- Nano bioactive HAP-nano bioresorbable β-TCP-PEG composite scaffolds and their biochemical activity for implant applications
K. Kalaa, M. Sundara Ganeasanb, V. Balasubramanib, J. Manovasukib, K. Valivittana, T.M. Sridharb and M.R. Kuppusamyc,*
aDepartment of Biotechnology, St. Peter’s University, Chennai 600054, India
bDepartment of Analytical Chemistry, University of Madras, Guindy Campus, Chennai-600025, India
cDepartment of Chemistry, RV Government Arts College, Chennai 603001, India
Recent efforts towards the
treatment of bone defects and diseases focus on the development of bone
scaffolds. Bioceramics provide strength, osteoconductivity and also imparts
flexibility and resorbability. In this study, the biodegradable composites were
fabricated using bioactive nano Hydroxyapatite (n-HAP) and bioresorbable nano β-Tricalcium
phosphate (n-β-TCP) taken in 1:1 proportion. The nano composite scaffolds were
synthesized using PEG (Poly Ethylene Glycol) by wet precipitation method. XRD
(X-ray diffraction) confirms the presence of crystalline structure of n-HAP and
n-β-TCP within the lattice. FESEM (Field Emission Scanning Electron Microscopy)
and EDS (Energy Dispersion X-ray Spectroscopy) confirms the micro porous nature
and the phase purity of the composite. Further, biochemical studies were
carried out using MG-63 Osteoblast cell line to evaluate their sustainability
after implantation. The viability of the cells and proliferation rate is
evaluated using 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide
(MTT) with different concentration and different incubation period. The ALP
(Alkaline Phosphotase) test reveals that the composite favours bone
regeneration through apatite layer formation. Further studies were carried out
to explore the DPPH (1,1-diphenyl-2-picrylhydrazyl) activity on the composites
and results reveals that the composite have an ability to trap the free
radicals in the biological surroundings. The antimicrobial studies indicate
that the composites shows no major inhibitory effects towards the most common
bone affecting bacteria Staphylococcus aureus. The studies indicate that
the concentration range of the composite is ideal for bone growth and can be
used as substituents in the scaffold synthesis for normal and cancerous
patients.
Keywords: n-HAP, n-β-TCP, Poly ethylene glycol, composite, MTT assay, DPPH assay, ALP activity
Treatment of bone diseases and multiple fractures is a
great challenge and tissue engineering offers a solution to patch-up and heal
the damaged hard tissue by using biomaterials. Among several biomaterials that
are available biodegradable polymers have attracted researchers to study its
potential as an alternative to metallic implants [1]. These
biodegradable polymers are commonly used
as the biological substitutes for treatment of damaged
bone, drug delivery, repair of tissues and organs of the human body [2].
Biocompatibility is the primary property that any biomaterial should possess
during the selection of substituted biomaterials in order to provide a platform
for cell growth and proliferation. These biomaterials should actively interact
with the cell and activate the osseointegration process leading to new bone
deposition [3]. In recent studies, considerable attention has been given to HAP
based composite biomaterials. Since, it has specific properties such as biocompatibility,
biodegradability, an intrinsic antibacterial nature and
the ability to mold into various forms or geometries which is suitable for cell
growth and osteoconduction.
Bioactive ceramics (n-HAP) are widely used in scaffold
preparation. n-HAP on implantation in the human body has the ability to react
with physiological fluids. This bioceramic on further contact with the fluids
and tissues for a few days to weeks leads to the formation of new bone with
n-HAP layers [4, 5]
.
The composite materials on implantation provokes immune
response in the body which elicits a cascade mechanism in the biological
environment. This makes it necessary to evaluate the biological properties of
the composites along with their characterization to prove their
biological efficacy. This would help us to determine the
longevity of the implant [6]. The improper choice of the substituent materials
during scaffold preperation limits its biological application [7]. Even the
minute level of injury during restoration period affects the performance of the
scaffold. This may be due to the leakage of the toxic substances from the
scaffold into the biological fluids. This may induce inflammation at the
implant site [8]. To avoid such a situation, it is highly essential to carry
out the in vitro biochemical evaluation before the clinical studies. Hence, in
vitro biochemical screening is the best choice to evaluate the biocompatibility
and toxicity [9]
.
In
bone tissue engineering, the biological behaviour of the composites namely its
osteoconductivity, osteoinductivity and degradability would help us to
analyse the ability of the implant to induce bone growth in the biological surroundings. This could be accomplished in the
lab by carrying out in vitro testing with an appropriate cell line to study the
mechanism of interaction. In humans,
when the composites are implanted the
ions present in the body fluids surround the surface of the materials forming the ionic layers. This is
followed by the interaction with
protein that would support us to understand the osteoinduction process
[10]. In case of osteoconduction, the bone tissue grows around the internal pores of the scaffolds which would aid
the conduction process. The evaluation of osteoconduction property and
osteoinduction property helps us to assess the behavior of the scaffold after
implantation in the biological system.
This osteoconductivity can be measured
by taking into account the bone coverage area using optical or FESEM
analysis [11]. Biodegradability is the another important property by which the
materials get degraded once inserted inside
the body. The biodegradability of the
biomaterials would determine the osteoconductive nature of the surrounding
cells that are in contact with the physiological environment [12].
In the view of
osteoconduction and osteointegration requirement nano Hydroxyapatite which is a
chief mineral component of bone matrix was selected. It is the most appealing
inorganic constituents that is widely used for the bone regenerative medicine.
n-β-TCP is a bioactive material that posses good biocompatibility and
biodegradability. The degradable property of n-β-TCP ensures the release of
elements such as calcium and phosphorus at the site of implantation. n-β-TCP
has limited direct clinical application due to its brittle nature
and difficulty of obtaining various implant shapes. This
problem can be overcome by using it as a one of the materials
in composites [13]. Bioceramic composites are prepared
in nano dimensions by mixing one or more phases of ceramics with mainly
polymers or other biocompatible materials. The typical microstructures of nanoceramic
composites result in exceptional properties (biological,
mechanical, electrical and electronic etc.). These composites can be used as
bulk materials for structural applications or can be developed as coatings on
different substrates for various applications [14]. The composites can be
prepared with Polyethylene Glycol (PEG) which acts as an important
additive component and provides hydrophilicity to the composite [15]
.
Nano form of biomaterials is preferable due to its small
grain size and large surface area that promotes the interfacial interaction
with bone. Moreover, nano ceramics are widely used in scaffold preparation as
they pocess controlled rate of degradation which could exactly match with new
bone growth rate. This can be acheived by controlling the crystalline grain
size of the nano ceramics and thus improve the bone graft healing without any
complication [34]. Further, researchers have reported that nano HAP can
suppress the cancer cells without affecting the normal cells [30]
.
Nano Hydroxyapatite reinforced polyphenylene sulfide biocomposites
were reported to have good cell proliferation with MG-63 cells. It shows the
osteogenic differentiation with higher ALP activity [16]. In
designing the synthetic scaffolds for bone tissue engineering the
materials can be prepared in various physical forms such as
powders, pastes or granules and porous scaffolds [3].
Scaffolds should be resorbable with time in the human body
with gradual replacement by newly formed bone tissue
and should ensure a high protein-adsorption capacity. n-HAP is stable against
dissolution in body fluids, whereas n-β-TCP has a much higher resorption rate
compared to that of n-HAP. One can easily control the resorption rate by
varying the ratio of n-HAP/n-β-TCP composites [17, 18]. Selection of
polymer is critical for preparing the composite and it should be
evaluated for its toxic nature. The systemic screening of scaffolds by invitro
method is reported in literature [31]. Invitro cytotoxicity and biochemical tests
are most ideal, reliable, simple and help us
to evaluate the toxic nature of the scaffolds. These tests aids in screening
the bone deposition properties such as osteoconduction, cell viability, cell proliferation and bone
regeneration. These tests are carried
out using the osteoblast cell line.
Cell viability and cell proliferation can be evaluated
using MTT assay. In this study the viable nature of the cell is confirmed by
the active presence of mitocondrial dehydrogenase enzyme. This enzyme inturn
reduces MTT and promotes cell proliferation. The reduction of MTT is confirmed
by the colour change. The cell proliferation is further confirmed
using the growth curve [19]. Implant-material related
infection is common and this could arise during surgery. This infection may affect
not only the surgical sites but also other organs of the body through the
migration of pathological microorganisms during blood circulation [20]. The presence of bacterial infection at the implanted
site often shows a delayed wound
healing and may sometimes result in
revision surgery.
The main aim of this study is to prepare n-HAP/ n-β-TCP/PEG
nano composite using different ratios of the bioceramics and polymer. This
would be followed by the evaluation of the composite for biochemical and
biological behaviour to identify the suitable ratio for the scaffold
preparation. Since both n-HAP & n-β-TCP are the constituents of bone. In
order to impart the hydrophilicity and antifouling property the PEG added the
composite. These composites were evaluated using invitro studies which includes
cell viability, cell proliferation, antimicrobial
activity, alkaline phosphatase activity and antioxidant activity.
The scaffolds prepared using 1:1:5 ratio of n-HAP/n-β-TCP/PEG was found to show
good cell attachment with least toxicity.
Materials
Calcium Nitrate Tetrahydrate [Ca(NO3)2·4H2O],
Diammonium hydrogen Phosphate [(NH4)2HPO4],
Poly Ethylene Glycol 6000 [PEG], Conc. Ammonia, Ethanol were purchased from SRL
and Finar. All the chemicals were of analytical grade and used further without
purification.
Synthesis
of n-HAP/ n-β-TCP/ PEG composites
The n-HAP and n-β-TCP was synthesized based on the earlier
established procedure in the lab [21]. The powders were dried and sintered at
800 °C for 2 h in air atmosphere using a muffle furnace. PEG solution was
prepared separately with deionized water by using a magnetic stirrer for 2~3 h
to obtain a homogenious solution. Synthesized n-HAP/n-β-TCP was added into the
solution to make a n-HAP/n-β-TCP/PEG by adding different concentrations of the
powders (0.5:1:5,1:1:5 and 2:1:5) mixture. The polymer
n-HAP/n-β-TCP mixture was stirred for 10 hours with
stirrer until n-HAP/n-β-TCP was completely dispersed in the solution and then
this mixture was dried at 100 °C in an hot air oven.
Material Characterization studies of n-HAP/n-β-TCP/PEG composites
The synthesized n-HAP/n-β-TCP/PEG composite materials were
characterized using different analytical techniques. The crystal structural
properties of n-HAP/n-β-TCP/PEG composites were characterized using X-ray
diffractometer (XRD) (model, Rigaku Ultima IV) with Cu Kα (λ = 0.154 nm)
radiation and 2θ scanning range of 10-80°. The morphology of n-HAP/n-β-TCP/PEG
were analyzed using Field Emission Scanning Electron
Microscope (FESEM) (model, Carl Zesis Supra 55).
Biochemical study of n-HAP/n-β-TCP/PEG
composites
Cytotoxicity assay
The assay was performed based on the Mosmann, 1983
procedure [22]. For this assay MG-63 cell line was obtained
from NCCS, Pune. Cells were plated in 24-well plates (Nucleon,
Roskilde, Denmark) and incubated at 37 °C with 5% CO2
under humid conditions. After the cell reached the
confluence, the prepared samples at different concentration was added and
incubated for 24 h, 48 h and 72 h. After incubation, the samples of each
concentration were removed from the wells and washed with phosphate-buffered saline
(pH 7.4) or DMEM (Dulbecco's modified eagle medium) without serum 100 µL/well
(5 mg/ml) of 0.5% 3-(4, 5-dimethyl-2-thiazolyl)-2,5-diphenyl-tetrazolium
bromide (MTT) was added and incubated for 4 h. After incubation,
1ml of DMSO (Dimethyl sulfoxide) was added in all the wells. The absorbance at
570 nm was measured with UV- Spectrophotometer using DMSO as the
blank. Measurements were performed and the concentration required for a 50%
inhibition (IC50) was determined graphically. The percentage of cell
viability was calculated using the following formula: 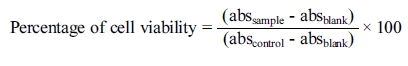
The time period is extended to 24 h, 48 h and 72 h in
order to determine the cell proliferation rate. Graphs are plotted using the %
of Cell Viability at Y-axis and concentration of the sample in X-axis. Cell
control and sample control is included in each assay to compare the cell
viability assessments.
Antibacterial activity
Antibacterial activity is done using Kirby-Bauer disk
diffusion susceptibility test (2009). [25] Staphylococcus aureus, a
common bone affecting gram positive bacteria is used. The
pathogenic organism is made to grow on Mueller-Hinton agar (MHA) in the
presence of anti-microbial impregnated filter paper disks. The
growth of the organism around the disk is an indirect measure of the compound ability against the pathogen.
Antibacterial activity of the composites was determined by disc
diffusion method on MHA medium. The
medium is then poured into the petri dishes. The inoculum was spread over the
solidified solid plate with the help of sterile swab moistened with the
bacterial suspension. Ampicillin (20 μL/disc) is taken as positive control. The
disc was placed in MHA plates consisting of 20 μl of sample (Concentration:
1,000 μg, 750 μg and 500 μg). The plates were incubated at 37 °C for 24 h.
The diameter of zone of inhibition gives the antibacterial activity.
DPPH (2,2 diphenyl 1 picrylhydrazil) Assay
The DPPH assay was performed based on Molyneux, 2004
method [24]. The main characteristics of an antioxidant are its ability to trap
free radicals. Highly reactive free radicals like ROS (Reactive Oxygen Species)
and RNS (Reactive Nitrogen Species) found in biological systems form a wide
variety of sources. A rapid and simple inexpensive method to measure the
antioxidant supply of a sample involves the use of free radical 1,1 diphenyl 2
picryl hydrazyl (DPPH). Three test tubes were labeled as blank, standard and
test. First 3.7 mL of absolute methanol (Sigma Aldrich) is added to all the 3
test tubes. This was followed by addition of 100 µL of standard Butylated
hydroxy toluene (Sigma Aldrich) to the standard test tube. 100 µL of composite
mixture is added to the test tube labeled as test. 200 µL of fresh DPPH
solution is added to all the test tubes including blank. The test tubes are
allowed to incubate at room temperature under dark conditions and the readings
were taken at 1, 5 and 30 min respectively. The results are expressed as
radical scavenging activity.
The molecule of DPPH were characterized as a stable free
radical by delocalization of electrons. This forms a deep violet colour,
characterized by the absorption band in methanol solution centered at 520 nm.
The antioxidant property is confirmed by the change from violet
to yellow colour on addition of DPPH. This change of colour is occurs due to
the reduction of DPPH by picryl group.
• The percentage of antioxidant
activity can be measured by,

Alkaline Phosphatase (ALP) assay
This assay was performed based on George N.Bowers,
Jr., and Robert B. McComb, 1966 procedure [23]. ALP at an
alkaline pH hydrolyses p-Nitrophenyl phosphatase to form
p-Nitrophenol and Phosphatase. The rate of formation of p-Nitrophenol is
measured as an increase in absorbance which is proportional to the ALP activity
in the sample.
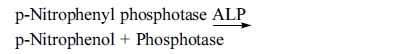
The
buffer selected for this work is 2-amino-2-methyl-l-propanol (2A2M1P) (Sigma
Aldrich). The substrate, p-nitro phenyl
phosphate as the disodium salt, tetra hydrate (Sigma Aldrich). To evaluate the
ALP activity, the bio-composites were incubated in 0.5 mg/ml p-nitro phenol
phosphate in glycine buffer solution every five minutes a 90 µL aliquot of the
solution was removed and placed in 96 well plates containing 10 µL of a stop
solution. 0.1 M NaOH (Sodium hydroxide) (Aldrich chemical) and 0.1 M EDTA
(Ethylene diamine tetra acetic acid) (Aldrich chemical). The absorbance was
measured at 405 nm. The enzyme activity was calculated using formula.
Activity = A405 (Vreaction / Valiquot) / β
A405: Absorbance at 405, Vreaction:
Volume of reaction, Valiquot: Volume of aliquot, β: Extinction
coefficient for para nitro phenol, λ -18.5 at 405 nm.
ALP Activity in micromol/min (U/L)
= ΔA/min./18.75
× Test volume/Serum Volume × 1/Temperature factor
Where, 18.75 is mill molar absorptivity, Temperature at
30°C is consider as temperature factor is 1 ALP in U/L can be calculated as,
ALP Activity in U/L = ΔA/min. × 2754
The overall summary of n-HAP/n-β-TCP/PEG com- posite is presented in Fig. 1 along
with the biochemical studies involving – (i) cell
proliferation (ii) antimicrobial activity (iii) Free radical trapping
(antioxidant) and (iv) cell regeneration (ALP activity) is given in Fig. 1.
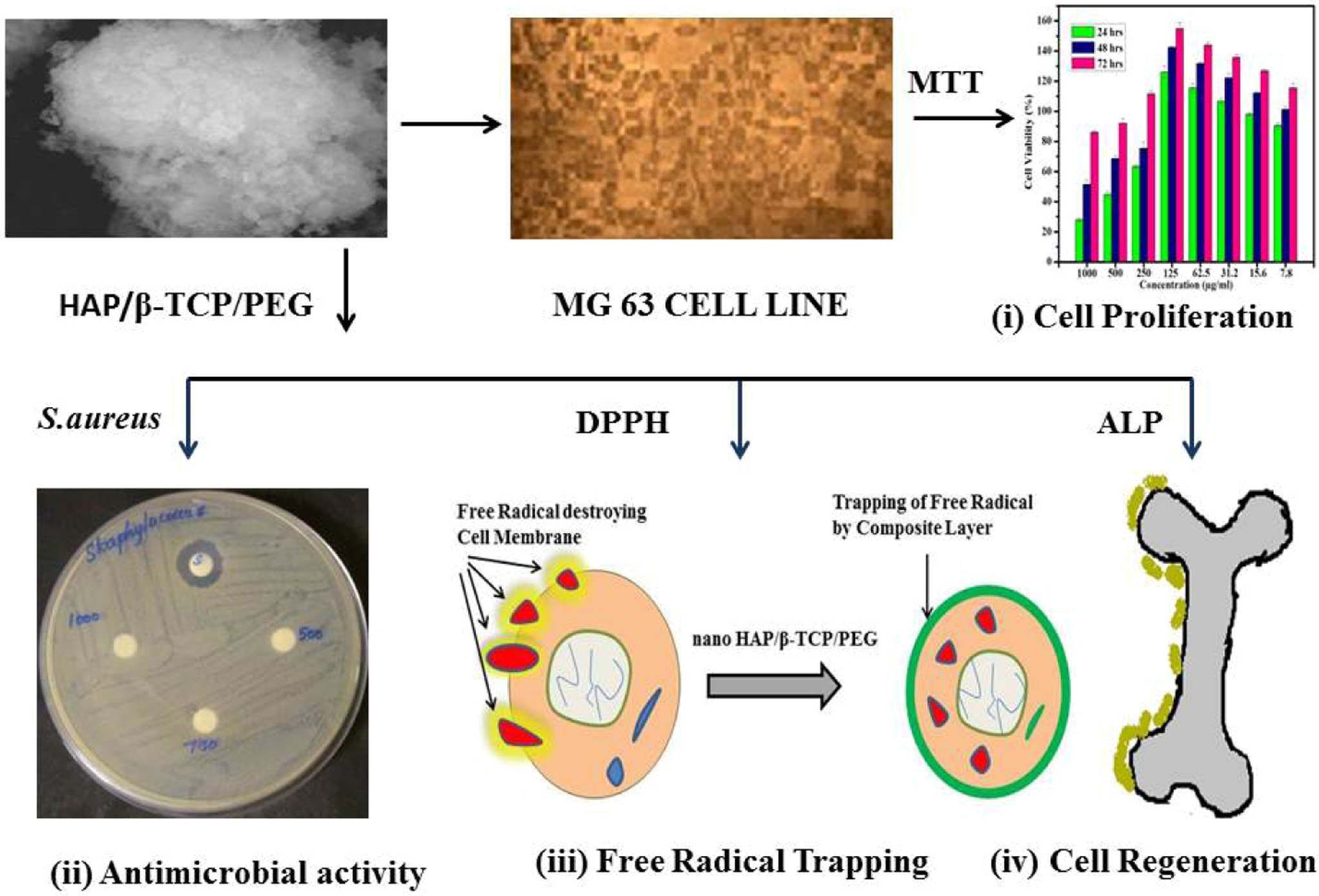
|
Fig. 1 Schematic representation of n-HAP/n-β-TCP/PEG composite osteoblast cells and bacterial cell culture. |
Bioceramics such as n-HAP, n-β-TCP are commonly used in
new bone generation. But, in order to obtain the specific and desired
properties such us porosity, bioactivity, bio resorbability and
biocompatibility, researchers have prepared composites in which
different biomaterials can be combined based on the specific
property depending on the nature of their application [26]. In this concept,
the current work focus on the choice of the material composition which includes
Nano- Hydroxyapatite, Nano-β-Tricalcium phosphate, and
Polyethylene Glycol which posses the basic properties such as
bioactivity, bioresorbability and hydrophilicity. The present work focuses on
the bone minerlization in scaffold materials, ALP activity which induces the
bone mineralization [27] was studied with the prepared scaffolds. The normal
value of ALP is 20-140 units per litre. If there is an increase or decrease in
the normal values it is termed as abnormality which leads to discomfort to the
patient [28]. Since, ALP is needed for the apatite formation the level should
be monitored and the values should not cross the maximum level. The report
suggests that the ALP can induce hydroxyapatite mineralization [29]. The
changes in ALP values of the composite would affect the formation of apatite
layers of new bone. Hence, n-HAP can be used in different proportion to
maintain the normal ALP activity. The study was further extended to select the
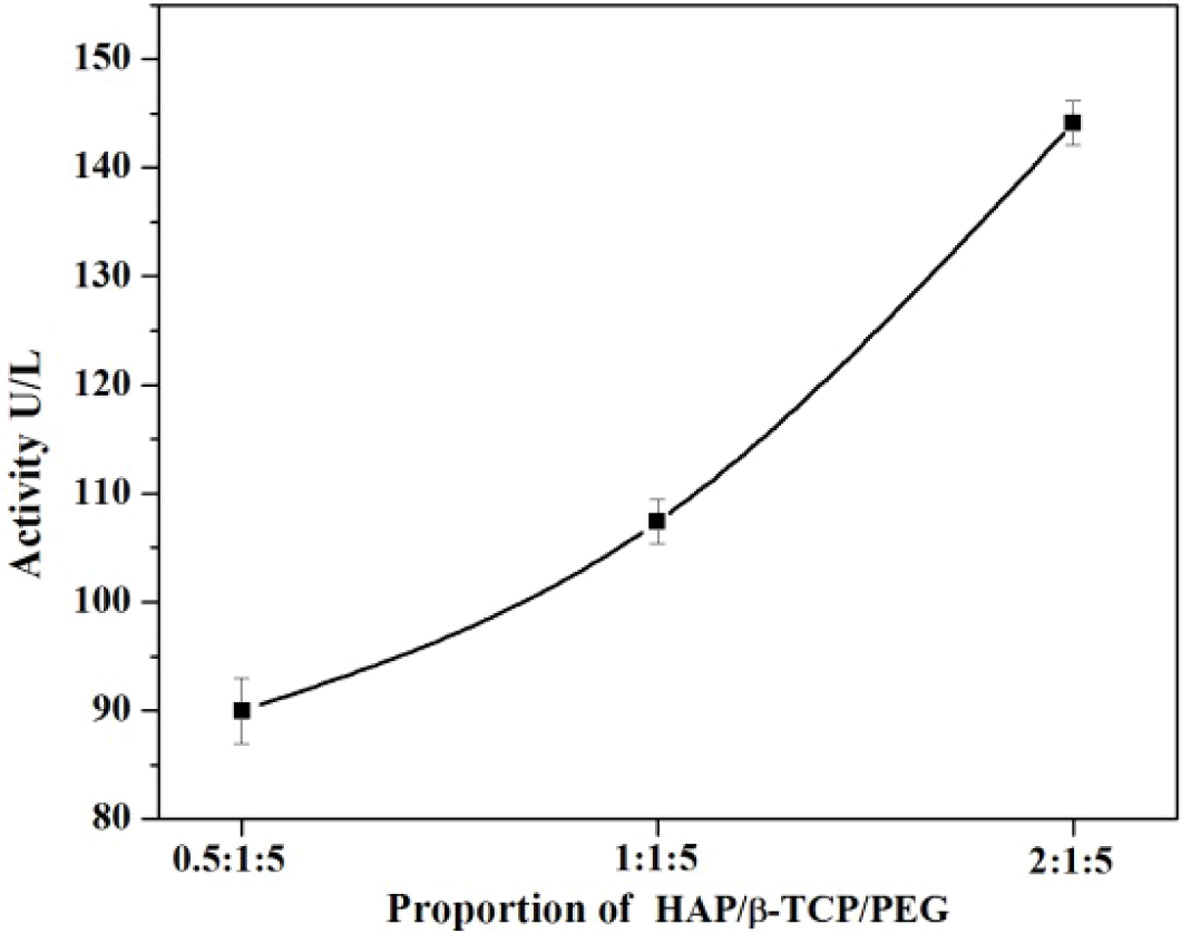
proportion of the components present in the composite. To fix the
concentration, three different proportions were taken in the composite mixture
of n-HAP/n-β-TCP/PEG . The proportion includes 1:1:5, 0.5:1:5, 2:1:5 which has
different concentration of n-HAP in n-HAP/n-β-TCP/PEG. The above proportions
were tried for different concentration to identify the concentration which can
be suitable for further studies. The changes in optical activity with changes
in the ratios of n-HAP/n-β-TCP/PEG is given in Fig. 2.
The ALP activity for the composite with n-HAP/n-β-TCP/PEG
in 0.5:1:5 ratio shows the ALP activity to be about
89.964. Different ratios of samples were prepared and tested
to obtain the highest ALP activity. As the ratio of n-HAP was increased from
0.5 to 2 the 2:1:5 composite shows an ALP activity of about 144.126 which is
normally higher than that of the optimum level that can be found in the blood.
For a n-HAP ratio of 1:1:5 the ALP was found to be 110.321. Hence, the current
study indicates that the n-HAP/n-β-TCP/PEG scaffold with 1:1:5 ratio has the
optimum and desired ALP activity and can be considered for further
intensive investigations.
Characterization study
of n-HAP/n-β-TCP/PEG composites
XRD analysis
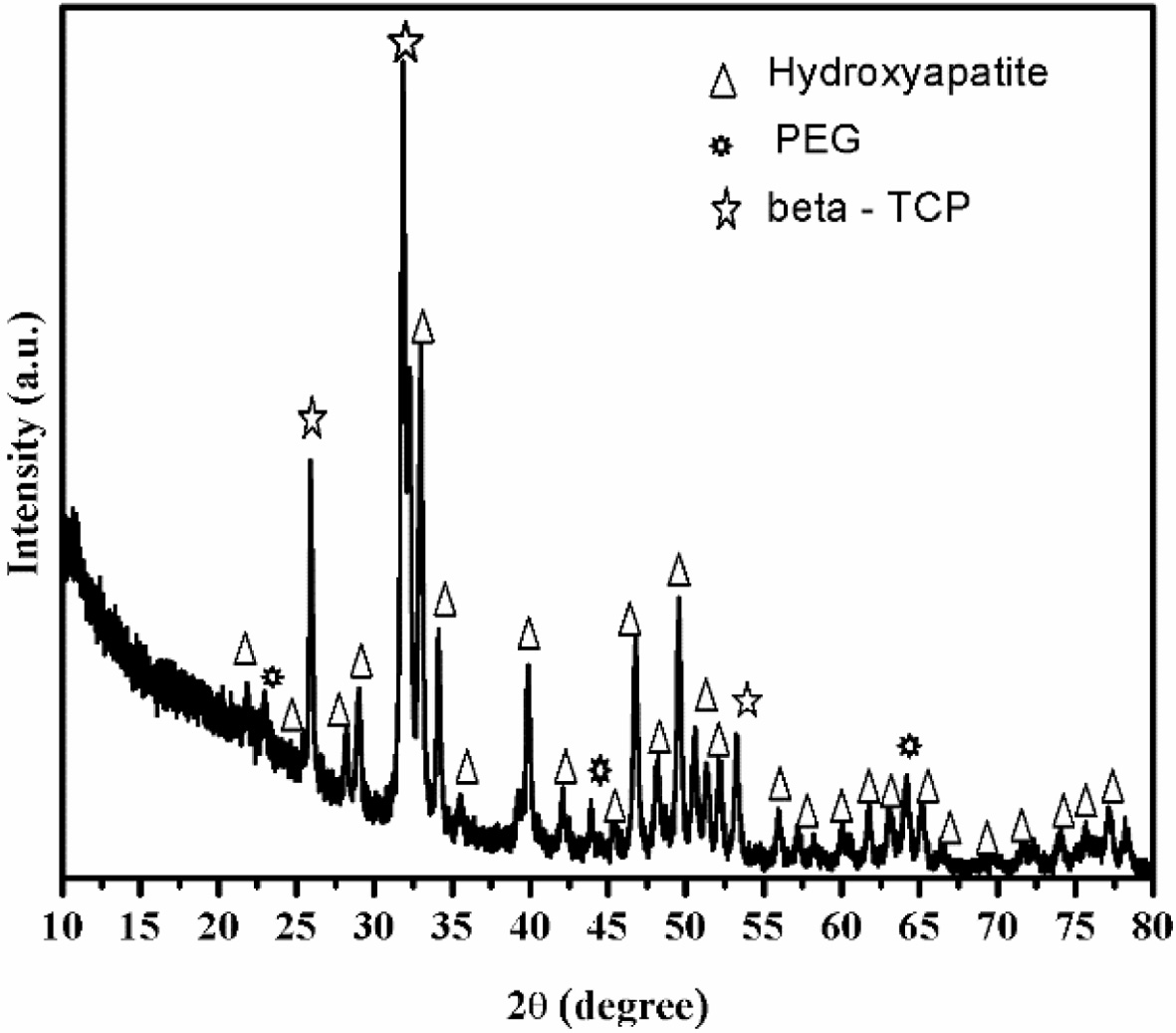
Fig. 3 shows the XRD pattern of n-HAP/n-β-TCP/PEG
composites which shows that HAP and β-TCP exists as nano form
and present together in the composite. The peaks corresponding to nano HAP
powder were found at the reflections of (002), (102), (210), (211), (112),
(300), (202), (310), (311), (222), (213), (321), (004) and (510). The
crystalline nature of nano HAP can be established by the presence of
diffraction peaks with high intensities and minimal line broadening after
indexing the two theta values with JCPDS file no.9-0432. The observed patterns
clearly indicate that no structural transformation of mono phasic nano HAP
occurs. This indicate that the bioactivity would be preserved. The intensity
peaks are assigned to n-β-TCP were obtained at (024), (1010), (214), (300),
(0120), (220), (1016), (3012) and (4010). These, hkl indices were
compared with JCPDS file no.09-0169. The intensity
peaks for PEG were obtained at the XRD patterns indicates that the
addition of polymer to the sample do not affect the crystal structure of n-HAP
and β -TCP nanoparticles. PEG is observed to show XRD peaks at 23.328 and a few
minor peaks are observed, which is
consistent with data in previous report [33].
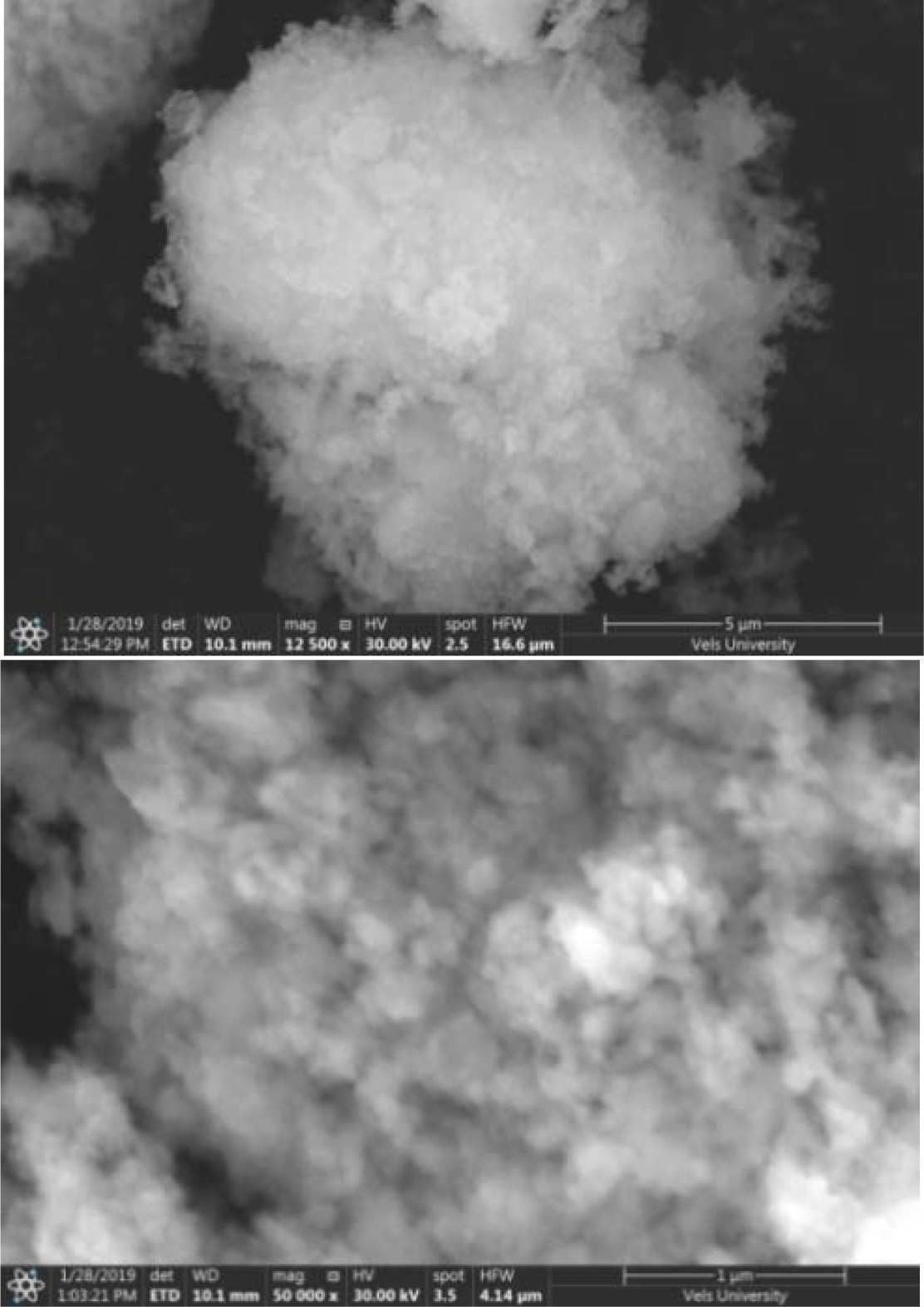
Field Emission Scanning Electron Microscope study (FESEM)
The
FESEM micrographic images of pure n-HAP/ n-β-TCP/PEG compositions are shown in Fig. 4. The FESEM image shows that particles are well agglomerated in the polymer matrix. The images show spherical particles of the ceramic to be embedded in PEG
indicating the composite nature of
the ceramics. The porosity of the samples were found to vary from 1.3 to 2%.
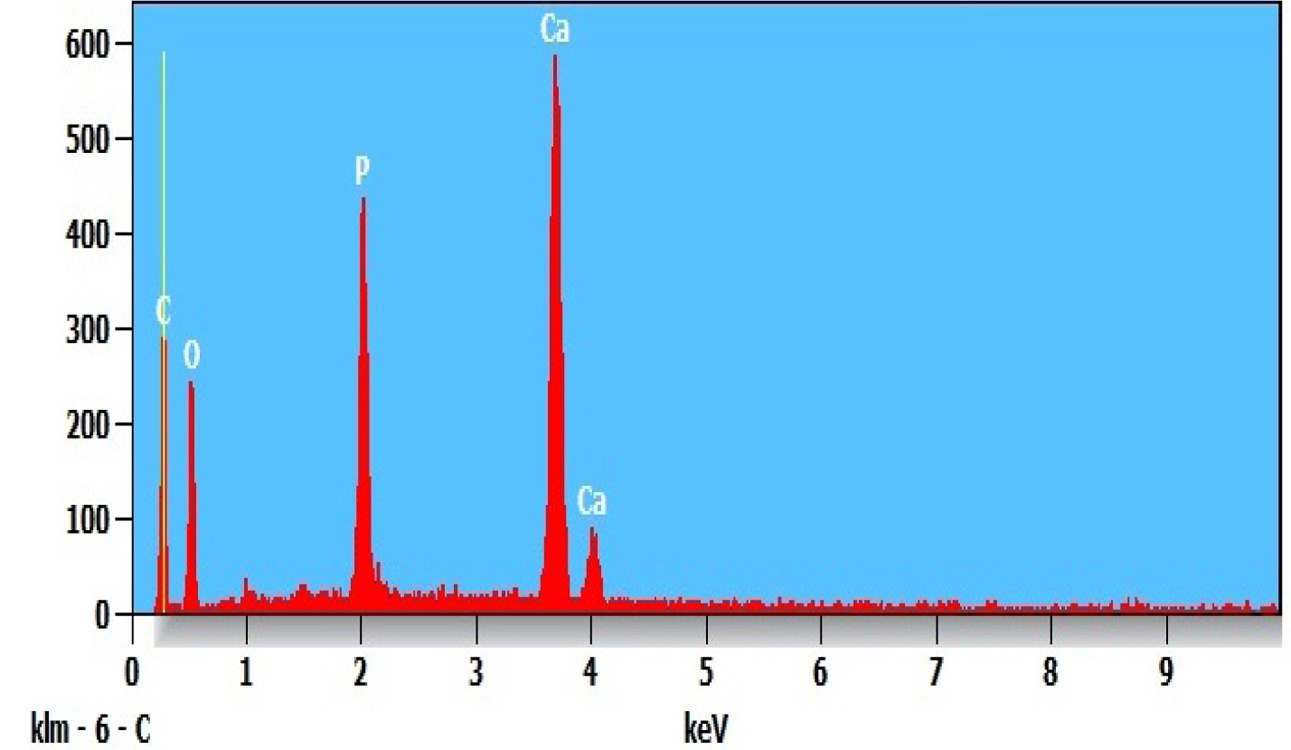
The EDS of n-HAP/n-β-TCP/PEG compositions shown in Fig. 5. This indicates the
presence of Ca, O, P and C in the nanocomposite which further confirms the
presence of n-HAP/n-β-TCP.
Biochemical study of n-HAP/n-β-TCP/PEG
composites
Cytotoxicity assay
The composite mixture prepared with n-HAP/n-β-TCP/PEG
(1:1:5) were taken in different dilutions. The linearity of MTT assay in the
MG-63 cells were plated out in doubling dilution using 0.1mL growth medium in
96 well plate. The MTT is added to all the cells immediately and the plates
were allowed to incubate at 37 °C for 4 h. The graph was plotted between the
obtained concentration and the percentage of
cell viability. The duration of
exposure is usually determined as the
time required for maximal damage occurs but is also influenced by the stability
of the composites. MTT is the most commonly used yellow colour substrate and
this converted into a dark blue formazan product when incubated with the live
cells. The viability percentage of the cells were measured
spectrophotometrically by the colour obtained after the addition of MTT [32].
The exposure time is prolonged to identify
the rate of proliferation of the cell.
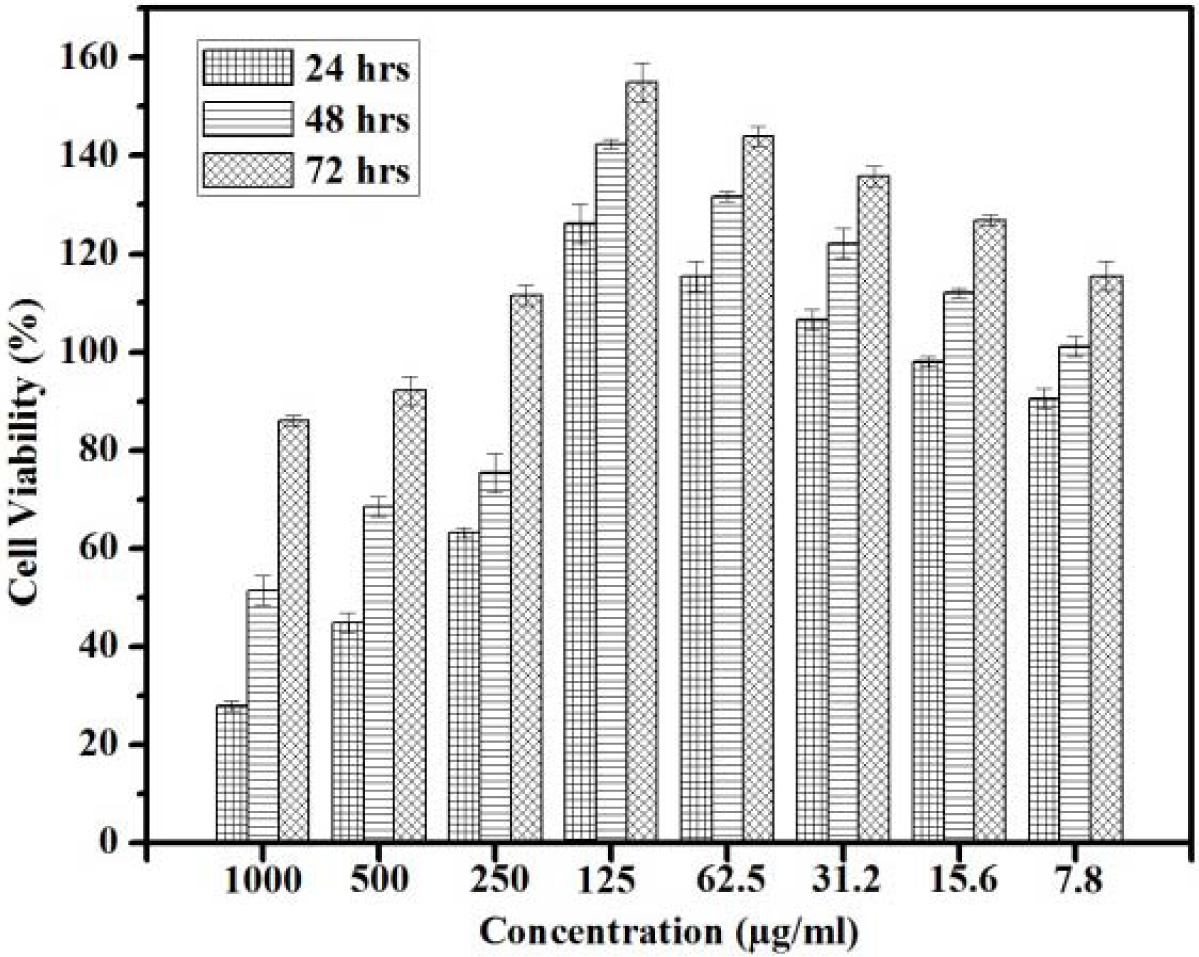
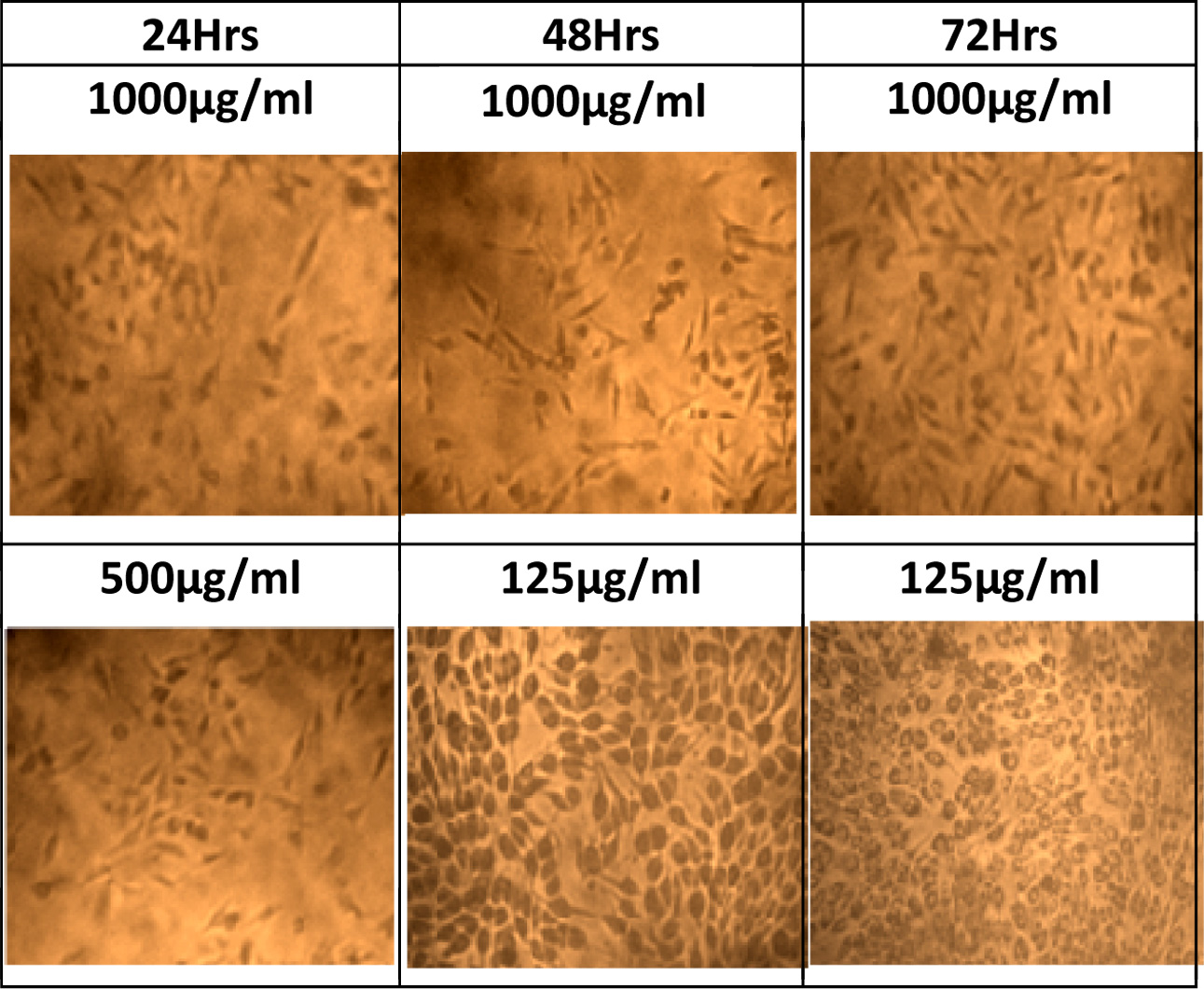
Fig. 6 indicates the percentage of cell viability IC50
that can be reached by the active cells after 24 h upto 250 µg/ml. This
indicates that the percentage of cell viability is found to be 50% and above.
The rate of cells were increased when the incuabtion time was extended to 72 h.
When the concentration increases the 50% of cell viability reached only in
extended period of incubation. When the concentration increases upto 1,000 µg/ml
the percentage of cell viability IC50 is found only after 48 h of
incubation. The proliferation of the cells are identified at different exposure
time. In all concentration when the time of exposure increases the rate of
proliferation increases The Fig. 6 shows that in each concentration 72 h peak
is higher than 48 h which inturn higher than 24 h. This suggests that when the
concentration increases to 500 μg/ml and 750 μg/ml, the increase in incubation
period is required to get better proliferation at 48 and 72 h respectively.
These results indicate that the level of cell viability
increases with time of exposure and it decreases with concentration. The cells
were allowed to proliferate for two to three population doubling times (PDTs)
in order to distinguish between cells that remain viable and are capable of
proliferation and those that remain viable cannot proliferate. The percentage
of surviving cells is then determined indirectly by MTT. The amount of MTT
formazan produced is directly proportional to the cell viability. The
proliferation of the cells were noticed from the concentration 125 μg/ml,
62.5 μg/ml, 31.5 μg/ml where the cell viability percentage is found to be
double and that confirms the population doubling through proliferation. Normally IC50 represents the con- centration of the composite materials that
is required for 50% of cell viability. This
50% viability is achieved at 1,000 μg/ml
and 500 μg/ml after 48 h of incubation.
The same 50% viability can be achieved in all other concentration (250, 500
and 750 μg/ml) after 24 h of incubation. Fig.
6 shows that IC50 can be seen upto 250 μg/ml concentrations with an
exposure time period ranging from 24 h, 48 h and 72 h. When the concentration is increased to 500 μg/ml and 1,000 μg/ml
the IC50 cannot be achieved in the initial time phase of 24 h and
this will get elicit only after the period of exposure 48 h and 72 h
respectively. This implies that at all the concentration the cells would
proliferate indicating that the sacffold is biocompatible. As the concentration range increases above 250 µg/ml it is
important to increase the incubation period for cell proliferation to take
place. The results indicate that the nano composite of n-HAP/n-β-TCP/PEG can be
used maximum of 250 μg/ml as the substituent for the scaffold preparation. The
concentration preferable for scaffold is found to be maximum at 250 μg/ml. As
the concentration crosses 250 μg/ml
the period of incubation should
increases.
Thus, it can be inferred that when the concentration exceeds,
the period of incubation also need to be increased for finding
both the cell viability and proliferation This assay suggest that the choice of
materials used in preperation of nano composite is good
to provoke the proliferation of cells. The scaffolds prepared indicate the
presence of osteoconduction property.
Antibacterial activity
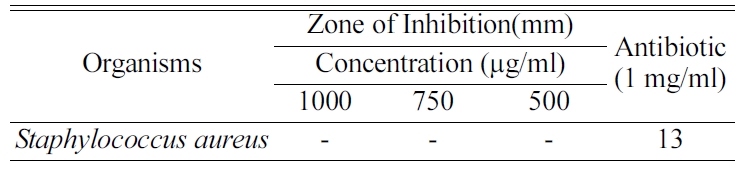
Table 1 shows that no zone of inhibition was found at
different concentrations of 1,000 µg/ml, 750 µg/ml and 500 µg/ml and the
inhibition zone found only in the antibiotic amphicilline disc. The
composite materials were tested with Staphylococcus aureus which
is the common pathogen that induces the bone infection. The composite materials
of different concentration such as 1,000 µg/ml, 750 µg/ml and 500 µg/ml were placed in
a disc and the disc placed over the microbial environment.
The zone of inhibition was calculated by measuring the diameter of the
diffusion around the disc. This confirms that the composite does not show any
inhibition towards the Staphylococcus aureus. The inhibition is not found with the increase in
concentration.
DPPH (2,2 diphenyl 1 picryl hydrazil) assay
Scientific evidence suggests that antioxidants reduce the
risk for chronic disease including cancer and heart diseases. The main
characteristics of an antioxidant are its ability to trap free radicals. The
antioxidant property of the composite n-HAP/n-β-TCP/PEG is measured with the
addition of 1,1 diphenyl 2 picrylhydrazyl (DPPH). The formation of reduced form
of DPPHH is due to the reaction of free radical with unpaired
electron of DPPH become reduced by capturing hydrogen ions which
was donated by the composite materials. The reduced state of DPPHH would form
yellow colour. The increase in the intensity of the colour confirms the higher
reducing activity and also more antioxidant property ie.
above 50% which is required for implantation inside the
biological medium.
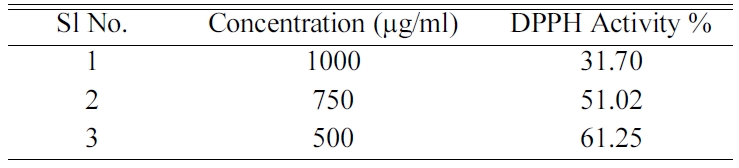
Table 2 explains the activity of the DPPH for the
different concentration of the composite n-HAP/n-β-TCP/PEG. A decrease in the
acitivity of the composite was found with the increase in concentration of the
composite. The activity above 50% can be viewed only in 750 μg/ml and also
below that concentration. This study concludes that the DPPH property can be
good for the composite when the concentration range is less than 750 μg/ml is
used for the scaffold preparation.
Alkaline phosphatase assay
The composites were further analysed for the bone
formation ability by using ALP activity. The analysis were taken for different
concentration of the n-HAP/n-β-TCP/PEG composites such as 1,000 µg/ml, 750
µg/ml, 500 µg/ml and 250 µg/ml respectively. The results were taken after 3
minutes incubation at the standard condition. The results indicate that ALP
activity of the composite increases when the concentration increases. This
is mainly due to the presence of increased levels of Alkaline phosphatase
enzyme in the hemolysis sample. The increase level leads to the breakdown of
the proteins in the body. This will convert more pyrophosphate to inorganic
phosphate which induces bone mineralization and this would in turn increase the
bone growth. Normally, healthy adult should have the ALP level 20-140 units per
litre (U/L) below and the above will leads to discomfort in healthy individual.
Since, ALP is need for the apatite formation the level should be monitored that
it should not cross the maximum level.
ALP Activity in U/L = ΔA/min. × 2754
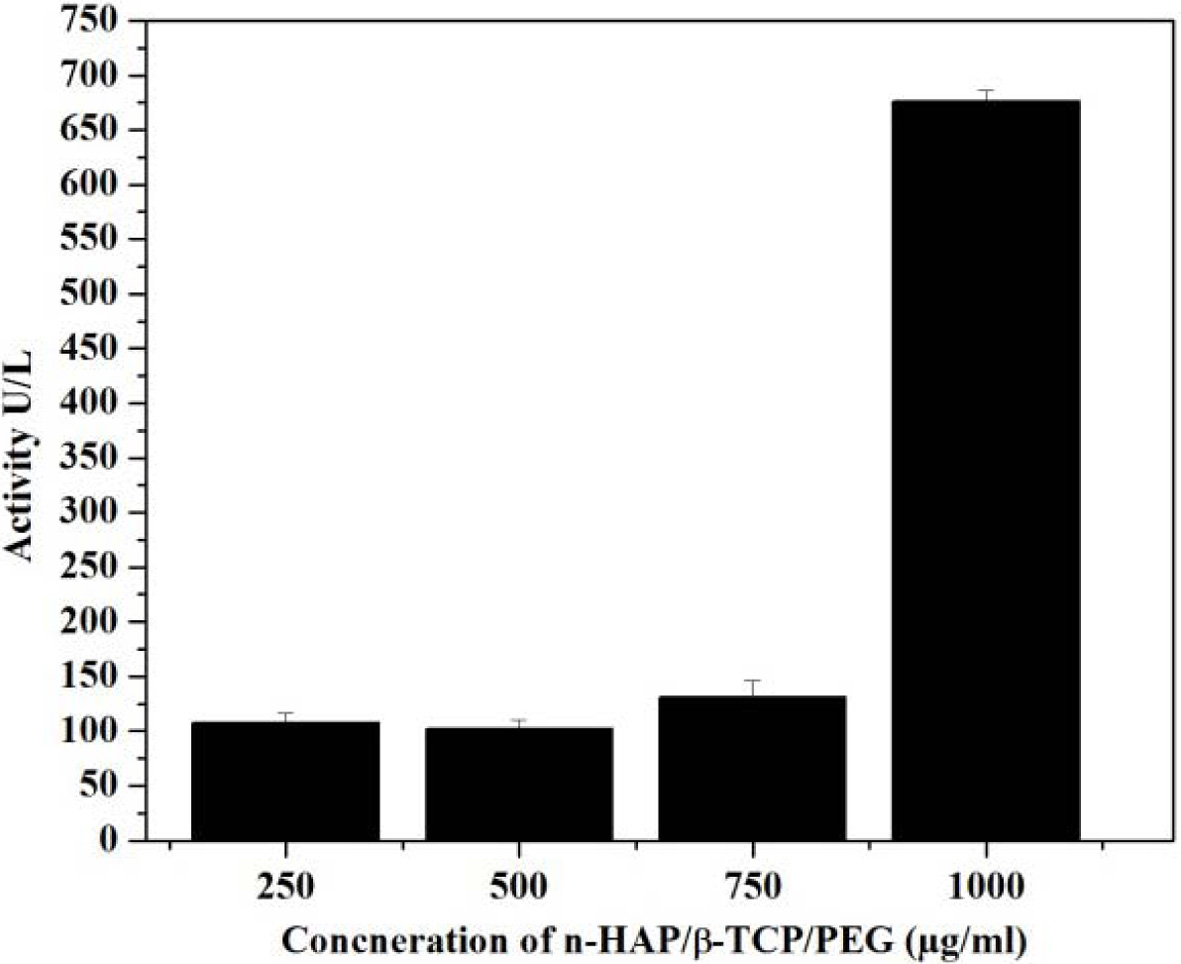
The results of ALP activity are given in Fig. 9 indicate
that the concentration up to 750 μg/ml, the ALP level is increased and does not
cross the optimum level. These reports confirms that the n-HAP/n-β-TCP/PEG
composite can be used up to 750 μg/ml concentration as scaffold
for implantation.
Fig. 7 Fig. 8 Fig. 10
|
Fig. 2 Alkaline Phosphatase (ALP) activity at different proportion. |
|
Fig. 3 XRD pattern of n-HAP/n-β-TCP/PEG composites. |
|
Fig. 4 FESEM analysis of n-HAP/n-β-TCP/PEG composites. |
|
Fig. 5 Elemental analysis of n-HAP/n-β-TCP/PEG composites. |
|
Fig. 6 Cell viability at different concentration and different time period. |
|
Fig. 7 MG-63- CELL LINE (40x ×100). |
|
Fig. 8 Cell proliferation image at different concentration and time period. |
|
Fig. 9 Alkaline phosphatase activity of n-HAP/n-β-TCP/PEG composites. |
|
Fig. 10 Porous spongy structure of scaffold. |
n-HAP/n-β-TCP/PEG composites indicate better biochemical
and bioactivity performance at 1:1:5 proportion. The presence of
n-HAP/n-β-TCP crystalline phases were confirmed from XRD patterns.
FESEM analysis of the prepared composites indicates that the n-HAP and n-β-TCP
are embedded in the polymeric matrix. The elemental analysis further confirms
that the compsotion of nano composites. The biological studies of the
composites indicate its biocompatibility, cell proliferation, antioxidant,
toxicity and ALP activity. MTT assay confirms the MG-63 cells when adhered to
the composite mixture at different concentration shows better viability and
also proliferation at exponential phase. The concentration range preferable is
250 μg/ml to 750 μg/ml. To get better proliferation it is suggested that when
the concentration range exceeds 250 μg/ml the incubtion period should
increases. The DPPH assay confirms that the composite can be used for cancerous
patients due to its anti oxidant property that results in the trapping of free
radicals. The increased alkaline phosphatase enzyme can be seen in the required
concentration for the scaffold preperaed in the ratio of 1:1:5 indicating its
osteoconduction and osteoinduction nature.
This work was supported by UGC
SAP DRS-I (no. F.540/16/DRS-I/2016 (SAP-I)) program, Department of Analytical
Chemistry, University of Madras, Guindy Campus,Chennai-600 025
There are no conflicts of interest to declare.
- 1. M. Deng, S.G. Kumbar, Y. Wan, U.S. Toti, H.R. Allcock, and C.T. Laurencin, Soft Matter 6[14] (2010) 3119-3132.
-

- 2. G. Turnbull, J. Clarke, F. Picard, P. Riches, L. Jia, F. Han, B. Li, and W. Shu, Bioactive Materials 3[3] (2018) 278-314.
-

- 3. M. Bongio, J.J. Van Den Beucken, S.C. Leeuwenburgh, and J.A. Jansen, J. Mater. Chem. 20[40] (2010) 8747-8759.
-

- 4. X. Du, S. Fu, and Y. Zhu, J. Mater. Chem. 6[27] (2018) 4397-4412.
-

- 5. N. Eliaz and T.M. Sridhar, Cryst. Growth Des. 8[11] (2008) 3965-3977.
-

- 6. A. Regiel-Futyra, M. Kus-Liśkiewicz, V. Sebastian, S. Irusta, M. Arruebo, A. Kyzioł, and G. Stochel, RSC advances 7[83] (2017) 52398-52413.
-

- 7. P. Kumari and P. Majewski, J. Nanomater. (2013) 6.
-

- 8. P.J. VandeVord, H.W. Matthew, S.P. DeSilva, L. Mayton, B. Wu, and P.H. Wooley, P.J. VandeVord, H.W. Matthew, S.P. DeSilva, L. Mayton, B. Wu, and P.H. Wooley, J. Biomed. Mater. Res. 59[3] (2002) 585-590.
-

- 9. G. Thrivikraman, G. Madras, and B. Basu, RSC Advances 4[25] (2014) 12763-12781.
-

- 10. J. Lu, H. Yu, and C. Chen, RSC advances 8[4] (2018) 2015-2033.
-

- 11. P. Weiss, P. Layrolle, L.P. Clergeau, B. Enckel, P. Pilet, Y. Amouriq, G. Daculsi, and B. Giumelli, Biomaterials 28[22] (2007) 3295-3305.
-

- 12. J. Schnieders, U. Gbureck, E. Vorndran, M. Schossig, and T. Kissel, J. Biomed. Mater. Res., Part B 99[2] (2011) 391-398.
-

- 13. K.R. Mohamed, A.M. Mohamed, and H.H. Beherei, J. Genet. Eng. Biotechnol. 9[2] (2011) 111-119.
-

- 14. I.M. Low, in “Advances in Ceramic Matrix Composites” (Woodhead Publishing, 2018) p.27-48.
-

- 15. B. Chen, Y. Zhang, J. Zhang, L. Zhu, and H. Zhao, RSC Advances 9[32] (2019) 18688-18696.
-

- 16. Y. Deng, Y. Yang, Y. Ma, K. Fan, W. Yang, and G. Yin,. RSC Advances 7[1] (2017) 559-573.
-

- 17. G. Daculsi, O. Laboux, O. Malard, and P. Weiss, J. Mater. Sci.: Mater. Med. 14[3] (2003) 195-200.
-

- 18. R.Z. LeGeros, S. Lin, R. Rohanizadeh, D. Mijares, and J.P. LeGeros, J. Mater. Sci.: Mater. Med. 14[3] (2003) 201-209.
-

- 19. J. Van Meerloo, G.J. Kaspers, and J. Cloos, In Cancer cell culture (2011) 237-245.
-

- 20. F. Anagnostou, A. Debet, G. Pavon-Djavid, Z. Goudaby, G. Hélary, and V. Migonney, Biomaterials 27[21] (2006) 3912-3919.
-

- 21. S.P. Vinodhini, R. Manonmani, B. Venkatachalapathy, and T.M. Sridhar, RSC Advances 6[67] (2016) 62344-62355.
-

- 22. D. Gerlier and N. Thomasset, J. Immunol. Methods 94[1-2] (1986) 57-63.
-

- 23. G.N. Bowers and R.B. McComb, Clinical Chemistry 12[2] (1966) 70-89.
-

- 24. P. Molyneux and J. Songklanakarin, J. Sci. Technol. 26[2] (2004) 211-219.
- 25. J. Hudzicki, Kirby-Bauer disk diffusion susceptibility test protocol. (2009)
- 26. M.J.E. Paulo, M.A. dos Santos, B. Cimatti, N.F. Gava, M. Riberto, and E.E. Engel, Clinics 72[7] (2017) 449-453.
-

- 27. E.E. Golub and K. Boesze-Battaglia, Current opinion in Orthopaedics 18[5] (2007) 444-448.
-

- 28. U. Sharma, D. Pal, and R. Prasad, Indian J. Clin. Biochem. 29[3] (2014) 269-278.
-

- 29. M. Hui, S.Q. Li, D. Holmyard, and P.T. Cheng, Calcif. Tissue Int. 60[5] (1997) 467-472.
-

- 30. C. Gao, Y. Deng, P. Feng, Z. Mao, P. Li, B. Yang, J. Deng, Y. Cao, C. Shuai, and S. Peng, Int. J. Mol. Sci. 15[3] (2014) 4714-4732.
-

- 31. C. Ribeiro, V. Sencadas, D.M. Correia, and S. Lanceros-Méndez, Colloids Surf., B 136 (2015) 46-55.
-

- 32. S.D. Abel and S.K. Baird, Food chemistry 241 (2018) 70-78.
-

- 33. M.K. Barron, T.J. Young, K.P. Johnston, and R.O. Williams, AAPS PharmSciTech 4[2] (2003) 1-13.
-

- 34. M. Demirel and B. Aksakal, J. Ceram. Process. Res. 19[1] (2018) 5-10.
- 35. B.W. Lee and I.G. Hong, J. Ceram. Process. Res. 20[6] (2019) 655-659.
-

 This Article
This Article
-
2020; 21(2): 240-248
Published on Apr 30, 2020
- 10.36410/jcpr.2020.21.2.240
- Received on Dec 13, 2019
- Revised on Feb 17, 2020
- Accepted on Feb 24, 2020
 Services
Services
- Abstract
introduction
materials and methods
results and discussion
conclusion
- Acknowledgements
- Conflict of Interest
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- M.R. Kuppusamy
-
Department of Chemistry, RV Government Arts College, Chennai 603001, India
Tel : +91-44-2743 1257 - E-mail: kuppusmr@gmail.com






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.