- Comparative studies on bentonite clay and peanut shell carbon recovering heavy metals from printed circuit boards
Murugesan Manikkampatty Palanisamya,* and Kannan Kandasamyb
aDepartment of Chemical Engineering, Erode Sengunthar Engineering College, Erode, Tamil Nadu 638057, India
bDepartment of Chemical Engineering, Kongu Engineering College, Perundurai, Erode, Tamil Nadu 638060, India
The quantity of electronic
waste subjected to disposal annually is increasing alarmingly and is of major
environmental concern due to the existence of heavy metals and other toxic
substances. In this present study, by combining leaching and adsorption the
recovery of heavy metals from Printed circuit boards (PCBs) has been performed.
The two stage aqua-regia leaching extracts Copper (Cu), Tin (Sn), Lead
(Pb), and Zinc (Zn) from PCB. Bentonite Clay (Bent) and Peanut Shell Carbon
(PSC) in their pristine, thermally and chemically activated forms were employed
as adsorbents to remove the heavy metals from leached solution. Effect of
parameters (contact time, temperature, adsorbent dosage and size) on %
adsorption was studied. Chemically activated bent (C-A Bent) has proven to be
an effective among all adsorbents studies with % adsorption for Cu 97%, Sn 98%,
Zn 96%, and Pb 96%. Leaching and adsorption combination can become a promising
methodology for handing electronic waste.
Keywords: Leaching, Adsorption, Bentonite clay, Peanut shell carbon, Printed Circuit Board (PCBs)
Nowadays, electronic devices are rapidly growing due to
human population and industrialization. E-waste has been increased every year
around 18.5 lakh metric tons [1]. Electronic waste like television (TV),
personal computer (PC), Mobile phone, DVD Player, Printed circuit boards
(PCBs), and Printed wiring board scraps contain heavy metals. PCBs are one of
the major important Electronic wastes. The PCB scraps have 50% of iron and
steel elements, 21% plastics, 13% non-ferrous metals and others 16% [2]. In
addition to that metals contain 10-30% of copper and further
metals such as Zn 2%, Pb 4%, etc., based on the source and category
of the circuit board [3]. Skin damage, headache, cardiovascular diseases, hair losses, brain effects are the impact of
heavy metals on human [4-8]. Due to these problems, heavy
metals should be disposed of properly. At first, incineration method was used
to dispose of the circuit boards [9-11]. Since it affects the environment by CO
and CO2 emissions, solidification and landfill methods were implemented to
dispose of the circuit boards [12, 13]. Later, these
practices were identified to be polluting the nature of soil by reducing the
interface mass transfer rate of soil. Cu2O and CuO were found from the oxidation method of the toxic metals from
PCBs by use of NaOH-supercritical water [14]. The preheating
method (Pyrolysis) recommended recovering the metals from electronic waste
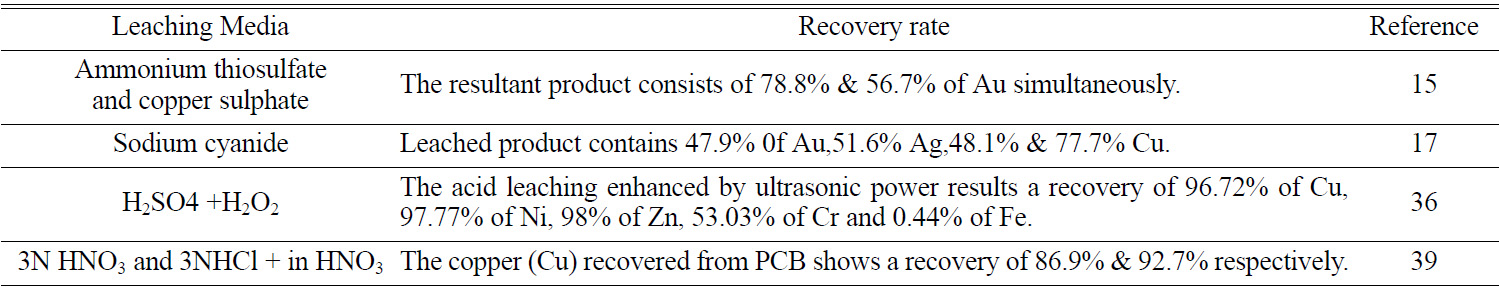
[15]. PCBs were dissolved in aqueous solution (HNO3, H2SO4, and HCL), the Electro-deposition techniques achieved more than 90% of Cu recovered [16]. Sodium cyanide solution was used as a leaching agent in leaching column technique and the
result obtained was recovery of Au 46%, Cu 62% and Ag 51% [17]. Leaching agents
Cyanide, Thiosulphate, Thiourea, and Halide were used to
separate the precious metals like Ni, Zn, Sn, Au, Cu, Pb and other elements
[18-20]. Several inorganic Chemicals were tested as leaching agents such as HCL
[21], H2SO4 [22, 23], Aqua Regia [24], HNO3 [25]. 85.4% of copper was leached from waste PCBs by the principle of leaching methodology. It was analyzed based on the
concentration and agitation time. The study emphasized that as the
concentration increases, the leaching of metal ions increased [25, 26].
There are different conventional
methodologies like Incineration, landfilling,
gasification, pyrolysis, hydro-
metallurgical and chemical leaching techniques have their own merits and
demerits in various aspects like cost, environmental effects and metal recovery. However, there are more difficulties to recover the heavy metals in
various processes like Electro-winning, Electro-deposition, Acids leaching and
others. Therefore, heavy metals like Cu, Sn, Zn, Pb, As, Zi and Cr are
recovered by adsorption techniques by Bent adsorbent [27, 28]. Natural
clay adsorbed Cu and Zn based on various parameters like temperature
(25 ℃), Agitation speed (200 rpm) and pHfrom aqueous solution [6, 29].
The result reveals that copper is recovered from phosphoric acid solution by
A-Bent. It summarizes that the effect of concentration decides the rate of
recovery by adsorbent [30]. The ability of the Bent was investigated by
adsorption of Cu and Ni. The characterization of bent is based on concentration
(5 ppm-30 ppm) and temperature (20-80 ℃) [28]. The activated carbon (AC) was prepared by using chemical
reagent (HCL). The adsorption capacity of A-Bent to recover the heavy metals
like Pb, Cadmium (Cd), and Cu was analyzed. The results showed maximum
adsorption capacities in natural and A-Bent and were found to be Pb 83 mg g-1 and 92 mg g-1 [31]. Natural clay (Sepiolite) can be used to adsorb the Cu and Zi
from aqueous solution [32]. The result gave the metal ions (Cu2+ and Zn+) adsorption efficiency with the respective parameter from aqueous
solution and it concluded that the Natural clay is effective one
for recovery of heavy metals and minerals [33-36]. Till now many studies were
done focusing on extraction of heavy metals from PCBs but have not focused on
handling the extracted solution and it is applicable to recover the metals from LCD monitors boards, LED, LCD Boards, mobile phone boards.
On the other hand several researchers used adsorption technique for recovery of
heavy metal from aqueous solutions. Here in this present study, it is focused
on the extraction and recovery of heavy metals from crushed
and sieved printed circuit boards using two stage leaching combined with
adsorption.
Materials
Raw Printed Circuit Boards (PCBs)
The waste (PCBs) raw materials were obtained from Global
E-Waste management services, a waste collection unit in
Tamil Nadu, India. For experimental purpose, 5 kg scraps of PCBs were utilized.
The sample initially cleaned manually to remove dust particles by Air Blower.
Later other elements such as capacitors, resister, integrated
circuits, diodes, transistor etc., were detached with the
help of mechanical tools (saw metal cutter, sheet metal cutter, metal lathe
cutting tool, cutting pliers and materials separation toolkit. The sample PCBs
and disassembled samples were taken out and shredded using scissors to a 15 mm
× 15 mm size [22, 37, 38]. Raw boards were crushed with the help of jaw crusher
having capacity 80 kg hr-1
with a clearance of 10 and 5 mm. PCBs of 5-10 mm sizes samples were obtained.
Then the samples were further subjected to Ball mill having a ball weight 500
gm at a speed of 60-120 rpm with a mill diameter 200 mm driven by 0.25 HP, 3
phase motor. Then the samples were further subjected to Ball mill having a ball
weight 500 gm at a speed of 60-120 rpm with a mill diameter 200 mm driven by
0.25 HP, 3 phase motor. Since, the samples obtained 5 mm sizes to 3 mm from
ball mill were it is not meeting the specified size requirements; these PCBs
are then subjected to heating operation at 700-900 ℃ using muffle
furnace to enhance the flexibility and crushing properties of PCBs [16]
followed by continues size reduction operations of Drop-weight crusher,
Pulverizer and ball mill for two hrs. The crushed sample thus
obtained were converted into powdered form using pulverizer having disc
diameter 175 mm driven by 3 phase motor at 1,400 rpm in 225-445 V supply. The
resultant powdered samples were screened under various mesh size and weight
fraction of bottom products (sieves from 52 B.S.S to pan) becomes higher but
not sufficient for expected recovery. The pulverized PCB powders were milled in
a ball mill which results high size reduction and highest weight fractions were
obtained at lowest sieves. The weight fractions obtained at
each sieves were separately collected and subjected for leaching operation.
Results obtained were in the range of 4 mm-0.05 mm particle sizes
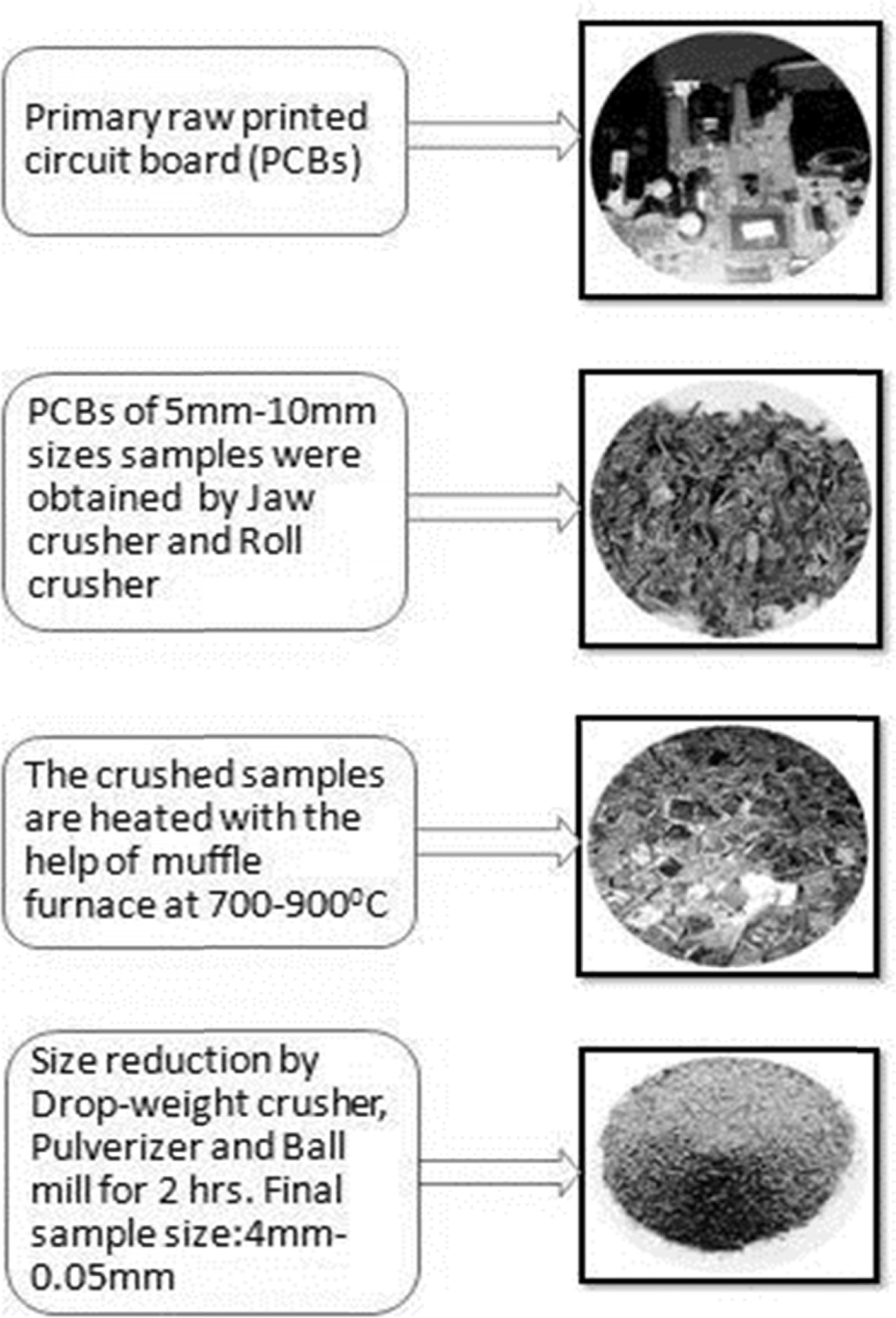
[26, 39]. Therefore, Fig. 1 showed that, Primary Raw PCBs; in to
stepwise size reduction under the various mechanical operations (Jaw
crusher, Roll crusher, thermal heater and pulverized mills
produced small sizes between 4mm-0.05mm).
Methods
Aqua Regia preparation and Metals leaching
The leaching media is an important factor that should be
considered while extracting heavy metals such as Zn2+, Sn2+,
Cu2+ and Pb2+ etc from PCBs. Different sort of leaching
agents given various leaching rates with respect to
the type of metal were deliberated in previous
studies [25]. The Previous studies have resulted in significant metal recovery;
the copper (Cu) recovered by 3N HNO3 and 3N HCL+ in HNO3
from PCB shows a recovery of 86.9% & 92.7% respectively [40]. It also
possesses demerits such as targeted extraction of specific metal leads to loss
of several other valuable metals (Table 1). By using aqua regia as leaching
reagent by two stages (first stage HCL and HNO3 and second stage HCL
and H2SO4) heavy metals can be extracted from PCBs with
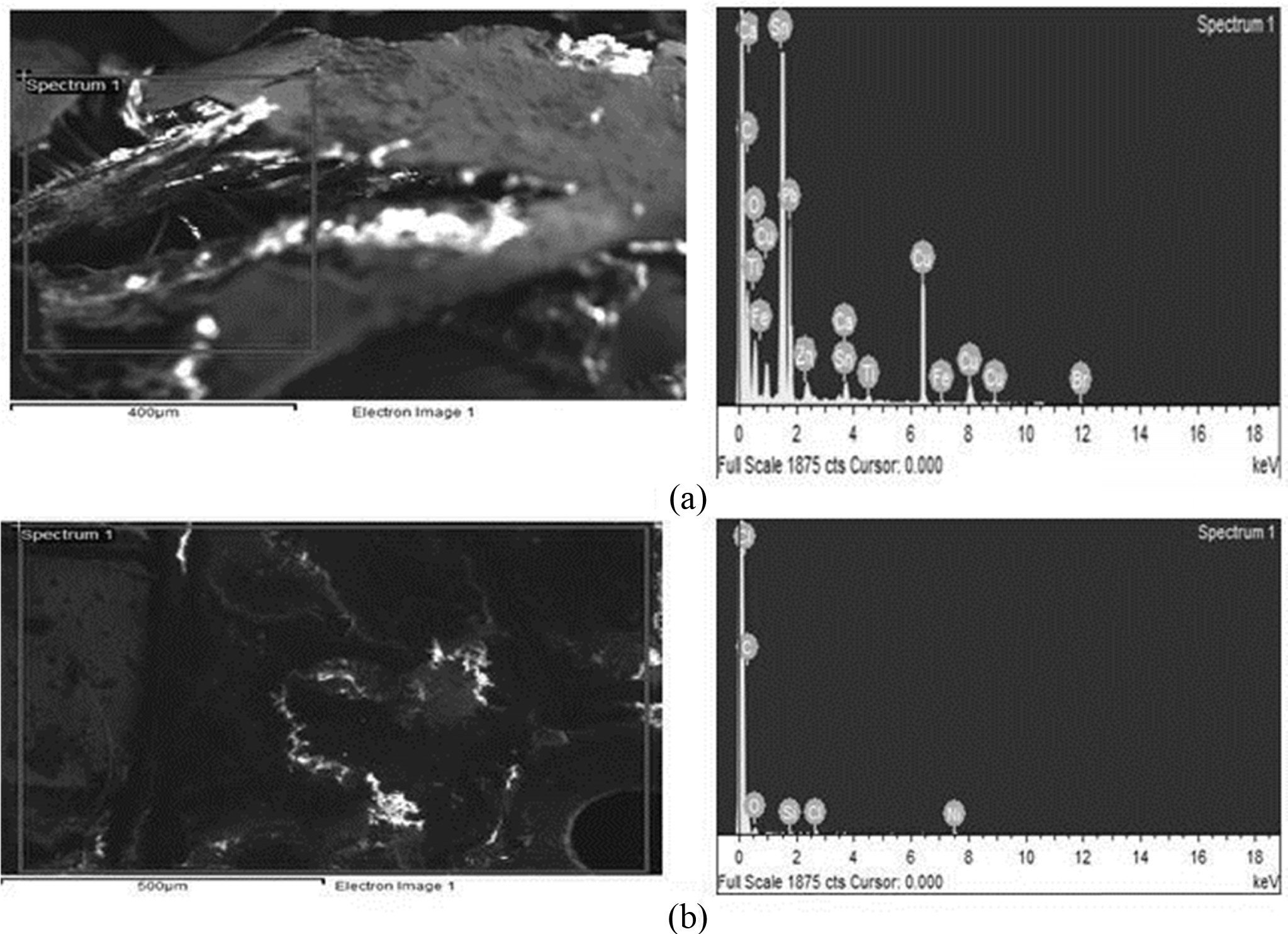
various operating conditions and samples were analyzed by Energy-dispersive
X-ray Spectroscopy EDXs [41, 42]. The dissolution of metal ions having PCBs
takes place according to the following reaction mechanisms:
Reaction of copper with Nitric acid
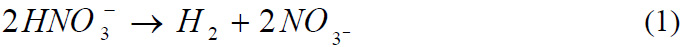
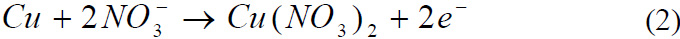
Reaction of Lead with Nitric acid
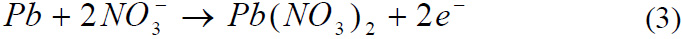
Reaction of Tin with Nitric acid
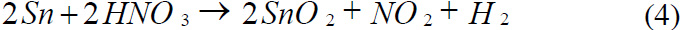
Reaction of Tin with Hydrochloric acid
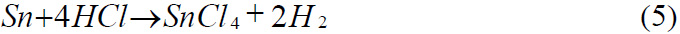
Reaction of with Hydrochloric acid

Reaction of Tin with Sulphuric acid
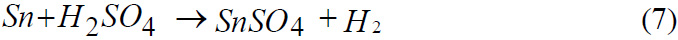
Reaction of Zinc with Sulphuric acid
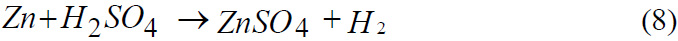
Adsorbentactivation
Bent clay carbon and peanut shell carbon were used as
adsorbents. Chemical and physical treatments are given to the
adsorbents initially. 250 g of both adsorbents were
taken in the thermal crucible and were dried for about five hrs for thermal
activation at 900 ℃. Therefore, the samples obtained are
of 1 µm to 5 µm. The higher specific surface area was obtained due to the
removal of unwanted gaseous molecules from the Non-activated adsorbents (NAC).
The C-A Bent & C-A PSC was prepared by using concentrated HCL and HNO3.
Then, it was shaken at 200 rpm for 5 hrs. The
acid-activated adsorbent was recovered and washed with deionized
water. After drying, both samples were grinded and
sieved. The preparedsamples were tested by the help of Scanning
Electron Microscope (SEM-FEI-Quanta FEG 200F) [41, 43].
Adsorption studies
The adsorption of heavy metals on PSC & Bent was
carried out in a batch system in both activated and NAC. 2 g of adsorbent was added to
20 ml of leached solution in a conical flask.
The mixture was shaken at 200 rpm for 5 hrs at 80 ℃ temperature.
After complete adsorption, the
samples were filtered and metals concentration was analyzed by using EDXs. It
is performing elemental analysis characterization of a sample in conjunction
with SEM. The energy of the beam
current is typically in the range of 100 Na, Schottky emitter
ranges between (-200 v – 30 kv), magnifications ranges 12X-105 X and resolution as 2
nm (Gold Nano-particles suspended on
carbon substrate). Then, adsorption efficiency of an adsorbent (PSC & Bent) was
determined by the following the
equation.
Removal efficiency (%) =
(Co - Ce)/Co × 100 (9)
Co is initial concentration of
metal ions from leached samples. Ce is the metal ions
concentration at the completion of adsorption operation [30].
Influence of various parameters on recovery
The studies carried out on recovering of metal ions from
leached solution with specified metals ions recovered by
PSC & Bent. Experiments were conducted with
pristine, thermally activated, chemically activated adsorbents to recover Zn2+,
Sn2+, Cu2+ and Pb2+ ions by varying the
operating parameters like adsorbent dosage (0-10 g), concentration (0-40 ml),
sizes of adsorbent (10-0.5 μm) contact time (0-4 hrs) and temperature (0-120 ℃) [25, 34, 35, 43]. Optimization studies were carried out to find the
maximum recovery.
|
Fig. 1 Image of Primary Raw PCBs; in to stepwise size reduction under the various mechanical operations (Jaw crusher, Roll crusher, thermal heater and pulverized mills produced small sizes between 4 mm-0.05 mm). |
Analysis
of printed circuit board
The primary samplefollowed three size reduction operations
namely crushing, pulverizing and milling and the weight fractions were
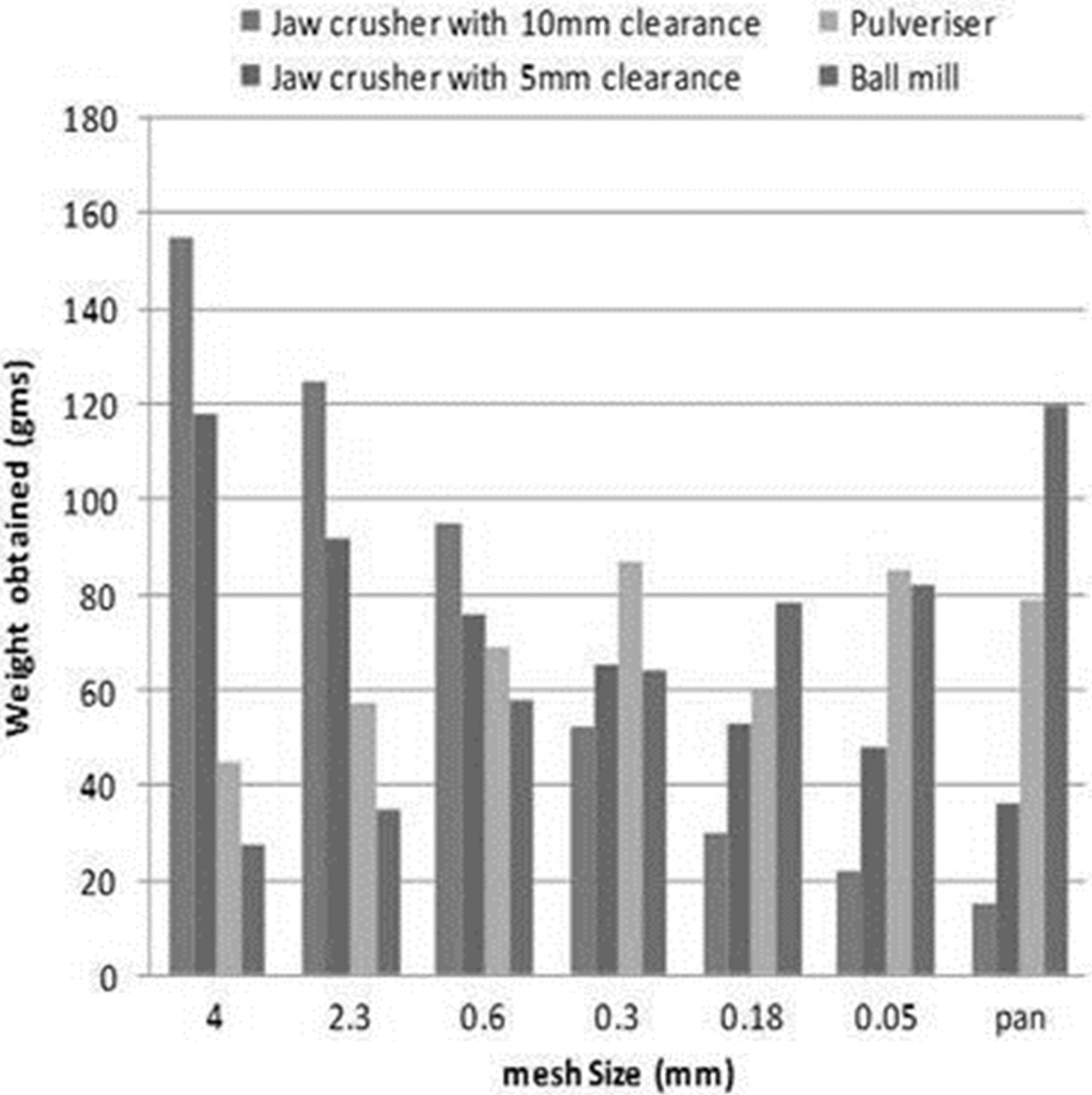
collected. The graphical representation (Fig. 2) of size analysis shows that
the fraction of the sample obtained in the sieves with larger mesh size has
been decreasing when subjected to a sequence of size reduction operations. From
the sieve analysis data of each operation, the sample obtained from ball mill
has many fractions of weight in the pan which is less than 0.05 mm [22]. The
sample sizes are most important for dilution of metal ions from leaching agent.
Here the minimum sizes of samples were used to recover the metals ions for
better dilution. Therefore, the results showed in graphical representation of
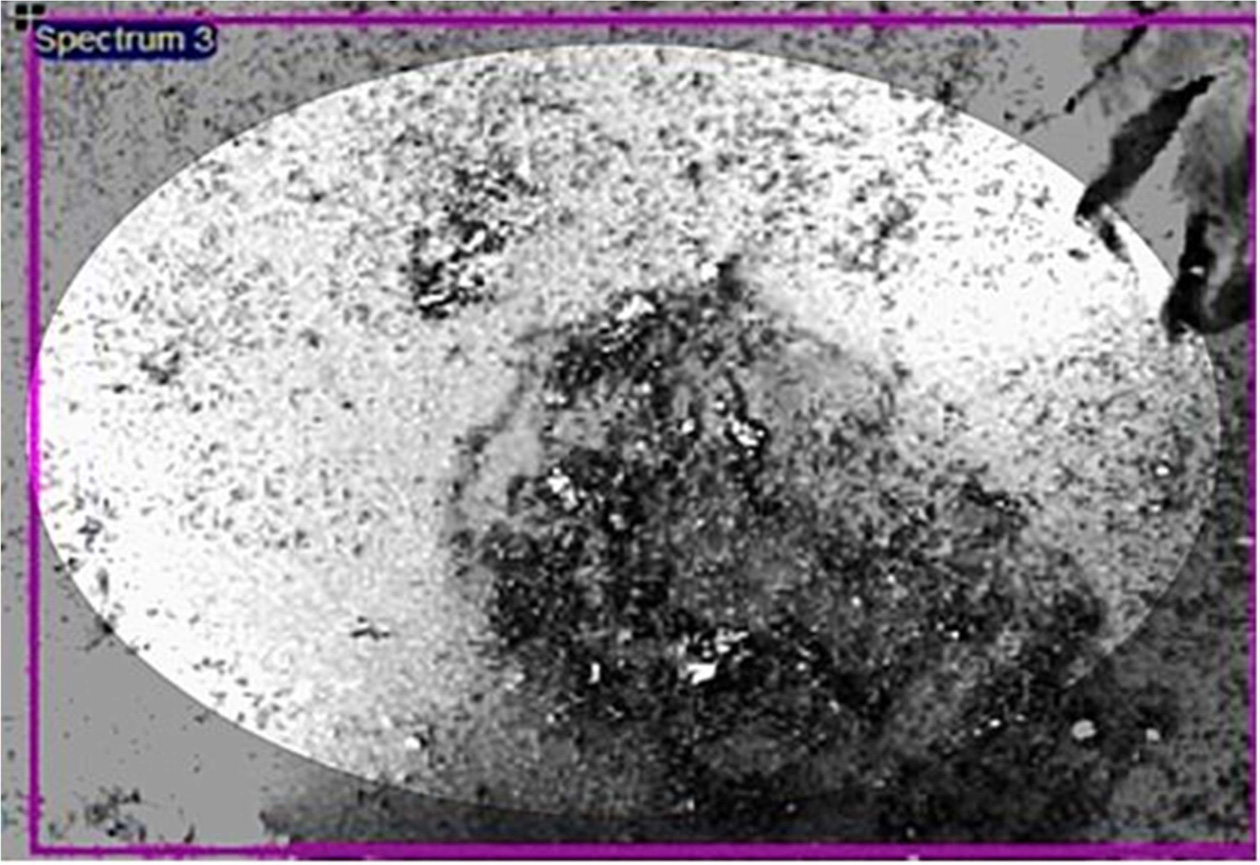
various size reduction operations (sizes up to 4-0.05 mm). The SEM images of
crushed samples were shown in (Fig. 3). Few studies were carried out based on
the effect of particle size to recover the metal ions from waste PCBs under
bioleaching and chemical leaching techniques. Present research comprises of
leaching used particle size 0.05 mm that is the sample retained just above the
pan.
Leaching
The optimization of leaching experiments carried out by
keeping optimized variable parameters based on previous
studies. The electro deposition method recovered 98% of hazardous metals (Cu, Pb, Zinc (Zn), Tin (Sn), Nickel (Ni),
Silver (Ag) and Gold (Au) etc.) from PCBs. It was done by using leaching
(acetic aqueous chloride) and electrodeposition method [44, 45] in
specific conditions of 80 ℃ of temperature, 0.05 mm of thickness, 3
hrs of contacting time, 80 rpm shaking speed and pulp density of PCB sample 20
g mL-1,
in bothstages having 3:1 ratio of HCL and HNO3
prepared as a leaching agent. The experimental results were
obtained under the above mentioned conditions and have been shown in (Table 2)
and (Fig. 4). Results found the optimum recovery rate for Cu 97.06%, Sn 94.66%,
Zn 96.64% and Pb 96.89% respectively.
Adsorbent characterization and studies
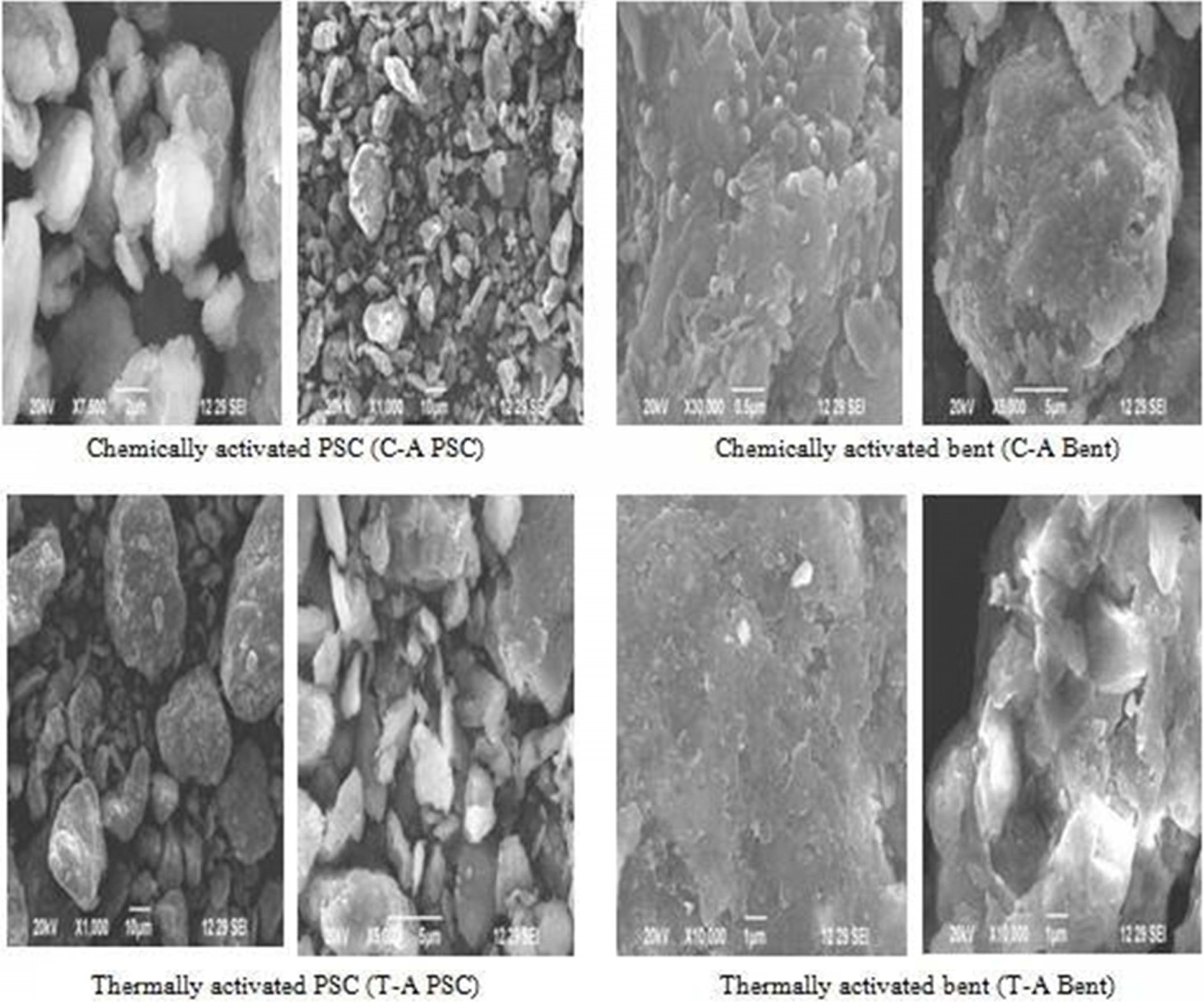
The SEM images of crushed and pulverized samples of Bent
and PSC subjected to thermal treatment and chemical treatment as shown in
Fig. 5 and it seems the C-A Bent particles
were slightly smaller compared with C-A PSC particles. It has been found by the
image of SEM. It reports the removal of moisturized contents and unwanted ions
from the adsorbents. In this regard, adsorbents improve the pore sizes and
enhance the recoverability rate. The adsorbent activation test were realized in
a experimental work, where the C-A bent and PSC are given maximum numbers of
pores like 2 μm of PSC and 0.5
μm of bent it should be comparatively
more rate of pores are created. The thermally activated adsorbents gave minimum
pore rates like 5 μm of PSC (T-A PSC) and 1 μm of bent (T-A Bent) due to the
recovery rate of unwanted minerals. Acid activation was more effective due to
the dissociation of both adsorbents by concentrated HCL and HNO3.
From the result shown in (Fig. 5), we observed that HCL and HNO3 are
strong acid to be able to improve the surface area of both adsorbents.
Therefore, many studies carried on the adsorption to recover of Zn2+,
Sn2+, Cu2+ and Pb2+ from various samples using
bent and PSC. During our tests, specified parameters were deliberate to see
their effects on the adsorbent performance in to the adsorbate (leached
solution) adsorption.
Effect
of contact time in the removal of metals
The contact time is an important factor affecting the
adsorption efficiency by agitation. To determine the effective time
ofadsorption, the sampleswere allowed to be leached for different interval of
time based on previous studies. The persistent condition was
maintained at 5 g sample adsorbents, size 0.05 mm shaken with 0.5
litter of aqua regia in a conical flask and shaking speed of 200 rpm while
temperature maintained at 34 ℃ for all category of adsorbents (C-A
Bent & C-A PSC and T-A Bent & T-A PSC). It is followed to be leached
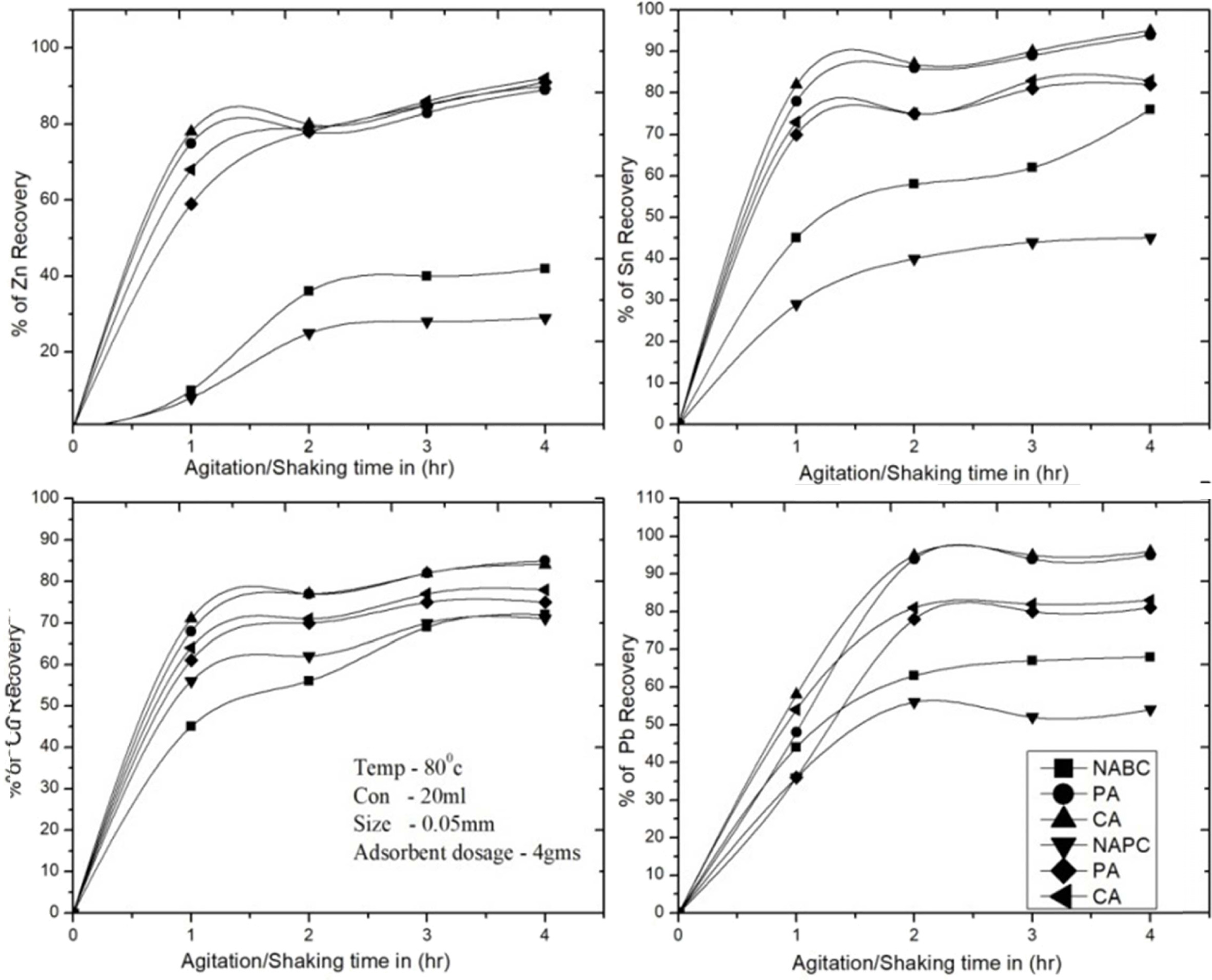
for 1, 2, 3, 4 and 5 hrs respectively. The test reported 1 to 4 hrs, for
adsorbent NAPC and NABC are adsorbed Zn 20-40%, Sn 35-65%, Cu 70% and Pb
40-60%, T-A PSC & T-A Bent are adsorbed Zn 83%, Sn 70-85%, Cu 70-80% and Pb
80-85%, C-A Bent & C-A PSC are adsorbed more recovery rate due to higher
surface area of adsorbents were the results as a both adsorbents are recovered
the metal ions at1 to 4 hrs more than 83% of Zn, 88% of
Sn, 89% of Cu and Pb 80-85% respectively. Therefore,
the tests are identified 1 to 3 hrs are gradually increased
adsorption rate and then linearly attain equilibrium.The optimum values for
removal of metal ions were determined at 2 to 4 hrs. Therefore, (Fig. 6) shows
that higher efficiency for Zn2+, Sn2+, Cu2+
and Pb2+ ions adsorption can be obtained at the time intervals
between 2 to 4 hrs. All remaining experiments were carried out at 4 hrs contact
time. The result shows at 3 hrs the maximum recovery of metals as 91.46%,
90.56%, 86.56% and 89.91% of Cu, Sn, Zn and Pb respectively.
Effect
of adsorbent dosage in the removal of metals
The experimental studies were carried out in the ratios
1:2, 1:4, 1:6, 1:8. 1:10 of leached solution and adsorbent. The adsorption rate
increased linearly with dosage and attained equilibrium. In order to determine
the optimum value of adsorbent dosage during the adsorption process, parameters
like contact time, concentration and temperature were kept constant. The
increased adsorbent dosage increased the surface area of adsorbent. This can be
attributed to the increase in the adsorption efficiency of adsorbent [42, 46, 47].
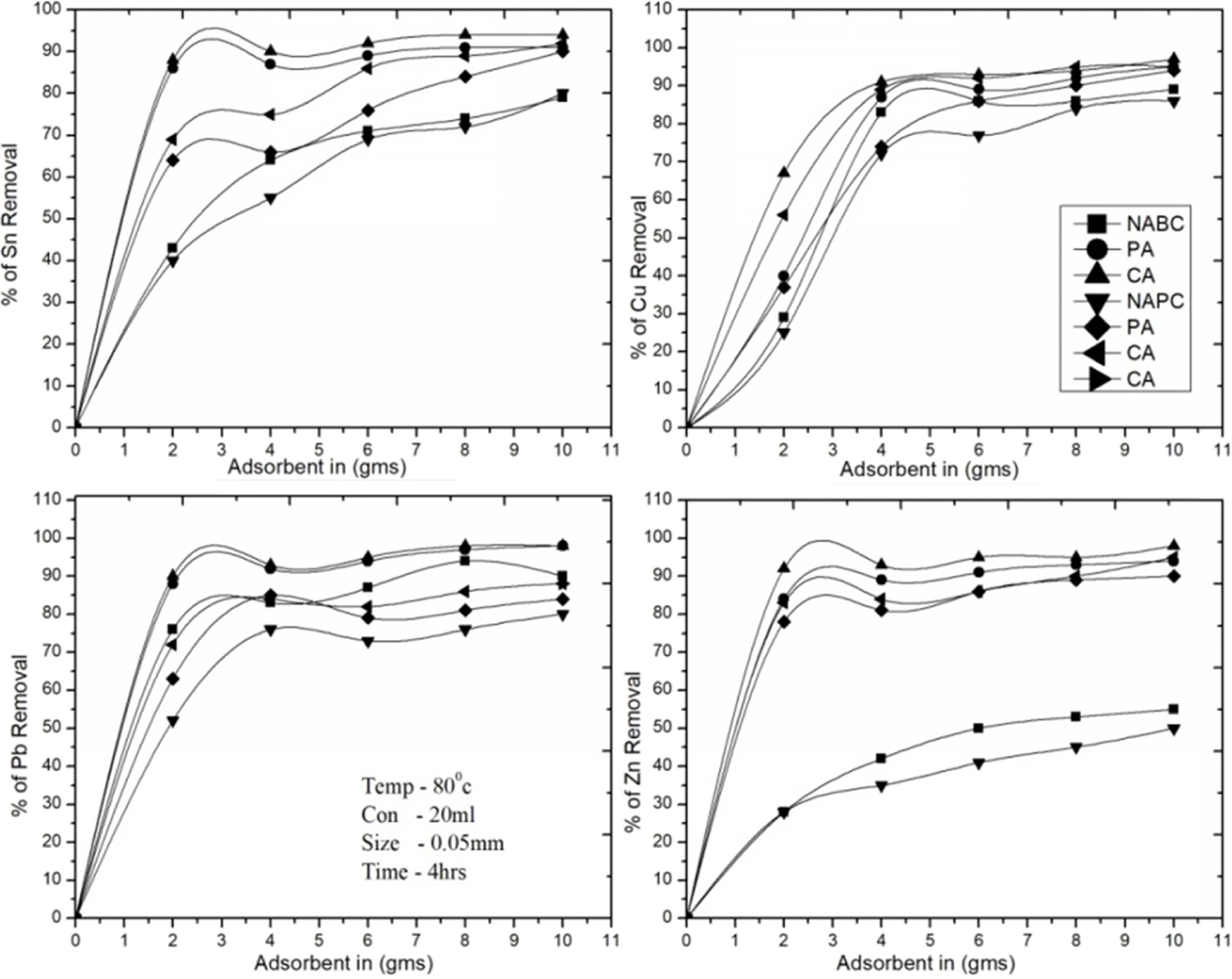
The experimental results were found with the dosage of both adsorbents at the
ranges from 0-10 g, NAPC and NABC are adsorbed linearly up to 5 g, after that
attain equilibrium, and finally 75% of Sn, 80-85% of Cu, 75-80% of Pb and
30-42% of Zn, T-A PSC & T-A Bent are studied and the results as 82-25% of
Sn, 85-92% of Cu, 85-88% of Pb, 80-89% of Zn and C-A Bent & C-A PSC are
much effective at 1-5 g of both adsorbent were found maximum recovery rate of
valuable metals like 93-95% of Sn, 96% of Cu, 94% of Pb, and 91-95% of Zn. The
present tests were analyzed linearly increased adsorbent dosage rate as well as
increased Zn2+, Sn2+, Cu2+ and Pb2+
ions recovering media it was increased adsorption
rate. Therefore, (Fig. 7) shows the adsorption efficiency
of activated and NAC adsorbents and optimum values
are found to be at 4 g per 20 ml of PCBs leached solution.
CA-Bent adsorbents were found maximum recovery of metallic composition as
95.06% of Cu, 97.87% of Sn, 92.39% of Zn and 96.60% of Pb.
Effect
of leached solution concentration
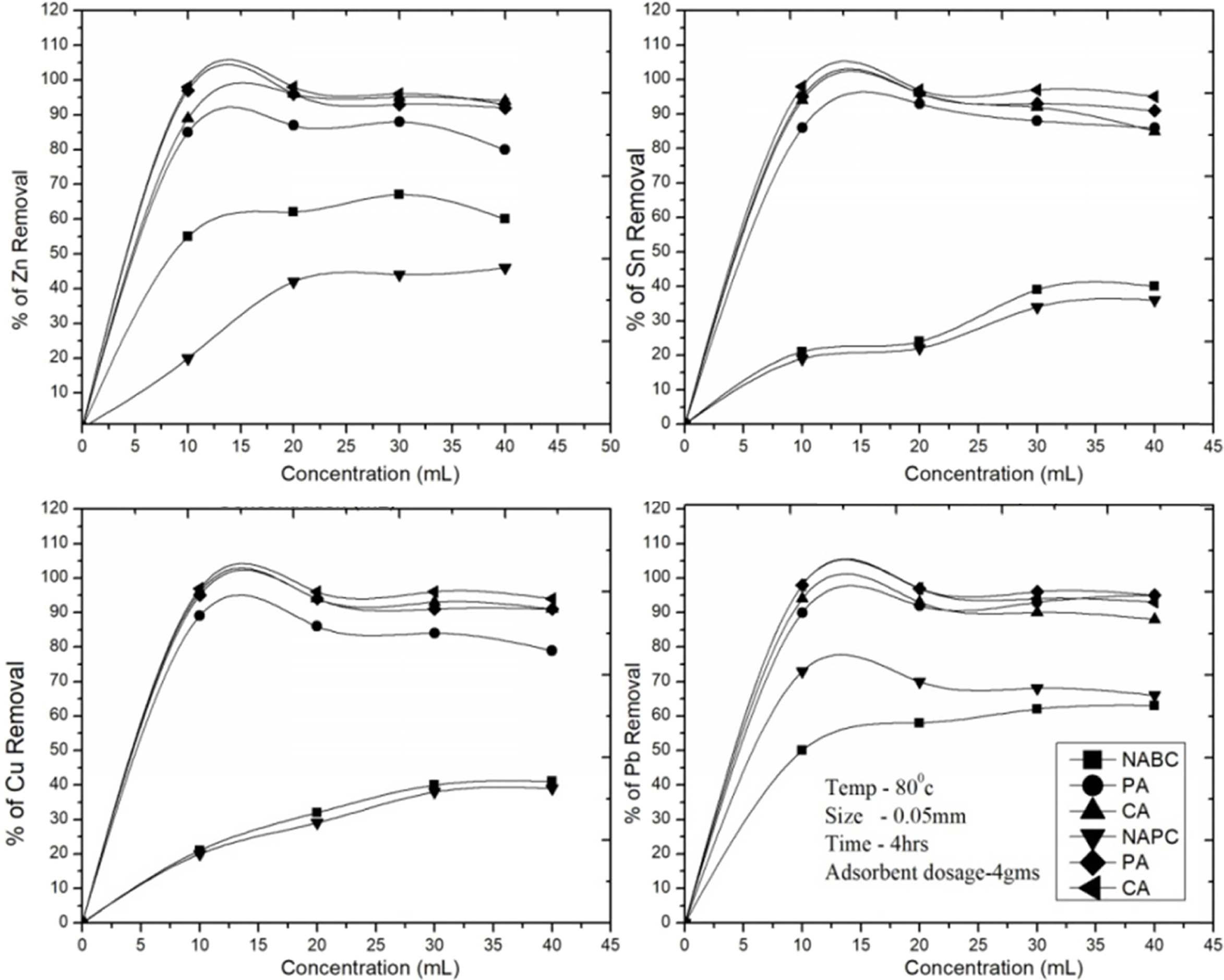
The effect of concentration on the adsorption of Zn2+,
Sn2+, Cu2+ and Pb2+ ions was carried out with
the con- centrations of 10, 20,
30, 40 and 50 ml under the operating temperature of 80 ℃, agitating
speed of 200 rpm and 0.5 μm of particle sizes within the equilibrium contact
time of 4hrs and adsorbent dosage of 4grams. As shown in (Fig. 8), the removal
quantity was increased with increasing themetal ions concentration until the
equilibrium at the adsorption. After that, leached solution metal ions will not
be adsorbed due to many more metal ions fully covered in the adsorbent surface.
The previous studies [27, 43] reported, the leached solutions 0 to 40 ml were
used in adsorption for heavy metals adsorption as aresult provides linearly
increase in the adsorption rate and attain saturation condition. The initial
metal concentration providesthe essential driving force to get over the
resistance tothe mass transfer between the aqueousand the solid phases. At
lower initial metal concentration, more sorption sites were available for metal
adsorption. Higher initial metal ion concentrations are enhances the
interaction among the adsorbate and adsorbent, hence the result was found
higher adsorption efficiency. Our results indicated
that 10-20 ml were the optimal concentrations for
Zn2+, Sn2+, Cu2+ and Pb2+ ions
removal, respectively. All the
studies carried out with the optimal concentration at 20 ml/4 g
of adsorbent. Bent (C-A sample) showed the maximum removal rate due to higher
pores and surface area. After the saturation time of 4 hrs, the removal of Sn
decreased from more than 96% to 85% at 20 ml of leached sample because of metal
recover ability reduction from both adsorbent. The same behavior
was observed with other metals ions removal Zn2+ (96-94%), Cu2+
(94-91%) and Pb2+ (98-93%), respectively. In comparison with PSC
(C-A sample) samples, the Bent (C-A sample) samples removed
maximum amount of metal ions but the treated PSC (C-A) and
untreated samples are showed much lower removal efficiency (Fig. 8). Thus,
it was clearly observed that the removal of metal ions
was strongly influenced by the concentration and
adsorbent surface recover ability rate.
Effect
of temperature on the adsorption
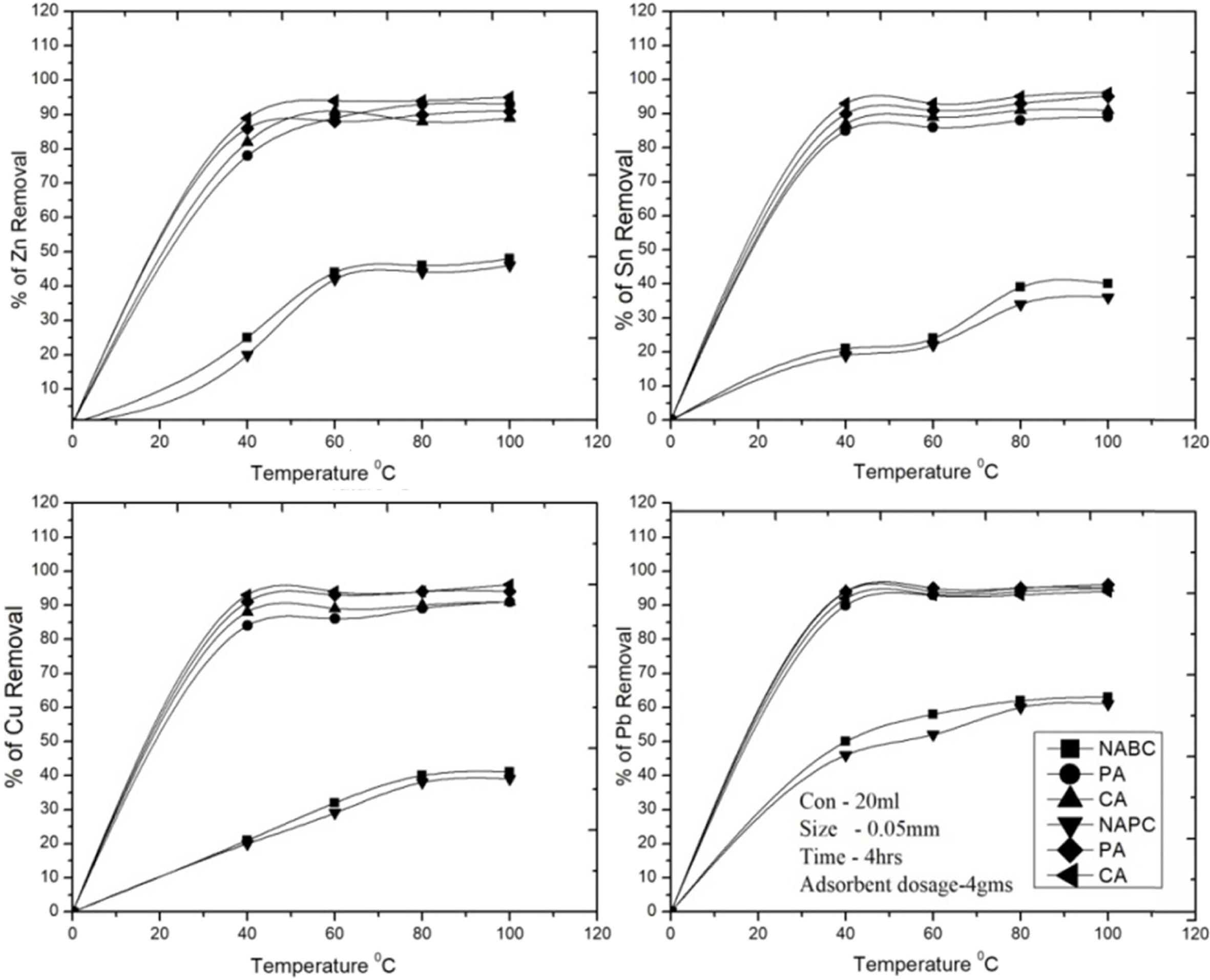
The study was carried out for the effect of temperature
on the adsorption of Cu2+, Zn2+, Sn2+ and Pb2+
ions on to Bent and PSC samples at 20, 40, 60, 80 and 100 ℃ with
other parameters kept constant with respective equilibrium conditioned level.
The adsorption capacity of metal ions by the both adsorbents increased as temperature
increases from 20 to 60 ℃ and then attained
equilibrium for endothermic sorption process. After attain
the equilibrium/saturation, adsorption rate decreased continuously and
stopped (temperature suggested
an exothermic sorption process) it were reported [29, 48]. The (Fig. 9) shows
the removal quantities of heavy metal ions by the activated and non activated adsorbent samples at highest removal
efficiency with respective
temperature. In this study shows that, the removal rate of C-A PSC
adsorbent has presence of results as Cu2+
(91%), Zn2+ (94%), Sn2+ (93%), Pb2+ (95%) and
C-A Bent presence of result as Cu2+ (88%), Zn2+ (82%), Sn2+ (86%), Pb2+
(94%) respectively. These results
demonstrate that the Bent is not effective for removal of metals byendothermic
temperature at 60 ℃. 20-40 ℃ temperature range was used
to get a good removal efficiency of about 90% for Pb removal. As a result of
increased adhesiveness in C-A Bent & C-A PSC adsorbents, both adsorbents
are showed higher adsorption rate at higher temperatures compare to T-A Bent
& T-A PSC adsorbents.
Effect
of particle size of the adsorbent
Effects of various sizes of adsorbents for both adsorbents
were studied. The different particle sizes of adsorbents such as 10 μm, 5 μm, 2
μm, 1 μm, and 0.5 μm were performed for metal ions adsorption and keeping
same operating procedure with studied parameters like
agitation contact time 4 hrs, adsorbent dosage 4 g, leached solution metal ion
concentration 20 ml and temperature 60 ℃ [49-51]. This study
analyzed adsorption rate with respect to various particle sizes for both
adsorbents. The small particle size of adsorbent gives maximum surface area so
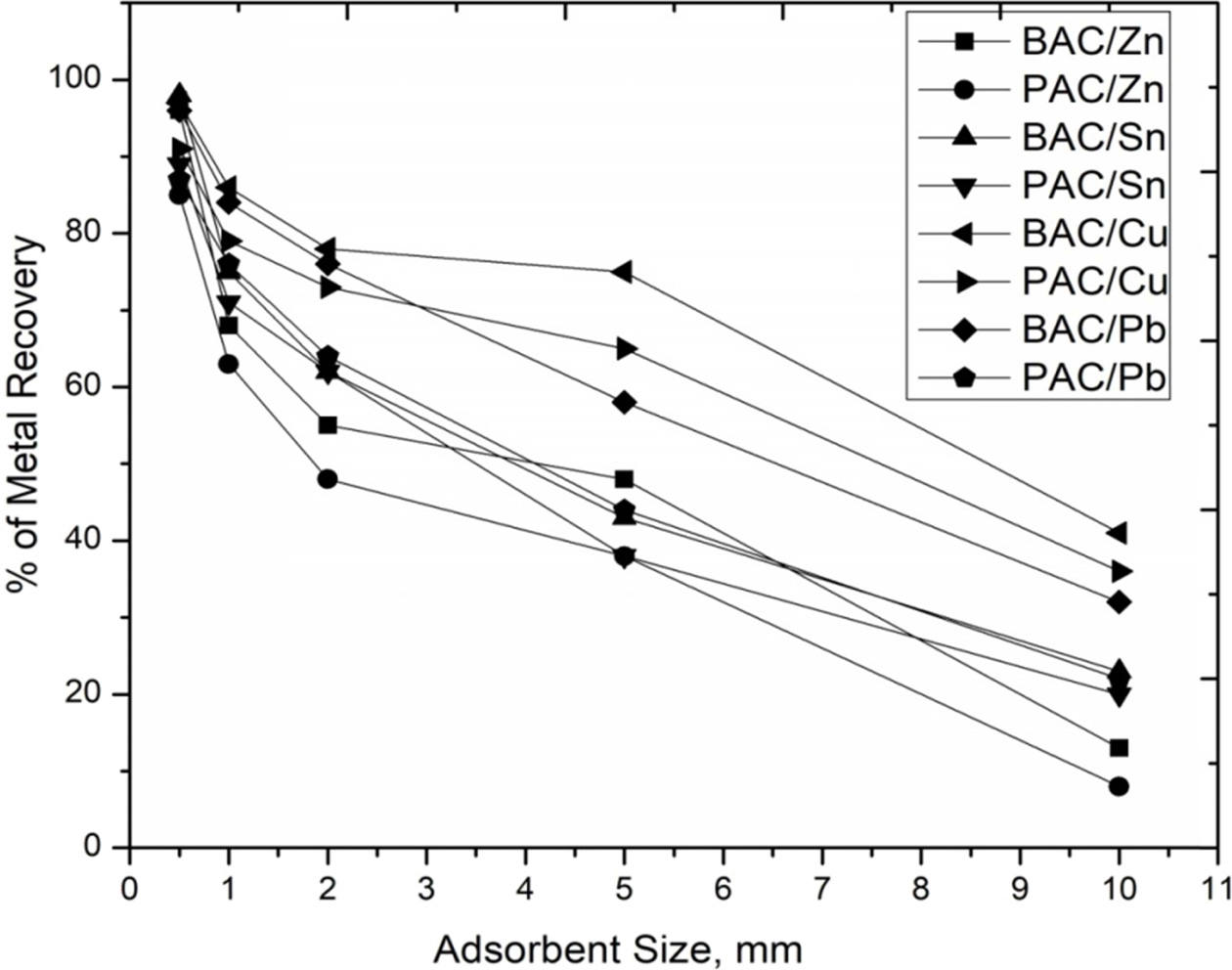
that metal ions easily adsorbed. Fig. 10 shows 0.5 μm
particle size adsorbent’s removal rate of BAC. It gives Zn
96%, Sn 98%, Cu 97%, Pb 96% and PAC gives Zn 85%, Sn 89%, Cu 91%, Pb
87%. Therefore, particle size is more important to recover
the heavy metals through adsorption.The previousresult also indicatesthat C-A
Bent samples gave the better removal efficiency [53-55]. The study revived
the recovery of heavy metal ions like Pb and Cu by different clay minerals.
Therefore, in this experimental work also confirm their beneficial
use for the removal of copper, tin, lead and zinc from heavy metals having
leached solution.
|
Fig. 2 Graphical representation of size reduction and analysis in various operations (jaw crusher, pulverize and Ball mill) sizes obtained 0.05 to 4 mm. |
|
Fig. 3 SEM images of metals present in powdered PCBs. |
|
Fig. 4 EDXs spectrum analysis for metal ions obtained before (a) Metals composition in before treatment of (PCBs) and after (b) Metals composition in after treatment of (PCBs) PCBs by Leaching. |
|
Fig. 5 SEM Microphotograph Surface images analysis of adsorbents (C-A Bent & C-A PSC and T-A Bent & T-A PSC). |
|
Fig. 6 Effect of contact time in various operating conditions for removal of Zn2+, Sn2+, Cu2+ and Pb2+. |
|
Fig. 7 Effect of adsorbent dosage on the removal of Zn2+, Sn2+, Cu2+ and Pb2+. |
|
Fig. 8 Effect of concentrations in various operating conditions for removal of Zn2+, Sn2+, Cu2+ and Pb2+. |
|
Fig. 9 Effect of temperature various operating conditions for removal of Zn2+, Sn2+, Cu2+ and Pb2+. |
|
Fig. 10 Effect of sample sizes in various operating conditions for removal of Zn2+, Sn2+, Cu2+ and Pb2+. |
|
Table 2 Metallic composition of leached PCBs at optimum conditions by stage-I & II. |
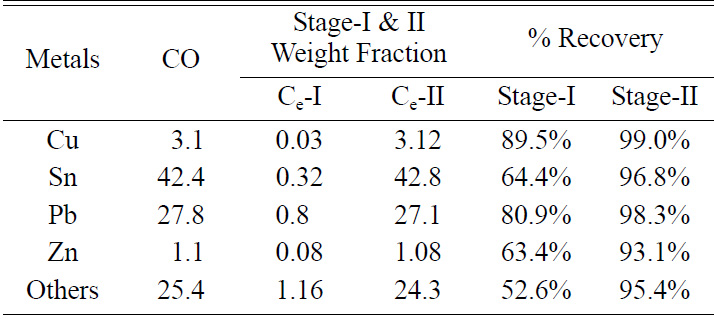
Heavy metals in waste PCBs were leached into corresponding
reagents during the two-stage leaching operations. The effectiveness of two leaching
media (HCL; HNO3, H2SO4; HCL) for recovery of
heavy metals during the treatment of PCBs was evaluated. The recovery rate was
nearly obtained for Cu - 99.04%, Sn - 96.88%, Pb - 98.34%, Zn - 93.1%, others -
95.44% (Ni, Mn, and Mg…) by aqua regia leaching. Hence the
leached metal ions were treated by adsorption. Activated
and NAC adsorbent (Bent & Peanut shell) were utilized to recover the heavy
metal ions (Zn2+, Sn2+, Cu2+ and Pb2+)
from PCBs and investigated by different significant parameters. The
experimental results showed that adsorption rate was linearly increasing
and later itbecomes stable due to changes in operating conditions. From the
result of this study, C-A Bent adsorbentsassist in effective separation of
heavy metal ions. Hence, it was concluded that the combination of aqua regia
leaching and bent adsorption is a more effective and economic way for the
elimination of heavy metals from PCBs.
PCBs : Printed
circuit boards
Bent : Bentonite
Clay
AC : Activated carbon
NAC : Non-activated
Carbon
NABC : Non-activated
Bentonite Clay
PSC : Peanut
Shell Carbon
C-A
Bent : Chemically
activated bent
C-A
PSC : Chemically
activated Peanut shell carbon
Co : Initial
concentration of metal ions from leached samples
Ce : Metal
ions concentration at the completion of adsorption operation
T-A
Bent : Thermally
activated bent
T-A
PSC : Thermally
activated Peanut shell carbon
Ce-I&Ce-II : Metal
ions concentration at the completion of adsorption in stage I & II
This study was carried out with utilization of the
laboratory facilities in Erode Sengunthar Engineering College and Kongu
Engineering College. The author would like acknowledge and thank his parents
for the support rendered.
- 1. D.-R. Pangavhane, M.Sadhana, A. Khandekar, and M. Rashmi, Ind. J.Res. 2(2013) 138-140.
- 2. D. Pant, D. Joshi, M.K. Upreti, and R.K. Kotnala, Waste Manag. 32 (2012) 779- 979.
- 3. S. Jaibee, N. Hisyamudin, B.M. Nor, A. Khalil, A. Rahim, F. Mohammad, S. Chee Kiong, F. Ahmad, S. Jamian, and Y. Seiji, ARPN J.Eng. Appl. Sci. 11 (2016) 2332-2335.
- 4. Monika and J. Kishore, Indian J. Community Med. 35 (2010) 382-385.
-

- 5. C.-S. Poon, Waste Manag. 28 (2008) 1449.
-

- 6. N. Padiyar, P. Tandon, and S. Agarwal, Int. J. Contemporary Dentistary. 2 (2011) 80-83.
- 7. Y.-C. Chien, H. Paul Wang, K.S. Lin, Y.J. Huang, and Y.W. Yang, Chemosphere. 40 (2000a) 383-387.
-

- 8. L.-M. Plum, L. Rink, and H. Hajo, Int. J. Environ. Res. Public Health. 7 (2010) 1342-1365.
-

- 9. H. Mattila and T. Virtanen, Chemosphere 25 (1992) 1599-1609.
- 10. N. Menad, B. Björkman, and E.G. Allain, Resour. Conserv. Recycl. 24 (1998) 65-85.
-

- 11. Y.-C. Chien and H.P. Wang, J. Environ. Sci. Heal. - Part A Toxic/Hazardous Subst. Environ. Eng. 4 (2000) 635-644.
- 12. Y. Ikushima, K. Hatakeda, N. Saito, and M. Arai, J. Chem. Phys. 108 (1998), 5855-5860.
-

- 13. Y.-J. Huang, H. Paul Wang, C.T. Li, and Y.C. Chien, Chemosphere. 40 (2000) 347-349.
-

- 14. Y.-C. Chien, H.P. Wang, K.S. Lin, and Y.W. Yang, Water Res. 34 (2000b) 4279-8283.
-

- 15. E. Kantarelis, W. Yang, W. Blasiak, C. Forsgren, and A. Zabaniotou, Appl. Energy. 88 (2011) 922-929.
-

- 16. I. Masavetas, A. Moutsatsou, E. Nikolaou, S. Spanou, and A. Zoikis-Karathanasis, Global Nest J.11 (2009) 241-247.
- 17. R. Montero, A. Guevara, and E. De La Torre, J. Earth Sci. and Eng. 2 (2012) 590-595.
- 18. M. Kerolli Mustafa, L. Ćurković, M. Ujević Bošnjak, and T. Rezić, Chem. Biochem. Eng. 31 (2017) 403-415.
-

- 19. J. Cui and L. Zhang, J. Hazard. Mater. 158 (2008) 228-256.
-

- 20. P. Xiang, Y. Zhang, and Q. Liu, IOP Conference Series: Mater. Sci. Eng. 394 (2018) 022001.
-

- 21. W. Lu, Y. Lu, F. Liu, K. Shang, W. Wang, and Y. Yang, J. Hazard. Mater. 186 (2011) 2166-2170.
-

- 22. H. Yang, J. Liu, and J. Yang, J. Hazard. Mater. 187 (2011) 393-400.
-

- 23. B. Bayat and B. Sari, J. Hazard. Mater. 174 (2010) 763-769.
-

- 24. P.-P. Sheng and T.H. Etsell, Waste Manag. Res. 25 (2007) 380-383.
-

- 25. B.-I. El-Eswed, O.M. Aldagag, and F.I. Khalili, Appl. Clay Sci. 140 (2017) 148-156.
-

- 26. M. Kumar, J.C. Lee, M.S. Kim, J. Jeong, and K. Yoo, Environ. Eng. Manag. J. 13 (2014) 2601-2607.
-

- 27. J.-P. Choudhury and M.A. Hashim, Chem. Biochem. Eng. 18 (2004) 295-302.
- 28. S.-A. Aljlil, Fares, and D. Alsewailem, Athens J. Sci. 1 (2014) 21-30.
-

- 29. A.-T. Sdiri and T.J.F. Higashi. Int. J. Environ. Sci. Technol. (2013) 1-23.
- 30. N. Abdennebi, M. Bagane, and C. Chtara, J Chem Eng Process Technol. 4 (2013) 166-170.
-

- 31. S. Budsaereechai, K. Kamwialisak, and Y. Ngernyen, KKU Res. J. 17 (2012) 800-809.
- 32. M.-F. Brigatti, L. Medici, and L. Poppi, Appl. Clay Sci. 11 (1996) 43-54.
-

- 33. S. Veli, and B. Aly, J. Hazard. Mater. 149 (2007) 226-233.
-

- 34. E. Helios-Rybicka and R. Wójcik, Appl. Clay Sci. 65 (2012) 6-13.
-

- 35. R. Sjöblom, H. Bjurström, and R. Pusch, Appl. Clay Sci. 23 (2003) 187-193.
-

- 36. Y. Kim, J. H. Kim, K. G. Lee, and S. G. Kang, J. Ceram. Process. Res., 6 (2005) 25-30.
- 37. E.-Y. Yazici and H. Deveci, Hydrometallurgy. 139 (2013) 30-38.
-

- 38. L. Flandinet, F. Tedjar, V. Ghetta, and J. Fouletier, J. Hazard. Mater. 2132 (2012) 485-490.
-

- 39. J. Hanafi, E. Jobiliong, A. Christiani, D.C. Soenarta, J. Kurniawan, and J. Irawan, Procedia - Soc. Behav. Sci. 57 (2012) 331-338.
-

- 40. R. Vijayaram, D. Nesakumar, and K. Chandramohan, Res. J. Eng. Sci. 2 (2013) 11-14.
- 41. Y. Chehade, A. Siddique, H. Alayan, N. Sadasivam, S. Nusri, and T.I.A., Int. Conf. Chem. Civ. Environ. Eng. 24 (2012) 226-234.
- 42. R. Souag, N. Kamel, M. Hammadi, Z. Kamel, D. Moudir, F. Aouchiche, Y. Mouheb, and S. Kamariz., J. Ceram. Process. Res., 16 (2015) 150-155.
- 43. T. -H. Ahn, K.B. Shim, K.H. So, and J.S. Ryou, J. Ceram. Process. Res., 15 (2014) 539-544.
-

- 44. R. Umapriya, V. Manisha, S. Vidyavathy, G. Arthanareeswaran, J. Rohan, and A.R. Poorna., J Ceram Process Res., 20 (2019) 291-300.
- 45. J. Zhu, V. Cozzolino, M.Fernandez, R.M.T. Sánchez, M. Pigna, and Q. Huang, Appl. Clay Sci. 52 (2011) 339-344.
-

- 46. F. Anjum, H. N. Bhatti, M. Asgher, and M. Shahid, Appl. Clay Sci. 47 (2010) 356-361.
-

- 47. N. Gomathi and I. Sridevi, Der Pharma Chem. 7 (2015) 219-224.
- 48. D.-H. Kwak and M. S. Kim, Mater. Sci. Appl. 4 (2013) 12-17.
-

- 49. P. Vanegas, J.R. Peeters, F. Plessers, D. Cattrysse, and J.R. Duflou, Kenya, in: Procedia CIRP. 15 (2014) 283-288.
-

- 50. B. Abeln and S. J. Lombardo, J. Ceram. Process. Res., 12 (2011) 515-520.
- 51. V.-L. Grudic, I. Boskovic, and A. Gezovic, Chem. Biochem. Eng. 32 (2018) 299-305.
-

- 52. S.-P. Kumar, S. Govindaradjane, and T. Sundararajan, Int. J. Eng. Res. Appl. (IJERA). 3 (2013) 469-473.
- 53. W.-E. Marshall and E.T. Champagne, J. Environ. Sci. Heal. Part A Environ. Sci. Eng. 30 (1995) 241-261.
-

- 54. A.- A. A Saad, Appl. Sci. Res. 5 (2010) 138-145.
- 55. V.-K. Gupta P.J. M. Carrott, M. Ribeiro Carrot, and M.L. Sauhas, Crit Rev Environ Sci Technol 39 (2009) 783-843.
-

 This Article
This Article
-
2020; 21(1): 75-85
Published on Feb 28, 2020
- 10.36410/jcpr.2020.21.1.75
- Received on Aug 27, 2019
- Revised on Nov 19, 2019
- Accepted on Dec 9, 2019
 Services
Services
- Abstract
introduction
material and methods
results and discussion
conclusions
nomenclature
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Murugesan Manikkampatty Palanisamy
-
Department of Chemical Engineering, Kongu Engineering College, Perundurai, Erode, Tamil Nadu 638060, India
Tel: +9566604416 - E-mail: engineermurugesh@gmail.com






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.