* and Farzana Khan Rony
Institute of Glass and Ceramic Research and Testing (IGCRT), Bangladesh Council of Scientific and Industrial Research (BCSIR), Dhaka-1205, Bangladesh
This paper describes the effect
of the ratio of two starting materials, eggshell (ES, source of Ca) and rice
husk (RH, source of silica) on the direct synthesis of wollastonite
via a facile two-step solid state method, i.e. ball milling of the raw
materials followed by calcination of the mixture at 1,000 oC.
The investigation was focused on optimizing the ratio of ES and RH to maximize
the formation of wollastonite. Keeping the wt.% of RH constant, four different
ratios of RH and ES (10:2.6, 10:3.0, 10:3.3 and 10:3.7) were used and the
observed result revealed that the initial ratio of RH and ES plays a key role
in controlling the formation of wollastonite as the major phase which was
confirmed by x-ray diffraction (XRD) and Fourier Transform Infrared (FT-IR)
techniques. The bioactive property of this wollastonite was studied in
simulated body fluid at 37 oC while the time dependent growth
of hydroxyapatite (HA) on the surface of wollastonite was examined by SEM and
XRD. Observed data supported the bioactive nature of wollastonite as
biomaterial.
Keywords: Wollastonite, Eggshell, Apatite, Bioactive
Wollastonite, a dazzling white to gray or brown coloured
calcium meta silicate (CaSiO3) has received enormous attention to
the researchers. The application of wollastonite in traditional ceramics e.g.
porcelain [1], heat insulating ceramics [2], concrete [3] and cement [4] is
quite well known due to its various notable properties (fluxing
characteristics, low shrinkage behavior and
better strength). However, in the arena of advanced ceramics, pure wollastonite
has been categorized as an innovative material which has proved its
potentiality to be used as a bone regenerative bioceramic material [5] and
presently it continues to be an eye-catching research field [6-11]. Furthermore,
composite format of wollastonite, is also developed for biomedical
applications [12]. However, the prime reason for the bioactivity of
wollastonite, is the ability to form Si–OH bond on its surface upon exposure in
simulated body fluid (SBF). Concerning the significance
of wollastonite as biomaterial, researchers have already developed many routes,
namely sol-gel [6, 9-11, 13-15], hydrothermal [16-18] solid
state [19-21], ultrasonic irradiation [5] microwave assisted solid
state [22] and wet chemical precipitation method
[23] etc. to synthesize CaSiO3.
Being a non-toxic, solvent free and environment friendly
method, hitherto, solid-state approach has ranked to be
preferable.
Nevertheless, in addition to the synthesis route, the
precursors used as calcium and silica sources are also important. Different combinations
of the starting materials have been used by
different researchers e.g. Udduttula et al. [6], Lakshmi et al. [9] and
Wang et al. [13] used [Ca(NO3)]2.
4H2O and tetra ethyl orthosilicate (TEOS) as calcium and silica sources respectively; Anjaneyulu et al.
[10] choice was eggshell and TEOS; eggshell and diatomite
were chosen by Puntharod et al. [16] while snail shell and rice husk ash (RHA)
were used by Phuttawong et al. [19]. The researchers of Vichaphund’s group
prepared wollastonite from eggshell and commercial grade (98%) silica [22]. In
combination with [Ca(NO3)]2. 4H2O researcher
also used Na2SiO3.9H2O as silica source [11].
However, researchers still continue
to explore different combination of
the raw materials to be used as the source of calcium and silica and numerous
recipes used to synthesize wollastonite [24-36] are summarized in Table 1.
In this era of globalization, scientists across the globe are
paying more and more attention to resolve environmental pollution problems and
this has led them to be concerned in developing new synthetic routes as well as
in utilizing waste materials. Keeping this view in mind, we have used waste ES
(as Ca source) and RH (as silica source) together to
synthesize wollastonite via solid state thermal treatment method.
RH, though categorized as waste/by-product but it
contains cellulose (38.3%), hemicellulose (31.6%), lignin (11.8%) and silica
(18.3%). Heat treatment or combustion of RH produces about 20-25 wt% of rice
husk ash (RHA), which contains more than 90% silica. [37]. On the other hand,
ES is enriched with CaCO3 (94-97%) [38]. Hence these two agro-wastes
are being getting the attention of the researchers to be used as the source of
silica and Ca respectively in synthesizing biomaterials and or ceramic
materials. Concerning the significance of RH and ES, to the
best of our knowledge, for the first time, we have studied
the effect of the ratio of ES:RH to synthesize CaSiO3
even though individually either ES or RHA was used as calcium
or silica source material in many previous studies
[10, 16, 19, 22, 33].
Bangladesh is categorically considered as Agriculture
or Agro based country having an annual production of 4-4.5 million
metric tons (MT) of paddy which gives about 9.0 million tons of RH as waste
product [39]. On the other hand, in Bangladesh the production of egg
is 10168 million per annum [40] and consequently every year ~113000 tons of ES
are thrown away to the environment. Hence, utilization of ES and RH will be beneficial
in two ways: (i) synthesized wollastonite will be cost
effective bioceramic material for biomedical applications, and (ii) it will be
an effective material-recycling pathway for waste management.
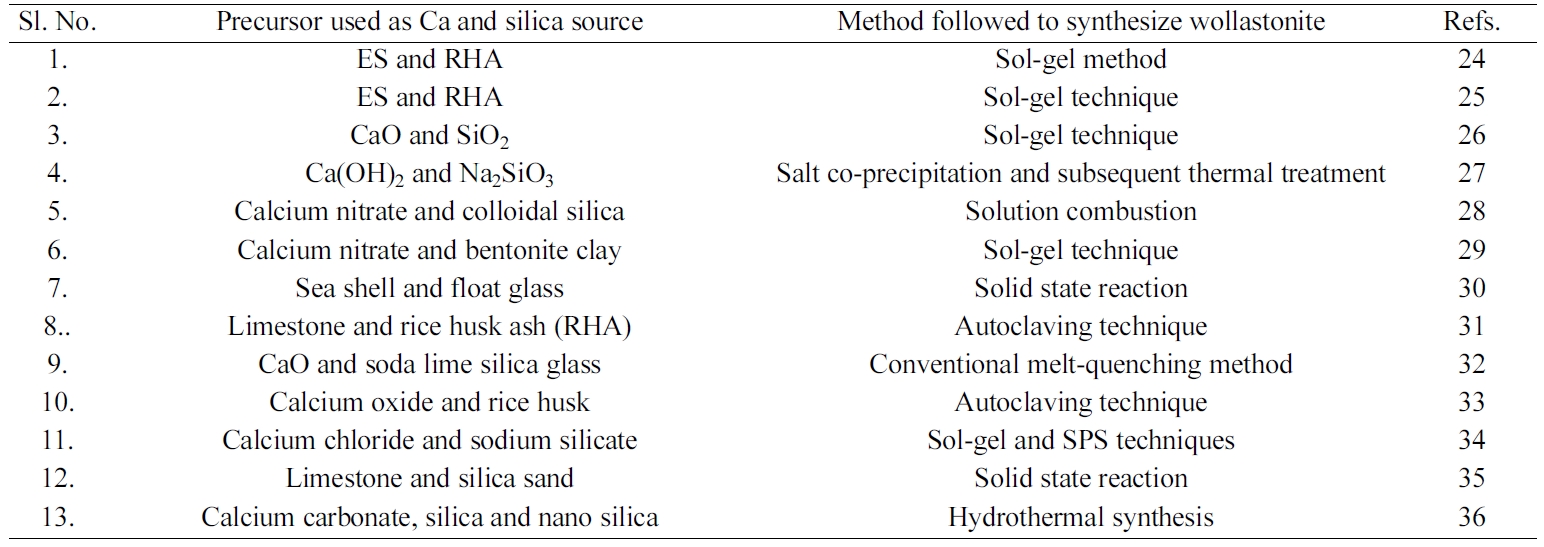
|
Table 1 Summary of the recepies used to synthesize wollastonite by different methods. |
Materials
and their processing
RH and ES were collected from rice mill and local
restaurant and used as the source of silica and calcium respectively. Prior to
using as source materials, RH was processed following a previously described
method [13]. Briefly, RH was soaked in water for 5 minutes,
maintaining the water to RH ratio at 20 L/kg. Then the mixture was kept
undisturbed for couple of minutes to allow the unwanted materials to be settled
down at the bottom. After that, RH was collected carefully from the upper
portion of the mixture using a sieve of 60-mesh. The cleaned RH was then dried
overnight in an oven at 100ºC followed by treating with 0.2 M H2SO4
(acid to RH ratio was 10 L/kg) at 100ºC for 2 hrs. After cooling and filtering,
the RH was washed with copious amount of water to remove any trace of sulphuric
acid and finally dried at 100 oC [1]. Entire
processing steps resulted about 40% weight loss of RH. On the
other hand, collected ES was washed thoroughly with tap water. Then the inner
shell membrane was removed from ES and boiled in water for 30 min. Finally, the
boiled ES was dried at 100 oC [2].
Synthesis
of wollastonite by indirect method
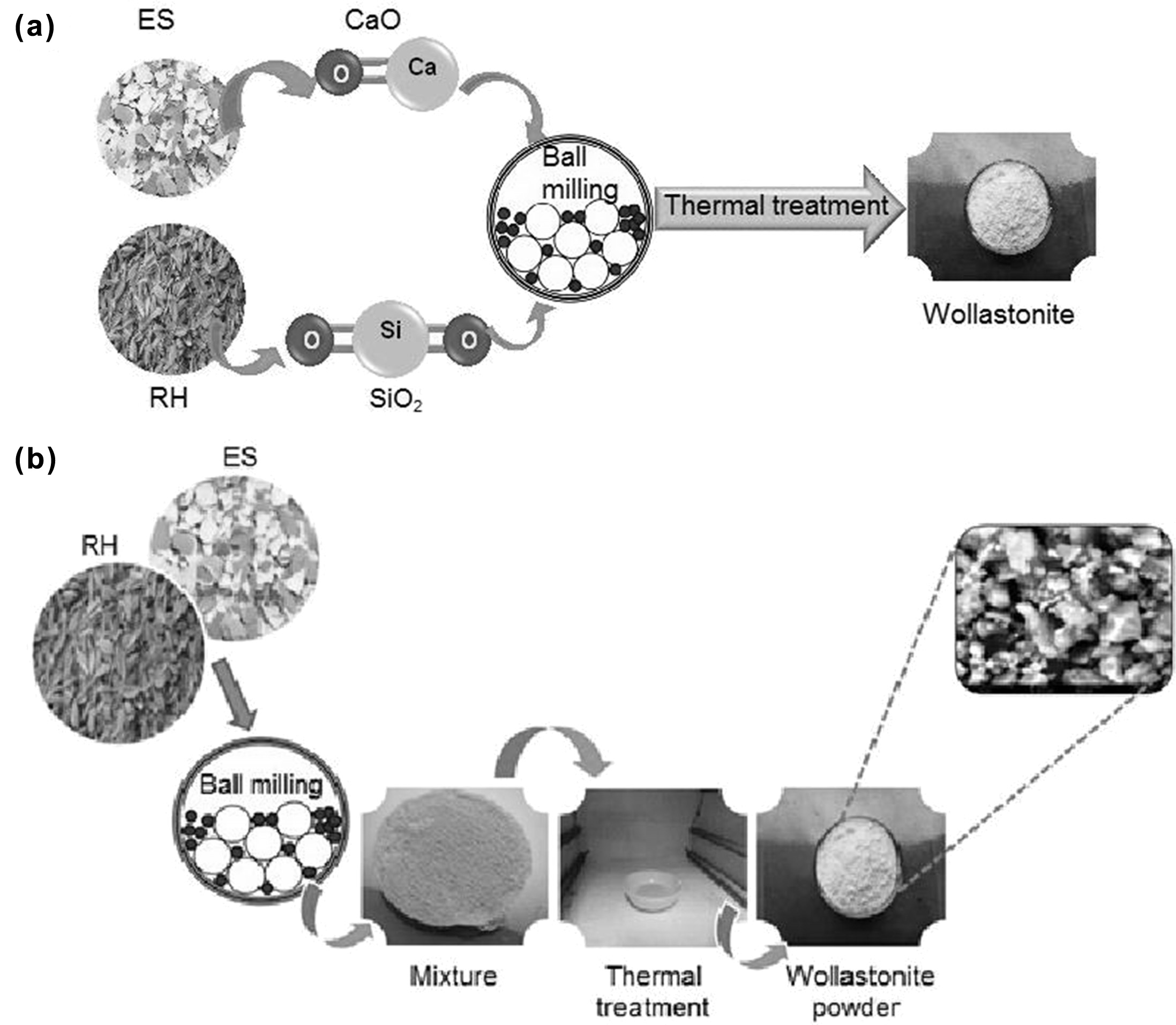
In order to synthesize wollastonite using RH and ES as the
starting materials, at first an indirect calcination method [21]
was followed which was then scaled up by developing the direct sintering
approach. Since theoretically the wt% of CaO and SiO2 in
wollastonite is 48% and 52% respectively [1-2], so to determine the exact wt.%
of CaO and SiO2 in ES and RH our primary attempt was confined with
the indirect method as adopted by Hossain et al. [21]. In the indirect method,
SiO2 was extracted from treated RH following the previously
described methods [1,21] but extending the
calcination temperature up to 1,000 oC. On the other
hand, 3 hours thermal treatment (at 1,000 oC) of ES resulted
the formation of CaO [21]. Then stoichiometric amount of
CaO and SiO2 were mixed homogeneously for 30 minutes at 1,000 rpm
using a high-energy ball mill (Model: MSK-SFM-1 QM 3SP2) followed by thermal
treatment at 1,000 oC for 3 hrs.
which facilitated the formation of desired wollastonite.
Synthesis
of wollastonite by direct calcination of RH and ES
In the direct method, a series of ratio of powdered RH and
ES (10:2.6, 10:3.0, 10:3.3 and 10:3.7) were chosen as the starting materials
and each selected proportion was straightaway ball milled for 30 minutes at
1,000 rpm to get the mixture in homogeneous form. Then the desired wollastonite
was produced by sintering the mixture in a muffle furnace at 1,000 oC
for 3 hrs. The schematic representations of the indirect and direct synthesis
process are displayed in Fig. 1.
Characterization
of synthesized wollastonite
Synthesized wollastonite was characterized by various
techniques e.g. X-ray diffractometer (XRD), Fourier transform
infrared spectrophotometer (FT-IR), Scanning electron
microscopy (SEM) and Thermogravimetric (TG/DTA) analyses.
The respective phases of wollastonite were identified by
the XRD (PANalytical X’Pert PRO XRD PW 3040). Intensity
data were collected by fixing scanning range, 2q = 10o – 75o
using Cu Kα radiation (λ = 1.5406 Å) with a step scan of 4o/min.
To confirm the presence of desired phases, recorded data were compared with
standard JCPDS files.
The presence of the functional group was explored by
recording the FT-IR spectrum using (Prestige 21; SHIMADZU) a FT-IR
spectrophotometer. The sample-to-KBr ratio was 1:100 while the scanning range
was 4000 to 400 cm-1
and the resolution was 4 cm-1 with
32 scanning.
The thermogravimetric analysis (TGA) was carried out on an
EXSTAR TG/DTA 6300 with alpha-alumina powder as a reference sample from 30 to
1,000 oC in nitrogen atmosphere (flow rate 50 nmL/min). The
heating rate was 20 oC/min. The surface morphology and
microstructural features of the synthesized material were observed by SEM
(Phenom Pro) applying an accelerating voltage of 15 kV.
Bioactivity
studies
In-vitro bioactivity response is the evaluation of
apatite-forming ability (mainly hydroxyapatite, HAp), on the surface of
bio-material in simulated body fluid (SBF). A previously published
method [1] was followed for this
purpose. About 0.2 g of synthesized wollastonite powder was
immersed into 30 ml SBF at 37 oC. The growth of HAp
was monitored at different time intervals (1, 3 and 7
days). To investigate the formation of HAp on the surface of wollastonite
powder, first the SBF solution was drained out carefully then the powder was
gently rinsed with deionized water to remove excess SBF followed by drying at
50 oC. The formation of apatite layers was examined by XRD and
SEM.
|
Fig. 1 Schematic representation of the synthesis of wollastonite (a) Indirect method, (b) Direct method. |
XRD
analysis
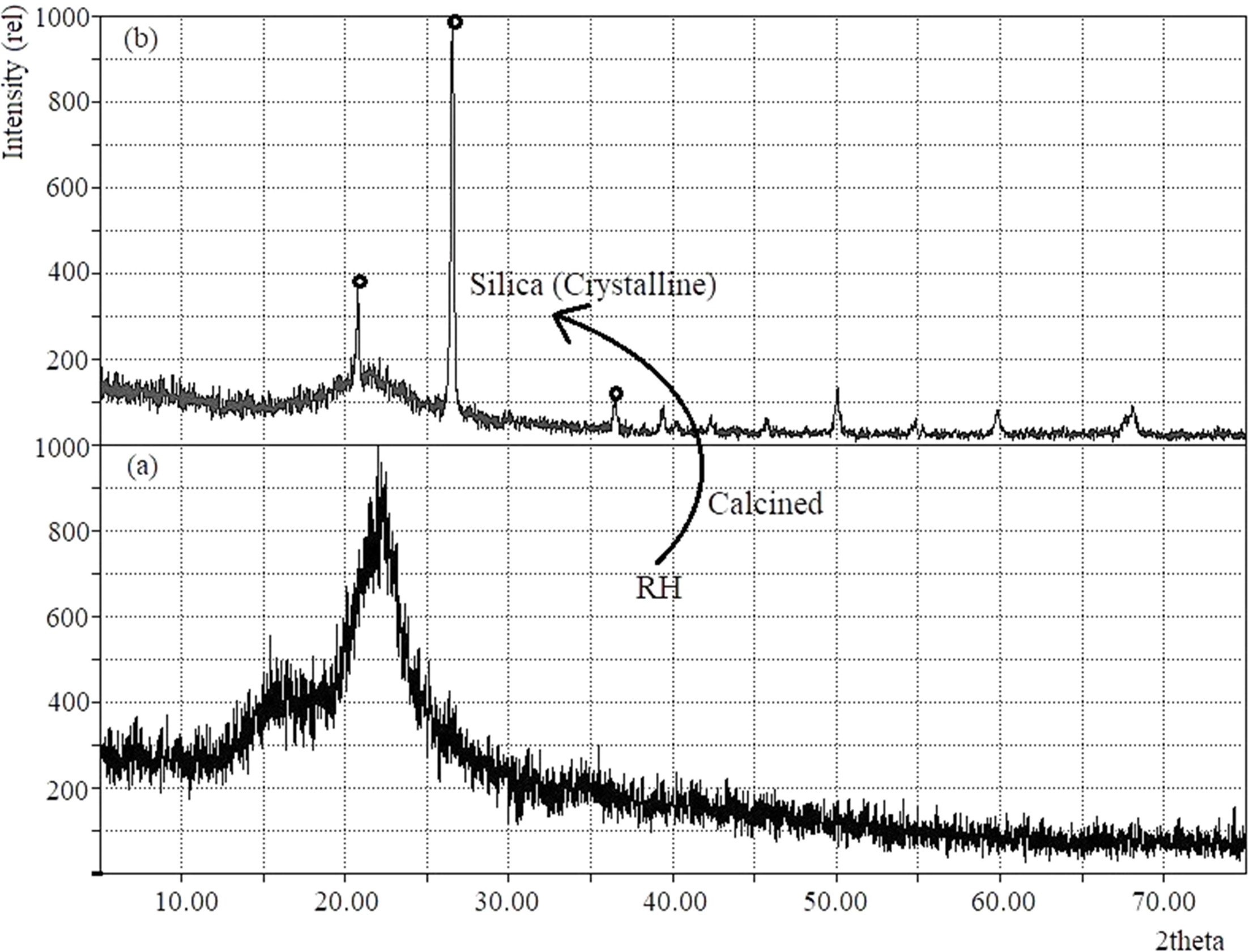
Given in Fig. 2 is the x-ray diffratogram of as received
RH and after calcination at 1,000 oC. The XRD pattern of SiO2
(Fig. 2b) having 2θ values of 21.9o, 26.6o and 36.0o
indicates that the silica formed in crystalline nature as expected. Because, it
is well known that the presence of amorphous silica, crystalline silica or both
phases strictly depends on the calcination temperature [41, 42]. The
characteristic peaks of tridymite (2θ = 21.6o) and cristobalite (2θ = 21.8o)
phases coincides giving a single peak at 21.9o. This could be due to
the melting of the surfaces of ash silica particles followed by formation of
bonding of the particles together [41, 42].
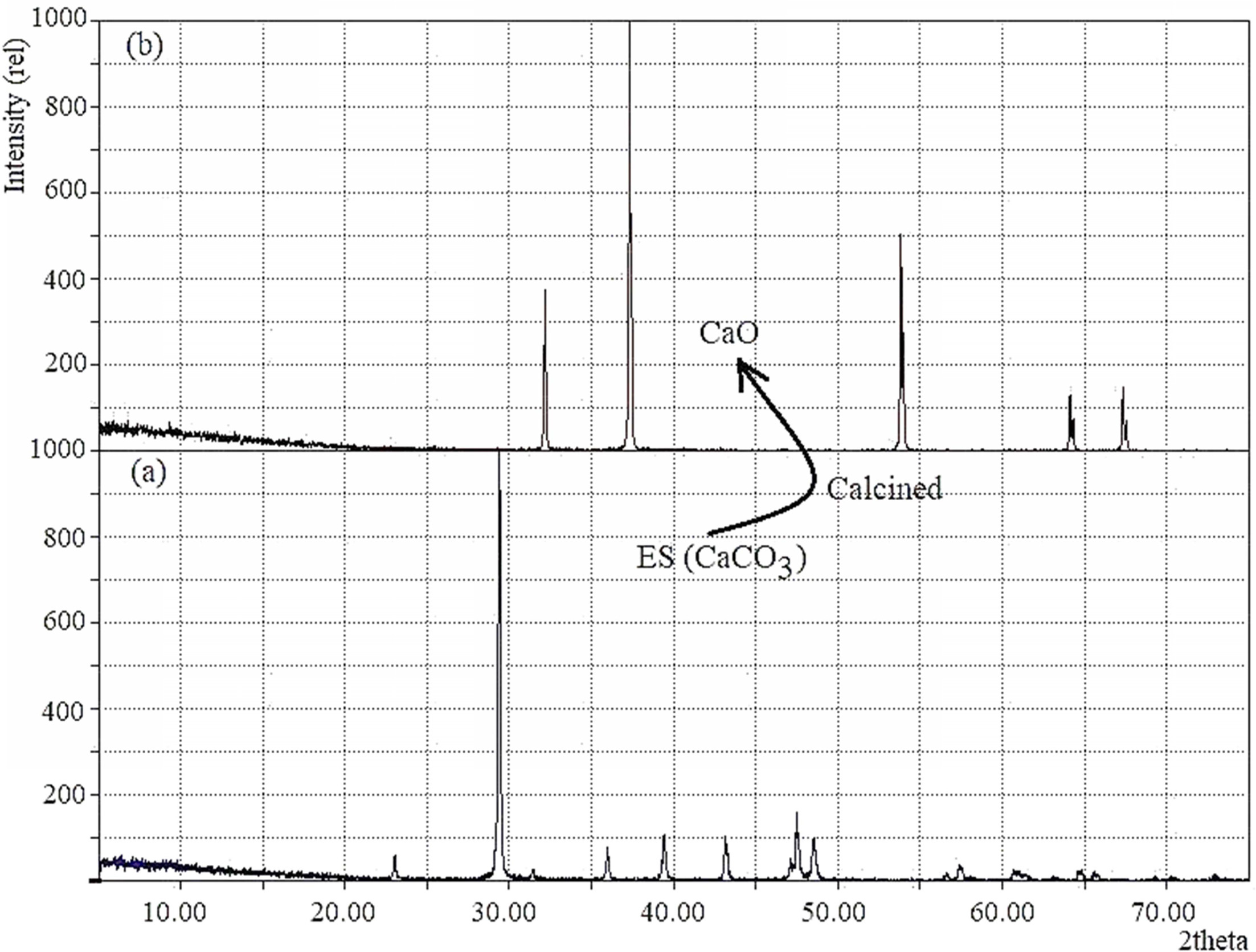
The XRD patterns of the ES before and after calcination
are displayed in Fig. 3(a, b). It is apparent from the
recorded diffraction data (Fig. 3a) that the ES without being
thermally treated shows the usual characteristic peaks for rhombohedral calcite
(File # 5-0586). The characteristic peak detected at 2θ position 29.485o
(1 0 4) plane coupled with several peaks at 23.07o,
36.06o, 39.48o, 43.26o, 47.64o and
48.6o confirmed the mineral phase of egg shell as
calcite and no other crystalline phase was observed.
Conversely, after calcination (Fig. 3b) of ES, existence
of the distinctive peak at 2θ position 37.09o (2 0 0) plane supports
the formation of CaO. Moreover, the calcined sample showed no peak at 2θ = 29.48o
which indicates that CaCO3 has completely changed to CaO [43].
However, the total percentage of
silica (SiO2) and CaO formed due to calcination of RH and ES at 1,000 oC were found to be 20% and 55% respectively. Following earlier studies
[14, 15, 19], our indirect approach used an equal ratio of these two calcined products (i.e. CaO and SiO2) to synthesize wollastonite. But as our
intention was to investigate the impact of the ratio of RH and ES on the direct
synthesis of wollastonite, we explored a facile pathway where blend of RH and
ES (at different ratios, 10:3.7; 10:3.3; 10:3.0 and 10:2.6)
were straight away calcined to get the desired wollastonite.
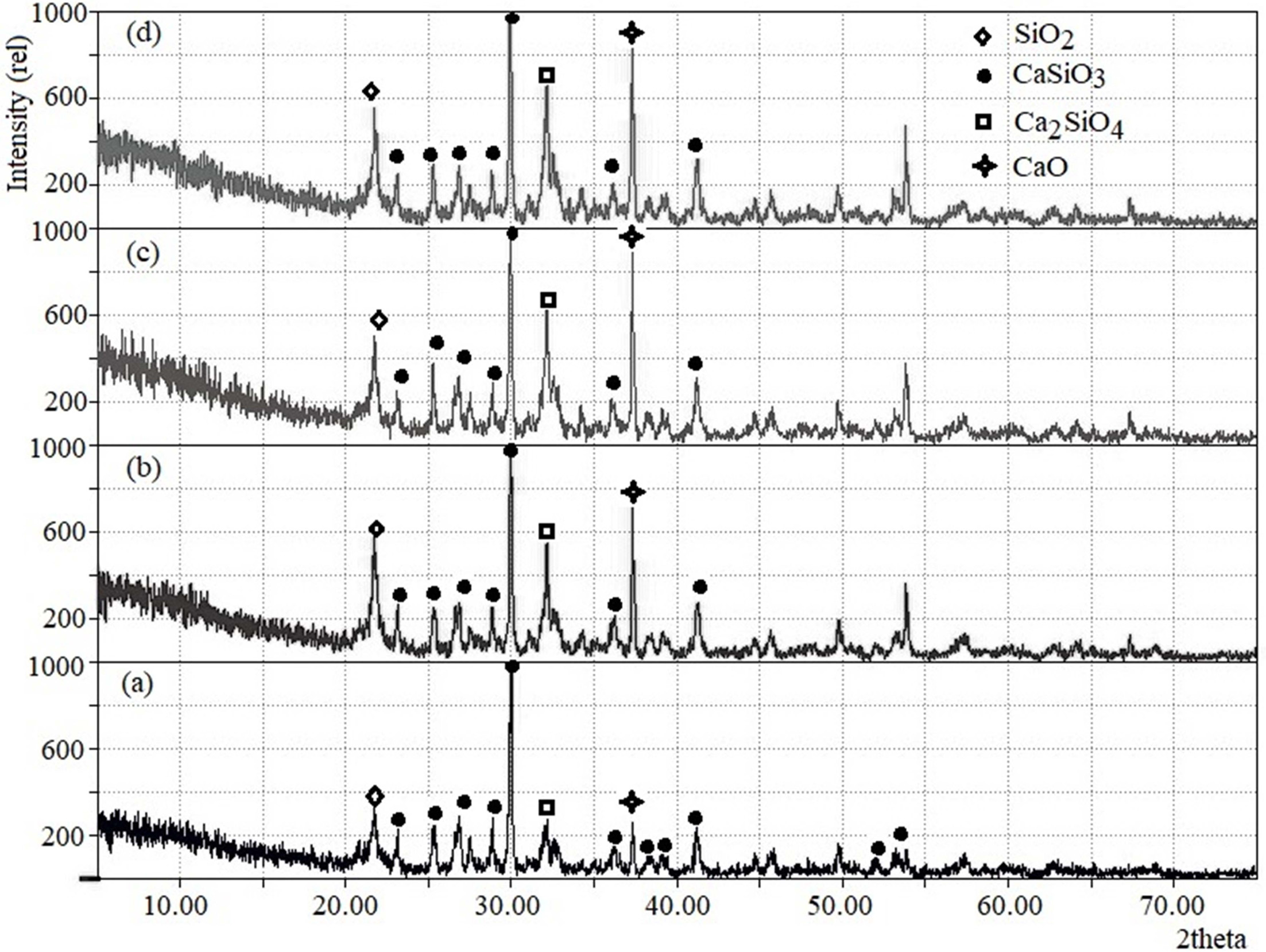
Fig. 4 demonstrates the corresponding x-ray diffractograms of
wollastonite as synthesized using the above-mentioned ratios of RH
and ES. It is clearly evident from Fig. 4 that the initial ratio of RH and ES
plays a key role in controlling the formation of wollastonite as the major
phase. Although the four chosen proportions of RH and ES effectively produced
wollastonite (consistent with the JCPDS file no. 043-1460) [10] but together
with wollastonite, larnite (Ca2SiO4) as well as unreacted
CaO and SiO2 were also indexed [13]. The wollastonite phase
(ICDD number: 00-043-1460) [10, 31] was recorded
in Fig. 4(a-d) at 2 theta positions 23.1, 25.4, 26.9, 28.9, 30.0, 36.2, 38.2,
39.2, 41.3 showed a good match with previous investigations [10, 31] while
calculated lattice parameters (a = 15·46, b = 7·33 and c =
7·19 Å) were also found to be in good agreement [10, 31].
Obviously, the formation of wollastonite with mixed phases
strictly depends on the fraction of RH and ES used. The characteristic peaks
for CaO, SiO2 and larnite were very prominent (as shown in Fig. 4b,
4c and 4d) when the ratios of RH and ES were 10:3.0, 10:3.3 and 10:3.7. On the
other hand, these three peaks appeared in suppressed state making the
wollastonite phase as predominant when the RH:ES ratio was fixed at 10:2.6
(Fig. 4a). As it has been observed from the XRD data that among the four
combinations of RH to ES ratios, better result was obtained at RH:ES ratio
10:2.6, we further proceeded with the FT-IR analysis of wollastonite
synthesized using this ratio of RH:ES.
FT-IR
analysis
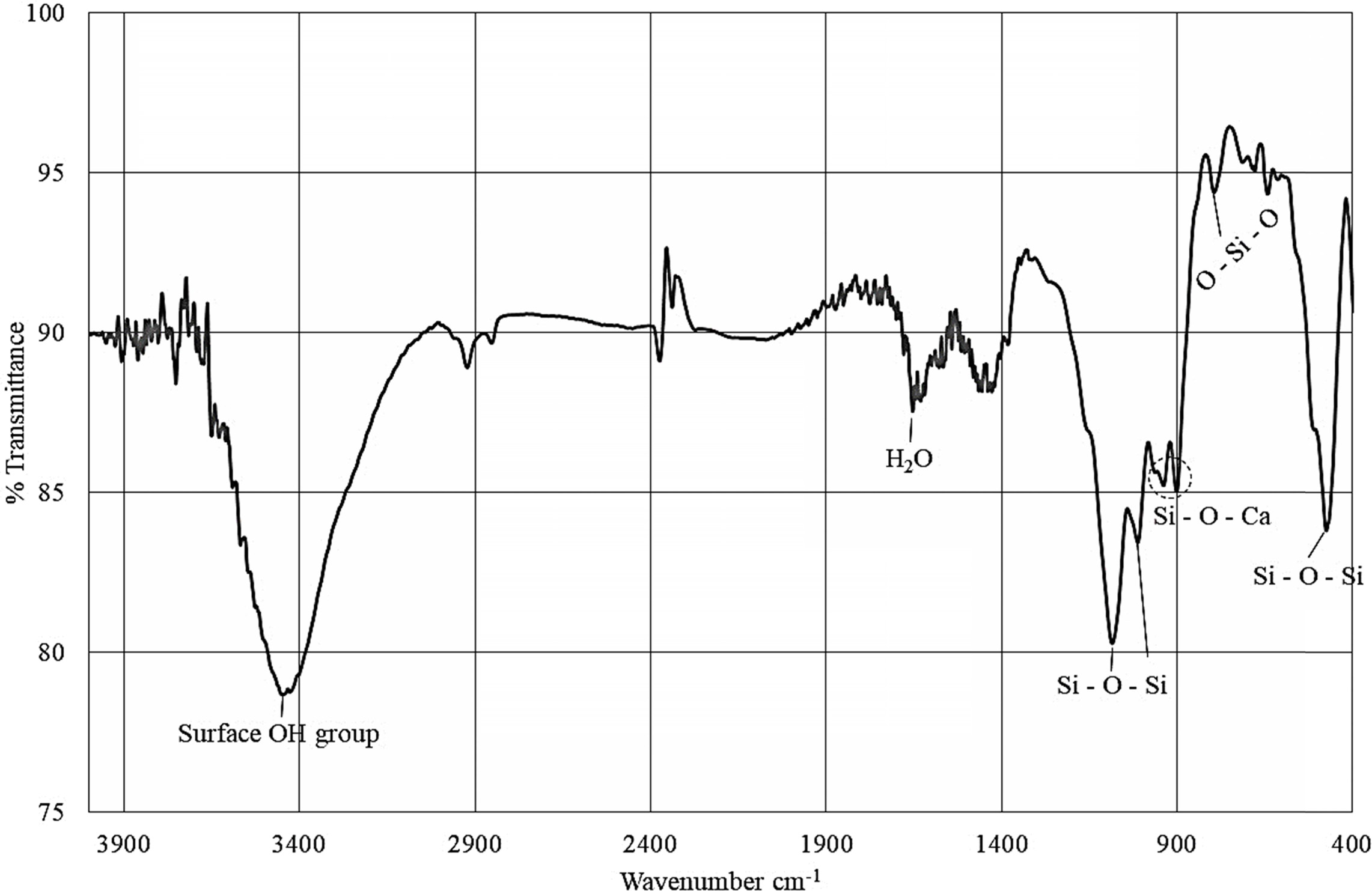
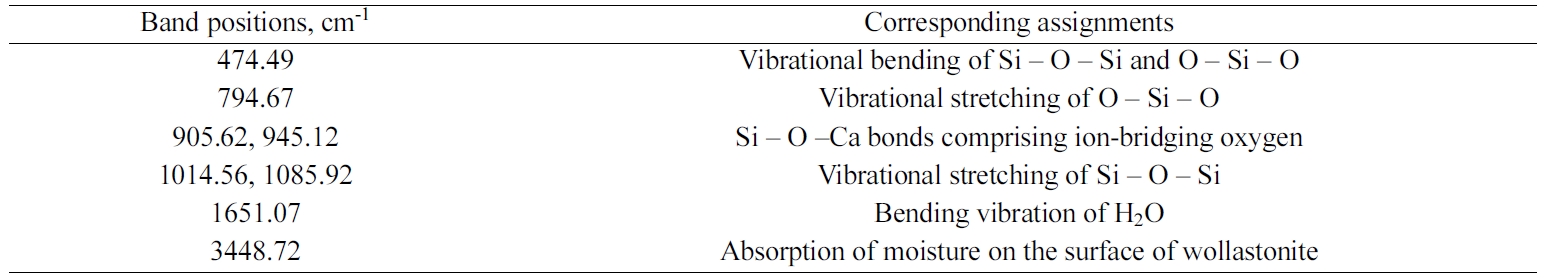
Fig. 5 represents a typical FT-IR spectrum of the synthesized
wollastonite. Recorded FT-IR band positions as
tabulated in Table 2, were fairly consistent with previous studies
[6, 10, 14]. Noticeably, the band position
observed around 476 cm-1
represents the vibrational bending mode of Si – O – Si bond while the
vibrational stretching mode of O – Si – O bond is positioned at 792.74 cm-1.
Si – O –Ca bond having ion-bridging oxygen is noticed at band positions 905-945
cm-1.
Vibrational stretching mode of Si–O–Si bond was observed in
the range of 1,014 to 1,091 cm–1. The bands
around 1,639 and 3,400 cm–1 are responsible for the moisture content
present in the sample.
Thermogravimetry
analysis
Since the RH and ES mixture of 10:2.6 ratio was the best
combination for synthesizing wollastonite by direct calcination,
further investigation was focused on the thermogravimetric analysis of this
mixture in raw state (i.e. without calcination). Given in Fig. 6 are the TG and
DTG behavior of RH and ES (10:2.6) blend which can be described consequently
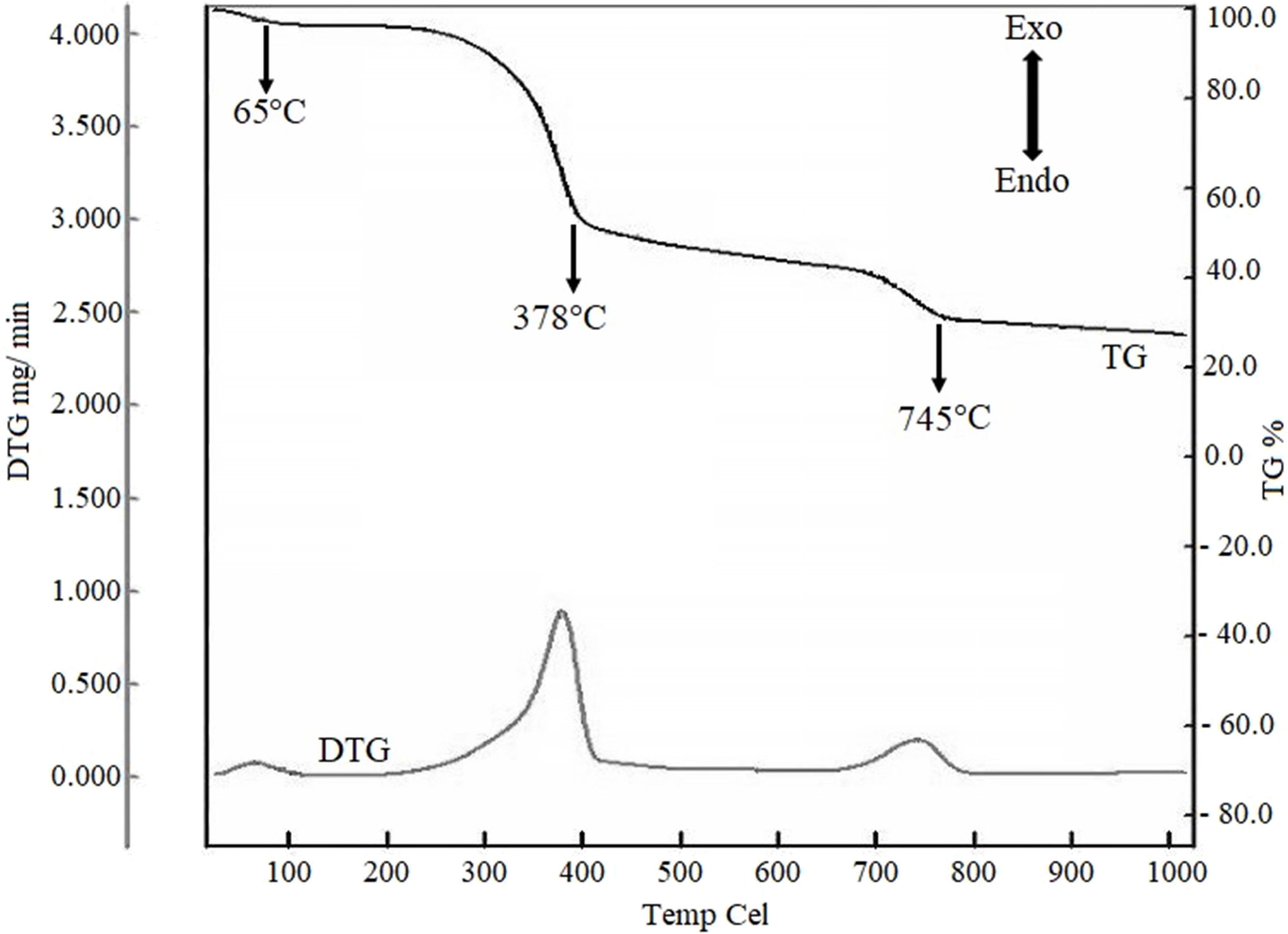
depending on different stages. It is apparent from the Fig. 6 that there are
three visible endothermic peaks at 65, 378 and 745 oC. The
first weight loss (~4%), as shown in TGA starts from ambient temperature to
65 oC, is responsible for the release of the moisture from the
sample during heating. The major decomposition occurred at 378 oC
where approximately 47% weight was lost. This is due to the degradation of
chemically bound water, cellulose, hemicelluloses and lignin from RH [44].
Finally, an endothermic peak at 745 oC is due to
high-temperature decarbonation i.e. due to the decomposition of calcium
carbonate from ES according to the reaction (1) [45]. The weight was constant
after 800 oC. Observed TG profile is in
line with many previous studies where such three steps
weight loss behaviour was well documented [33, 46-48].
No
significant weight change was observed in TG curve after 800 oC. Surprisingly, the DTG
curve also featured three exothermic peaks
at 65, 378 and 745 oC. The exothermic hump at 65 oC could be the
repre- sentative for the elimination
of physically adsorbed humidity water. The
2nd exothermic signal at 378 oC is comparatively strong than
the other two exothermic peaks. This is probably due to the burning of organic
matter from ES and RH [21]. The 3rd exothermic peak recorded at around 750 oC could be due to the formation
of CaSi2O4 and
CaSiO3 crystalline phases [13]. It should be
mentioned here that Sreekanth et al. [48] also obtained an exothermic peak of the DTA at 890 oC,
which is the preparatory temperature for the crystallization of CaSiO3.
In-vitro Bioactivity
Response of wollastonite synthesized using RH:ES ratio 10:2.6
The wt % ratio of RH and ES appeared significant in
producing the desired wollastonite. When the combinations
of RH and ES were 10:3.0, 10:3.3 and 10:3.7, formation of larnite
(Ca2SiO4) became significant with
wollastonite. Additionally, CaO and SiO2 were also present with the
desired product. On the other hand, formation of wollastonite as major phase
was visualized when the RH:ES ratio was fixed at 10:2.6 while
larnite formation was suppressed. Since, wollastonite shows
high biocompatibility as compared to other silicate based
ceramics, so it is presumed that wollastonite synthesized using this optimum
ratio will show better bioactive properties as larnite
formation was suppressed in this case. However, further
investigation in this direction is yet to be explored.
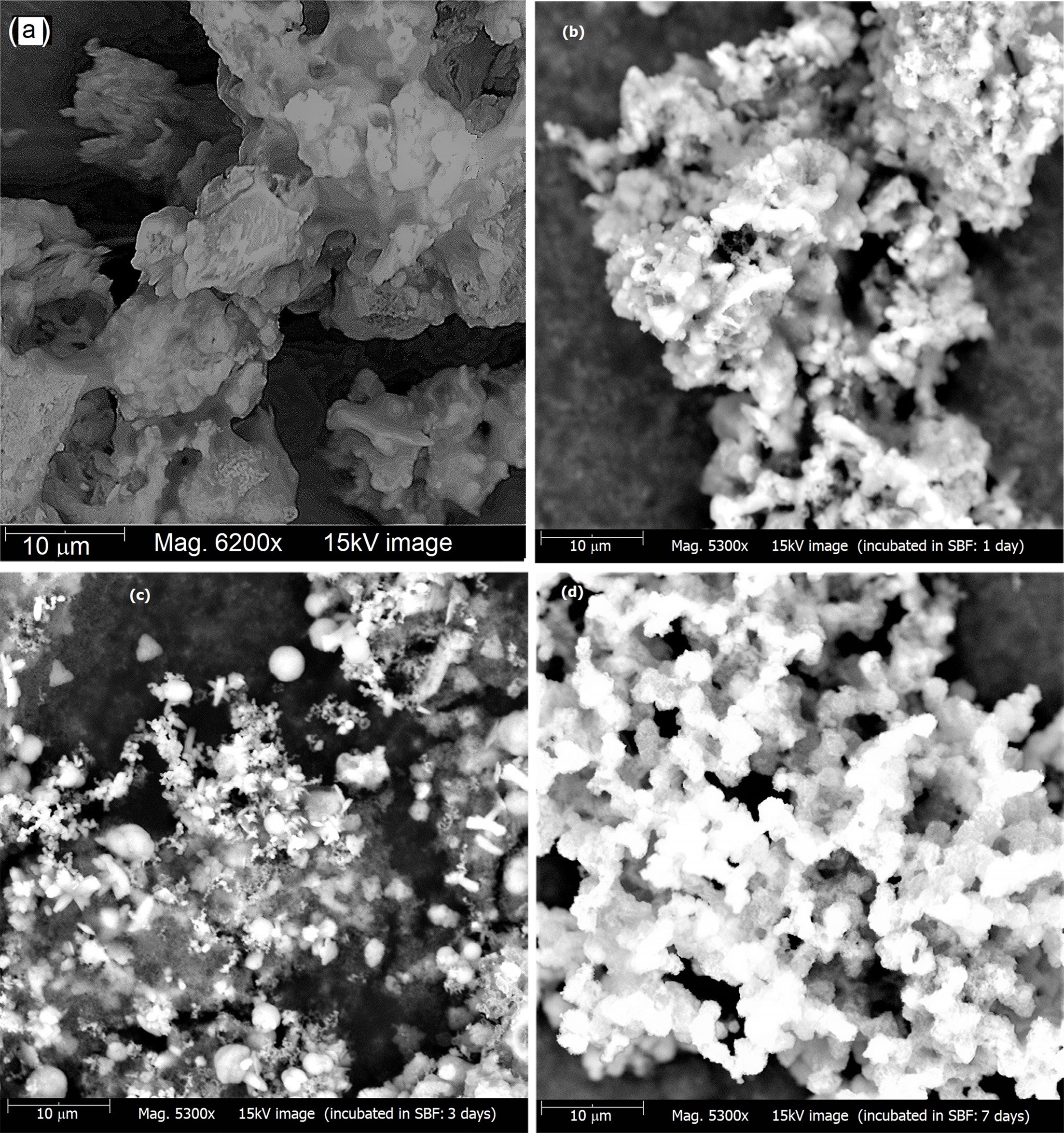
SEM images of wollastonite powder showing the in-vitro
bioactivity response i.e. the apatite forming ability are depicted in Fig.
7(a-d). Fig. 7(a) illustrates the surface morphology of bare wollastonite which
is in agglomerated form. However, in comparison with this sample, it is clearly
evident from Fig. 7(b, c, d) that as a result of immersing in SBF
the surface of wollastonite samples come to be covered by newly
formed apatite (HAp) layers and a continuous deposit of dense apatite takes
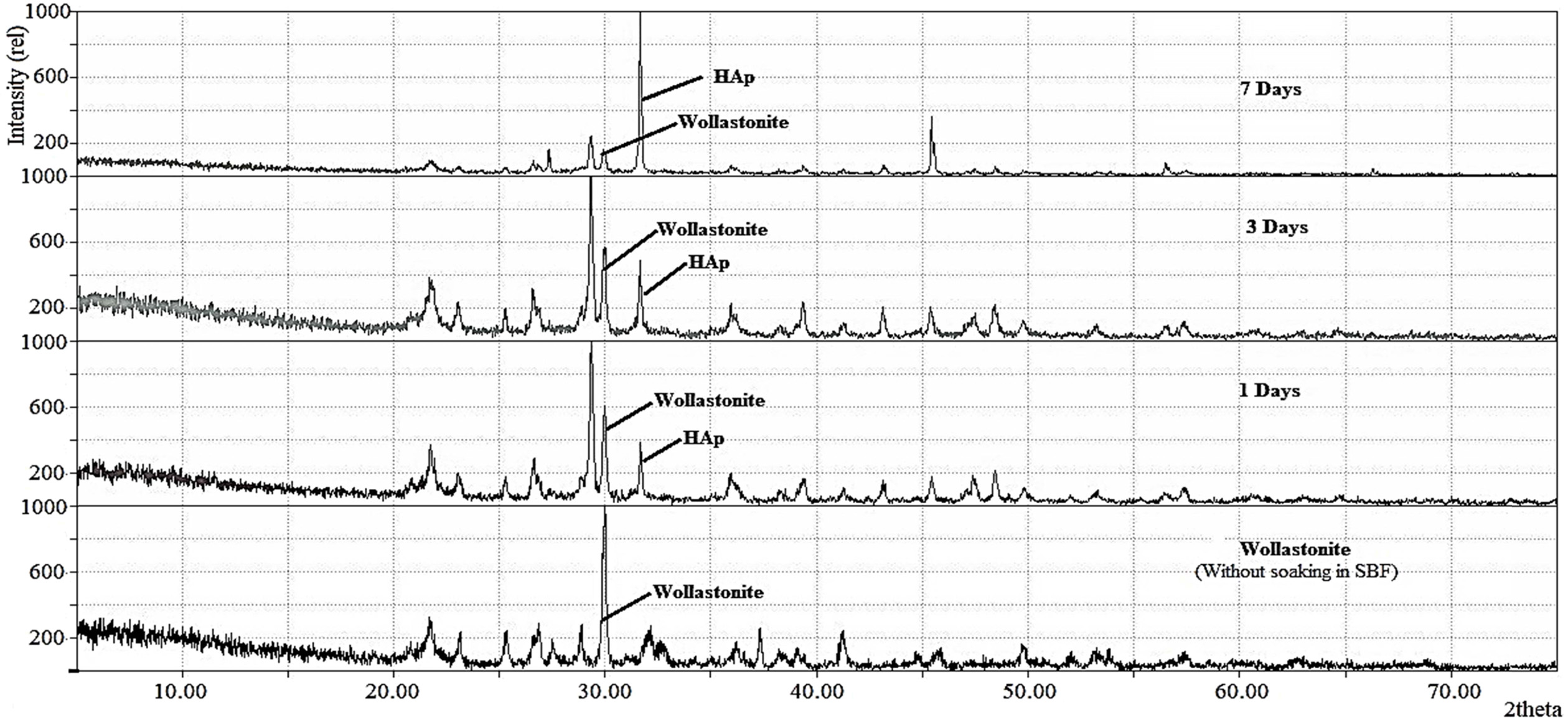
place with time. This was further validated by XRD data. The
stacked XRD diffractograms of wollastonite before and after soaking in SBF are
shown in Fig. 8. In SBF treated wollastonite, coupled with wollastonite peak,
the characteristic peak of HAp representing the (2 1 1) plane is also detected
at 2θ position 31.79o and this observation is in good agreement with
earlier studies [11,49]. Supporting the SEM observation, it is evident that as
more and more apatite forms, intensity of this freshly formed peak goes toward
the upward direction with the course of time. On the other hand, the intensity
of wollastonite peak gradually declines. This result supports that the
wollastonite synthesized by thermal treatment of two waste
materials e.g. RH and ES shows bioactive properties by inducing direct bone
ingrowth while incubated in physiological environment.
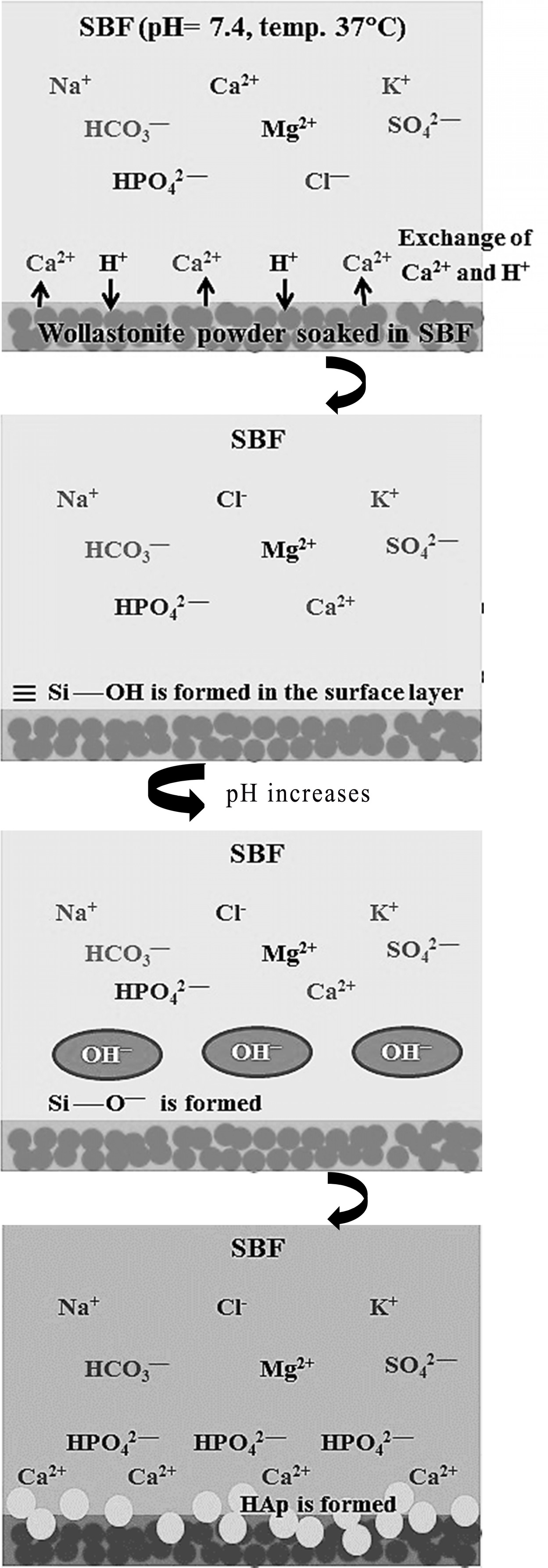
Mechanism of HAp formation
The bioactive response of wollastonite is accredited to
the nucleation of hydroxyapatite (HAp) and this phenomenon becomes activated by
the dissolution of calcium and silicate ions. Hence, the mechanism of HAp
formation on the surface of wollastonite powder soaked in SBF can be elucidated
following previous studies [6, 15, 49, 50]. Briefly, according
to Equation 2, due to immersion in SBF, Ca2+ present in wollastonite
tends to exchange with existing H+ of SBF forming silanol (Si – OH)
in the surface layer. This process increases the pH at wollastonite-SBF
interface and consequently a negatively charged surface having functional group
(Si – O–) is formed (Equation 3). The Ca2+ ions present
in SBF solution are firstly attracted to the solid - liquid interface which
causes the ionic activity product (IP) of apatite to be high enough at the
interface. Such an environment favours the growth of apatite on wollastonite
surface. Once the apatite begins nucleation, spontaneous growth occurs with the
aid of calcium and phosphate ion consumption from the mother SBF solution
(Equation 4). Fig. 9 depicts the pictorial representation of HAp formation
mechanism on wollastonite surface.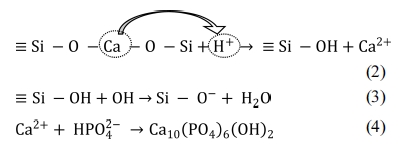
|
Fig. 2 XRD pattern of (a) treated RH and (b) silica obtained from RH treated at 1,000 oC. |
|
Fig. 3 XRD pattern of (a) ES and (b) CaO obtained from ES treated at 1,000 oC. |
|
Fig. 4 XRD pattern of RH-ES powders calcined at 1,000 oC. RH:ES = (a) 10:2.6. (b) 10:3.0. (c) 10:3.3 and (d) 10:3.7. |
|
Fig. 5 FT-IR spectrum of the wollastonite synthesized by calcining the mixture of RH and ES (ratio = 10:2.6) at 1,000 oC for 3 hrs. |
|
Fig. 6 TGA and DTG representation for the TGA for RH and ES (10:2.6) mixture |
|
Fig. 7 SEM images of (a) bare wollastonite samples and (b, c, d) after soaking in SBF for 1, 3 and 7 days respectively |
|
Fig. 8 XRD diffractograms of bare wollastonite samples and after soaking in SBF for 1, 3 and 7 days. |
|
Fig. 9 Schematic representation of apatite formation on wollastonite surface in SBF. |
|
Table 2 FT-IR band positions of wollastonite synthesized at RH:ES ratio 10:2.6. |
The
research work reported here in particular demonstrated
the influence of initial ratio of the raw materials, RH and ES in synthesizing
wollastonite directly by solid state method. Although different combinations of the starting materials have been
used by different researchers, but effect of the ratio of ES and RH has not
been explored yet. Hence the purpose of this research work was to develop a
protocol to synthesize wollastonite
from RH and ES and to the best of our knowledge, for the first time we have
investigated such effect on the
formation of wollastonite using RH and ES. Our approach revealed that the
initial ratio of RH and ES plays a
key role in controlling the formation
of wollastonite as the major phase. The optimum ratio of RH: ES to synthesize
wollastonite as the major phase was found to be 10:2.6 which was confirmed by x-ray diffraction (XRD) and Fourier Transform
Infrared (FT-IR) techniques.
Moreover, the bioactive response of the wollastonite synthesized using above
mentioned ratio of RH:ES showed excellent in-vitro bioactive properties. Such
observation revealed its suitability to be used as biomaterials.
Since, it is well-known that fine particles of the
ingredients speed up the diffusion reaction for the phase transition during
calcination and also helps to get the product in pure form thus further
advancement of our research focuses on investigating the effect of particle
size (ranging from fine to course) of the raw materials in synthesizing
wollastonite.
This research work was supported by BCSIR through R&D
project (Ref. 39.02.0000.11.014.007.2017/848, Dated 26/09/2017). Sazia Sultana
and Md. Maksudur Rahman also acknowledges BCSIR for ‘Nurul Abser Khan’ Post
Graduate Fellowship. Thanks to Dr. M. A. Gafur,
Principal Scientific Officer, Pilot Plant & Process
Development Centre, BCSIR, Dhaka for providing TGA facilities.
- 1. S. Ke, X. Cheng, Y. Wang, Q. Wang, and H. Wang, Ceram. Int. 39[5] (2013) 4953-4960.
-

- 2. M. Felipe-Sesé, D. Eliche-Quesada, and F.A. Corpas-Iglesias, Ceram. Int. 37[8] (2011) 3019-3028.
-

- 3. P. Kalla, A. Misra, R.C. Gupta, L. Csetenyi, V. Gahlot, and A. Arora, Constr. Build. Mater. 40 (2013) 1142-1150.
-

- 4. M.R.F. Gonçalves, C.K. Fillipeto, J. Vicenzi, and C.P. Bergmann, Constr. Build. Mater. 25[1] (2011) 320-327.
-

- 5. M. Mehrali, S.F.S. Shirazi, S. Baradaran, M. Mehrali, H.S.C. Metselaar, N.A.B. Kadri, and N.A.A. Osman, Ultrasonics Sonochemistry 21[2] (2014) 735-742.
-

- 6. A. Udduttula, S. Koppala, and S. Swamiappan, Trans. Ind. Ceram. Soc. 72[4] (2013) 257-260.
-

- 7. H. Begam, S. Mandal, J. Mukherjee, and S. K. Nandi, Trans. Ind. Ceram. Soc. 73[4] (2014) 284-292.
-

- 8. K. Maji and S. Dasgupta, Trans. Ind. Ceram. Soc. 73[2] (2014) 110-114.
-

- 9. R. Lakshmi, V. Velmurugan, and S. Sasikumar, Combust. Sci. Technol. 185[12] (2013) 1777-1785.
-

- 10. U. Anjaneyulu and S. Sasikumar, Bull. Mater. Sci. 37[2] (2014) 207-212.
-

- 11. R. Morsy, R. Abuelkhair, and T. Elnimr, Silicon 9[4] (2017) 489-493.
-

- 12. R. Morsy, R. Abuelkhair, and T. Elnimr, Silicon 9[4] (2017) 637-641.
-

- 13. H. Wang, Q. Zhang, H. Yang, and H. Sun, Ceram. Intl. 34[6] (2008) 1405-1408.
-

- 14. N. Tangboriboon, T. Khongnakhon, S. Kittikul, R. Kunanuruksapong, and A. Sirivat, J. Sol-Gel Sci. Technol. 58[1] (2011) 33-41.
-

- 15. H. Ismail, R. Shamsudin, M.A.A. Hamid, and R. Awang, J. Aust. Ceram. Soc. 52[2] (2016) 163-174.
- 16. R. Puntharod, C. Sankram, N. Chantaramee, P. Pookmanee, and K.J. Haller, J. Ceram. Proc. Res. 14[2] (2013) 198-201.
- 17. K. Yanagisawa, X. Hu, A. Onda, and K. Kajiyoshi, Cement and Concrete Research, 36[5] (2006) 810-816.
-

- 18. A.Yazdani, H.R. Rezaie, H. Ghassai, and M. Mahmoudian, J. Ceram. Proces. Res. 14[1] (2013) 12-16.
- 19. R. Phuttawong, N. Chantaramee, P. Pookmanee, and R. Puntharod, Adv. Mater. Res. 1103 (2015) 1-7.
-

- 20. S. Chehhlatt, A. Harabi, H. Oudadesse, and E. Harabi, Acta Physica Polonica A 127[4] (2015) 925-927.
-

- 21. S. S. Hossain and P. K. Roy, J. Asian Ceram. Soc. 6[3] (2018) 289-298.
-

- 22. S. Vichaphund, M. Kitiwan, D. Atong, and P. Thavorniti, J. Euro. Ceram. Soc. 31[14] (2011) 2435-2440.
-

- 23. K. Xiong, H. Shi, J. Q. Liu, Z. Shen, H. Li, and J. Ye, J. Am. Ceram. Soc. 96[3] (2013) 691-696.
-

- 24. S. Palakurthy, K.V. Reddy, R.K. Samudrala, and P.A. Azeem, Mat. Sci. and Eng. C 98 (2019) 109-117.
-

- 25. S. Palakurthy, P.A. Azeem, and K.V. Reddy, Ceramics International Part B 45[18] (2019) 25044-25051.
-

- 26. F.A.A. Azam, R. Shamsudin, M.H. Ng, A. Ahmad, M.A.M. Akbar, and Z. Rashidbenam, Ceramics International 44[10] (2018) 11381-11389.
-

- 27. A.P. Solonenko, A.I. Blesman, and D.A. Polonyankin, Ceramics International 44[18] (2018) 17824-17834.
-

- 28. I.V. de S.R. Nascimento, W.T. Barbosa, R.G. Carrodeguas, M.V.L. Fook, and M.A. Rodŕıguez, International Journal of Chemical Engineering, Article ID 6213568 (2018) 1-8.
-

- 29. L.A. Adams, E.R. Essien, and E.E. Kaufmann, J. Asian Ceram. Soc. 6[2] (2018) 132-138.
-

- 30. Heriyanto, F. Pahlevani, and V. Sahajwalla, J. Cleaner Production. 172 (2018) 3019-3027.
-

- 31. R. Shamsudin, F.A.A. Azam, M.A.A. Hamid, and H. Ismail, Materials 10[10] (2017) 1188.
-

- 32. K.A. Almasri, Hj. Ab A. Sidek, K.A. Matori, and M.H.M. Zaid, Results in Physics 7 (2017) 2242-2247.
-

- 33. H. Ismail, R. Shamsudin, and M.A.A. Hamid, Mat. Sci. and Eng. C 58 (2016) 1077-1081.
-

- 34. E.K. Papynov, O.O. Shichalin, E.B. Modin, V. Yu. Mayorov, A.S. Portnyagin, S.P. Kobylyakov, A.V. Golub, M.A. Medkov, I.G. Tananaev, and V.A. Avramenko, RSC Adv. 6[40] (2016) 34066-34073.
-

- 35. R. Shamsudin, M.A.A. Hamid, and A. Jalar, J. Asian Ceram. Soc. 2[1] (2014) 77-81.
-

- 36. A. Yazdani, H.R. Rezaie, and H. Ghassai, J. Ceram. Proc. Res. 11[3] (2010) 348-353.
- 37. D. Battegazzore, S. Bocchini, J. Alongia, and A. Frachea, RSC Adv. 4[97] (2014) 54703-54712.
-

- 38. S. Ahmed, F. Nigar, A.I. Mustafa, and M. Ahsan, Trans. Ind. Ceram. Soc. 76[4] (2017) 215-221.
-

- 39. S.J. Nipa and M.A. Hossain, Intl. J. Sci. & Engg. Res. 6[10] (2015) 387-391.
- 40. M.A. Hamid, M.A. Rahman, S. Ahmed, and K.M. Hossain, Asian J. Poult. Sci. 11[1] (2017) 1-13.
- 41. Y. Shinohara and N. Kohyama, Industrial Health 42[2] (2004) 277-285.
-

- 42. J.F. Saceda, R.L. de Leon, K. Rintramee, S. Prayoonpokarach, and J. Wittayakun, Quim. Nova 34[8] (2011) 1394-1397.
-

- 43. E.M. Rivera, M. Araiza, W. Brostow, V.M. Castaño, J.R. Díaz-Estrada, R. Hernández, and J.R. Rudriguez, Mat. Lett. 41[3] (1999) 128-134.
-

- 44. P.M.K. Reddy, S. Mahammadunnisa, B. Ramaraju, B. Sreedhar, and C. Subrahmanyam, Environ. Sci. and Poll. Res. 20[6] (2013) 4111-4124.
-

- 45. M.N. Freire and J.N.F. Holanda, Ceramica 52[324] (2006) 240-244.
-

- 46. D.S. Klimesch and A. Ray, Thermochimica Acta 306[1-2] (1997) 159-165.
-

- 47. R.P. S. Chakradhar, B.M. Nagabhushana, G.T. Chandrappa, K.P. Ramesha, and J.L. Rao, Materials Chemistry and Physics 95[1] (2006) 169-175.
-

- 48. S.K.S. Hossain and P.K. Roy, Boletín de la Sociedad Española de Cerámica y Vidrio 58[3] (2019) 115-125.
-

- 49. A. Harabi and S. Chehlatt, J. Therm. Anal. Calorim. 111[1] (2013) 203-211.
-

- 50. X. Liu, C. Ding, and P.K. Chu, Biomaterials 25[10] (2004) 1755-1761.
-

 This Article
This Article
-
2020; 21(3): 285-295
Published on Jun 30, 2020
- 10.36410/jcpr.2020.21.3.285
- Received on Jun 25, 2019
- Revised on Sep 16, 2019
- Accepted on Sep 26, 2019
 Services
Services
- Abstract
introduction
experimental details
results and discussion
conclusion
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Samina Ahmed
-
Institute of Glass and Ceramic Research and Testing (IGCRT), Bangladesh Council of Scientific and Industrial Research (BCSIR), Dhaka-1205, Bangladesh
Tel : +8801817549816
Fax: +88-02-58613022 - E-mail: shanta_samina@yahoo.com






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.