- Study on precipitation of microcrystalline boehmite from bayer process solutions
Gwang Hee Shina, Chan Woong Parka, Jong Hyeok Kangb, Sangyun Seoc, Tam Tranb and Myong Jun Kimb,*
aKorea Chemical Corp, Mokpo, Korea
bDepartment of Energy & Resources Engineering, Chonnam National University, Gwangju, Korea
cKorea Resources, Kangwon, Korea
This study was carried out to develop a process for
synthesising ultra-fine boehmite powders by precipitation from the Bayer
process liquor at a temperature within the range 85 ~ 95 oC.
The study was conducted to investigate whether boehmite produced by this
technique has the same physical properties as those prepared by hydrothermal
synthesis at elevated temperature and commercially available for use as fire
retardant in plastic making. As with gibbsite, the precipitation of boemite
requires seeding in which α-boehmite seeds were first produced by pyrolysis of
a plant ultra-fine gibbsite at 420 oC for 60 min. The boehmite
seeds of mean particle size (D50) of 0.30 μm were obtained through
subsequent hydrothermal synthesis.
This process would yield a high purity
well-crystalline boehmite of 1.30-1.47 μm mean particle size of low surface
area (2 ~ 4 m2/g) as required for commercial fire
retardants.
Keywords: Boehmite, Bayer process, Hydrolysis, Pyrolysis, Precipitation
Boehmite (α-AlOOH or Al2O3.H2O,
alumina mono- hydrate,
AMH) has found many applications in industry, including
use as a precursor for making special alumina ceramics,
as catalysts or fire retardants in plastic production. When
boehmite used as a precursor is calcined for making specialty alumina such as α-Al2O3
catalysts its crystal morphology (of different shapes such as
rhombic, hexagonal, etc.), purity and size
determine the transfor- mation
temperature and other properties of the final product [3]. As a fire retardant
boehmite decomposes to alumina (Al2O3), releasing water
and absorbing heat at a higher temperature (> 350 oC)
compared to alumina trihydrate/ATH (Al(OH)3, gibbsite) or magnesium
hydroxide (Mg(OH)2). Boehmite
fire retardants require specific particle properties (< 2 μm particles,
specific crystal shape, low BET specific surface area, and high purity, etc.).
It is well known that under hydrothermal conditions
gibbsite is converted to boehmite at a temperature in the range
100 ~ 350 oC, depending on pressure in the range
1 ~ 100 MPa [5, 15]. As an example, Panda et al. [9] could produce
nano-crystalline boehmite (crystallite size 35 ~ 75 nm) by treating
gibbsite (particle size range of P80 of 3 μm) hydrothermally at
temperatures < 350 oC and 15 ~ 55 MPa pressure.
In the temperature range 350 ~ 450 oC,
both boemite and α-Al2O3 are formed. One of many
techniques developed for the synthesis of boemite as nanowire, nanotube or as
nanoparticles (1 ~ 20 nm) of various crystallite shapes (hexagonal,
rhombic, ellipsoidal, etc.) employs hydrothermal conditions at temperature in
the range 150 ~ 250 oC under pressure for several days [7,
12-13, 16]. This was done by using different precursors as seeds,
ranging from bayerite Al(OH)3, gibbsite Al(OH)3,
to pseudo-boehmite sol gel, etc. As an example, flaky spherical boemite could
be produced from sodium aluminate (Bayer) liquor at
44.7 g/L Na2O (caustic NaOH) and alumina
set at A/C (alumina to caustic mass ratio, with A as g/L Al2O3
and C as g/L Na2O) of 1.41, using 266 g/L gibbsite seed at 180 oC
[6].
Several synthesis techniques were also proposed to produce
boehmite directly from Bayer or sodium aluminate liquors at temperatures lower
than 100 oC. Although the solubility of boehmite
(30 ~ 40 g/L Al2O3 in the temperature range
80 ~ 100 oC) is generally lower than for gibbsite
(60 ~ 120 g/L Al2O3) [6] the precipitation of
boehmite is slow and controlled via a kinetic step [14] with an
activation energy of 89 kJ/mol, compared to easier gibbsite
formation over the same temperature range 70 ~ 90 oC. To
produce pure boemite without gibbsite contamination the
precipitation has to be conducted at a very high total caustic
concentration (equivalent to 210 g/L caustic as Na2CO3)
equivalent to an A/C ratio of 0.67 [4]. Attempts to improve the
reaction rate, particle size (via promoting applomeration)
and strength (increasing via crystal growth) of boemite crystals have been made
during the crystallisation step from aluminate liquors. While
agglomeration of boemite is dependent on operating parameters
and can be enhanced by longer aging time, higher seed ratio, higher liquor
supersaturation (ie large difference of liquor Al2O3
concentration from equilibrium value), etc. crystal growth to control
crystallite size to within a certain range is difficult.
Wang and co-workers [18] found the crystal growth rate of
boehmite is independent of seed sizes or seed ratio and is in the range
0.08 ~ 2.4 μm/h at 80 oC, 4 ~ 10 times less than that
of gibbsite (2 ~ 7.2 μm/h). Panias and Krestou
[11] first precipitated nano-crystalline boehmite
(3 ~ 8 nm crystallites) by neutralising a supersaturated Bayer liquor
to pH 5 ~ 7 at 30, 60 and 90 oC using
nitric acid. Precipitates with higher crystallinity were formed
at pH 7 and 90 oC, which after aging for one week would yield
well crystalline boehmite of 22 ~ 37 μm
mean particle size. At 90 oC, nano-crystalline boemite
(30 ~ 80 nm) was produced using a concentrated sodium
aluminate liquor (120 g/L Na2O, 132 g/L Al2O3) within
24 h using 230 ~ 1200 g/L seeding with boehmite,
without which seeds the reaction is extremely slow [14]. Dash et
al. [1] found that to produce gibbsite within 8 h at a
reasonable yield (~ 10 g/L boehmite) and at atmospheric conditions
(85 ~ 95 oC, 1 atm). The Bayer liquors have to be
supersaturated at high aluminate concentration > 150 g/L Al2O3,
and the A/C ratio has to be in the range 1.0 ~ 1.2
with at least 100 g/L boehmite seeds of < 10 μm size added.
By adding an organic modifier, boehmite formation is
enhanced, minimising the co-precipitation of gibbsite. Dash et al.
[1] found that by adding 100 ~ 300 mg/L tartaric acid the nucleation
of gibbsite is prohibited and boehmite is produced at a lower temperature of
80 oC. Wang et al. [18] also realised that by adding ethanol
the activation energy of precipitation is reduced to 13.7 kJ/mol, shifting the
reaction kinetics to a diffusion control mechanism, thus promoting boehmite
formation.
The techniques studied and reported to date have been
difficult in scaling up for plant production due to long residence time
required for aging, either during hydrothermal processing or via precipitation
at 85 ~ 95 oC. These methods could not produce
microcrystalline boehmite with high
crystallinity in the narrow size range 1 ~ 2 μm nor having
a low surface area. Therefore, precipitation under Bayer
process conditions was studied to determine optimum conditions of liquor composition, temperature and reaction time for producing
micro- crystalline boehmite suitable
to be used as a fire retardant. The materials synthesised from this study are
also compared with existing commercial products currently on the market.
Materials
and reagents
The Bayer liquor used in this study was prepared from
a bauxite ore sample from Weipa, Australia (Table 1), the same
material has been used in the production of gibbsite/alumina trihydrate (ATH)
at the Daejoo-KC alumina refinery at Mokpo, South Korea. As in plant operation
the liquor used for the study was achieved by digesting bauxite to a caustic
solution to achieve a caustic concentration of 210 ~ 220 g/L (as Na2CO3)
and A/C ratio of 0.65 ~ 0.68.
All other reagents used in this study are of analytical
grade.
Analytical
techniques
Aluminum and caustic contents of the synthetic mother
liquors and precipitation filtrate were analysed by acid-base
neutralization/titration method. The precipitation (%) of boehmite/AMH based on
the alumina analyses of the mother liquor and the filtrate was calculated from
the following equation:
Precipitation (%) = [Alumina of mother liquor (g/L)
– Alumina in filtrate (g/L)] / Alumina of mother liquor
(g/L) × 100%
The prepared α-boehmite seeds used in this study were
analysed using a Particle Size Analyzer (PSD, S3500,
Microtrac), and the crystal phase was determined by
an X-Ray Diffractometer (XRD, Ultima IV, Rigaku). A
Surface Area Analyser (Quadrasorb-SI, Quantachrome) was used to
confirm the specific surface area of the seeds. The thermal decomposition and
composition of boemite (AMH) was evaluated using a thermal analyzer (DTG-60H,
Shimadzu). A Scanning Electron Microscope (SEM, SSX-550, Shimadzu) was also
used to evaluate the shapes of the particles produced. Chemical analysis was
performed on digested samples using an Inductively Coupled Plasma Optical
Emission Spectrometer (ICP-OES, ICPS-7510, Shumadzu).
Experimental
procedures
The following stages were conducted for the experiments:
(a) Liquor used: The Bayer mother liquor from which
boehmite was precipitated from was prepared by varying the A/C ratio, seed mass
ratio (mass of boehmite seeds/mass of Al2O3 in the
liquor),
(b) Precipitation of seeds: α-boehmite seeds were
prepared for the precipitation of boehmite by calcination of a KC superfine
(< 2 μm) gibbsite/ATH product (alumina trihydrate, Al(OH)3)
at 420 oC. The calcined material was then subjected to a hydrothermal
reaction, filtered and ground to produce nano-AMH
(boemite) material as shown in Fig. 1.
(c) Boehmite precipitation – The boehmite precipitation experiments were carried out by adding the above
freshly prepared seeds into the Bayer sodium aluminate liquor at different
temperatures, caustic soda concentrations, and amounts of seeds used.
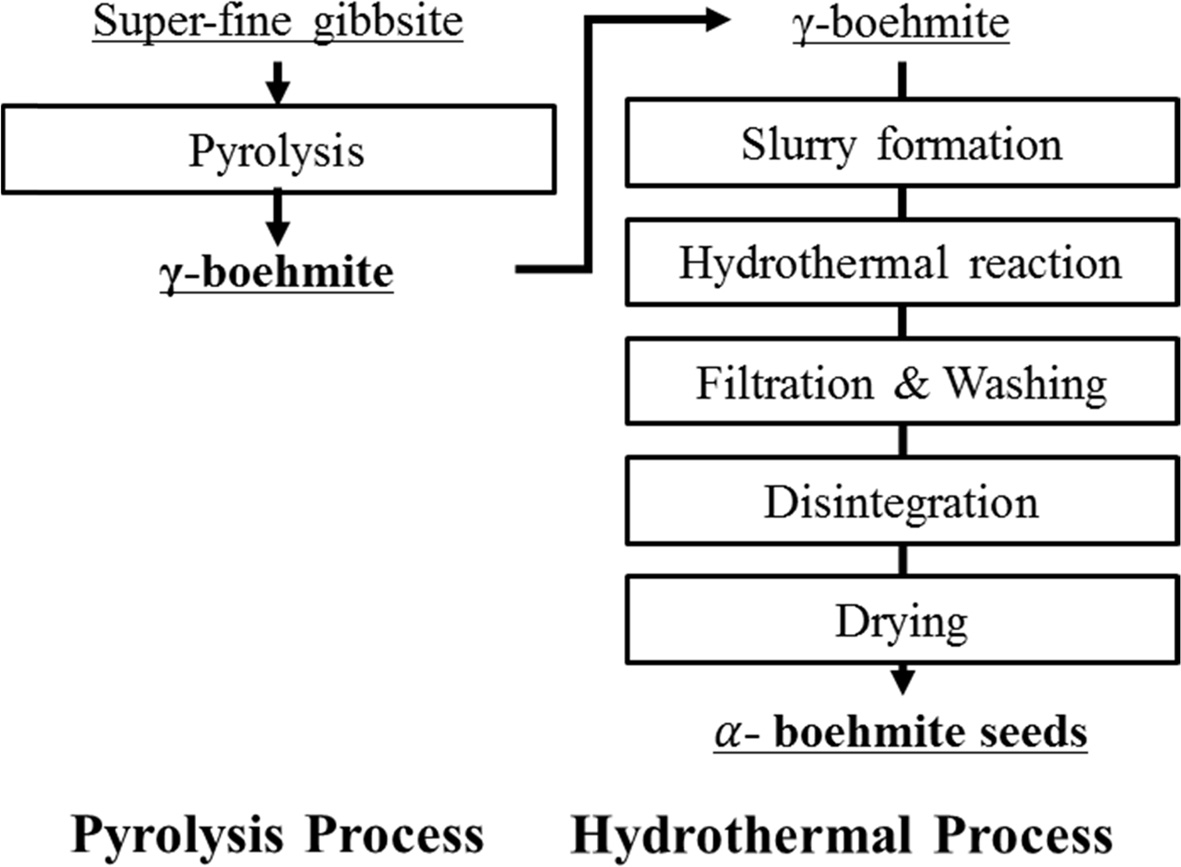
|
Fig. 1 Flowsheet of manufacturing α-boehmite seeds. |
|
Table 1 Chemical composition (%) of
the raw material (bauxite from Weipa, Australia). |

Thermodynamic
Study
A thermodynamic simulation of the precipitation conditions
was conducted using HSC program [8]. Data obtained (Fig. 2) confirm that
boehmite could be thermodynamically precipitated from 95 oC,
in preference to gibbsite. The formation of gibbsite peaks at
110 oC and decreases slowly as the temperature rises to 200 oC.
More boehmite is formed in this temperature range (95 ~ 200 oC).
Seed
preparation
Seed preparation is a critical step for this process.
Seeds were produced by roasting gibbsite (superfine < 2 μm gibbsite/ATH
from KC plant) at different temperatures to determine the condition for making
boemite. XRD analysis (Fig. 3) shows that gibbsite remains stable at less than
350 oC during roasting. Above
400 oC γ-boehmite/AMH is formed. The pyrolysis
of superfine gibbsite to form g-boehmite seeds was therefore conducted at
420 oC subsequently for the study. After hydrolysis to
190 ~ 200 oC this material is converted to α-boemite/AMH.
Optimum
boehmite precipitation temperature
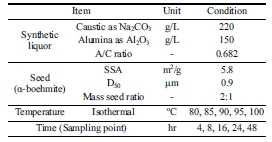
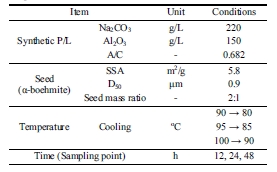
To determine the optimum precipitation temperature, tests
were conducted at the caustic NaOH concentration of
220 g/L (as Na2CO3), pregnant liquor A/C ratio 0.68,
mass seed ratio (boemite AlOOH seed/Al2O3 in the liquor,
both in g) of 2.0 with the isothermal (fixed) temperature
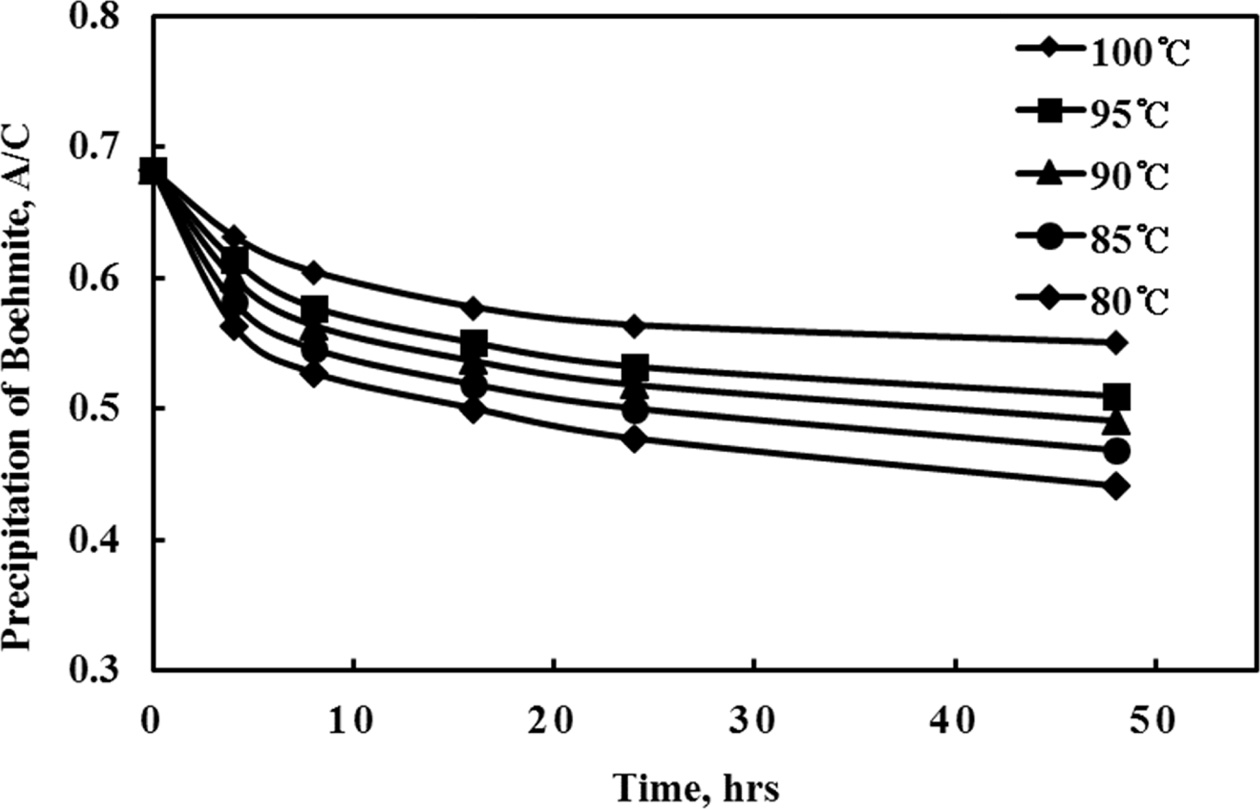
varying from 80 to 100 oC. It was found that gibbsite
was formed when the precipitation temperature was 85 oC or less and
boemite could only be produced when the temperature was set at higher than 90
oC. These results were confirmed by XRD analysis of the boehmite products
as shown in Fig. 4. It was also observed that the precipitation rate was very
slow, up to 48 h at which time the A/C ratio still has not reached a steady
state (ie. equilibrium has not been reached) as shown in Fig. 5.
Conditions for the tests to determine the optimum
precipitation temperature are tabulated in Table 2.
Precipitation
with slow cooling
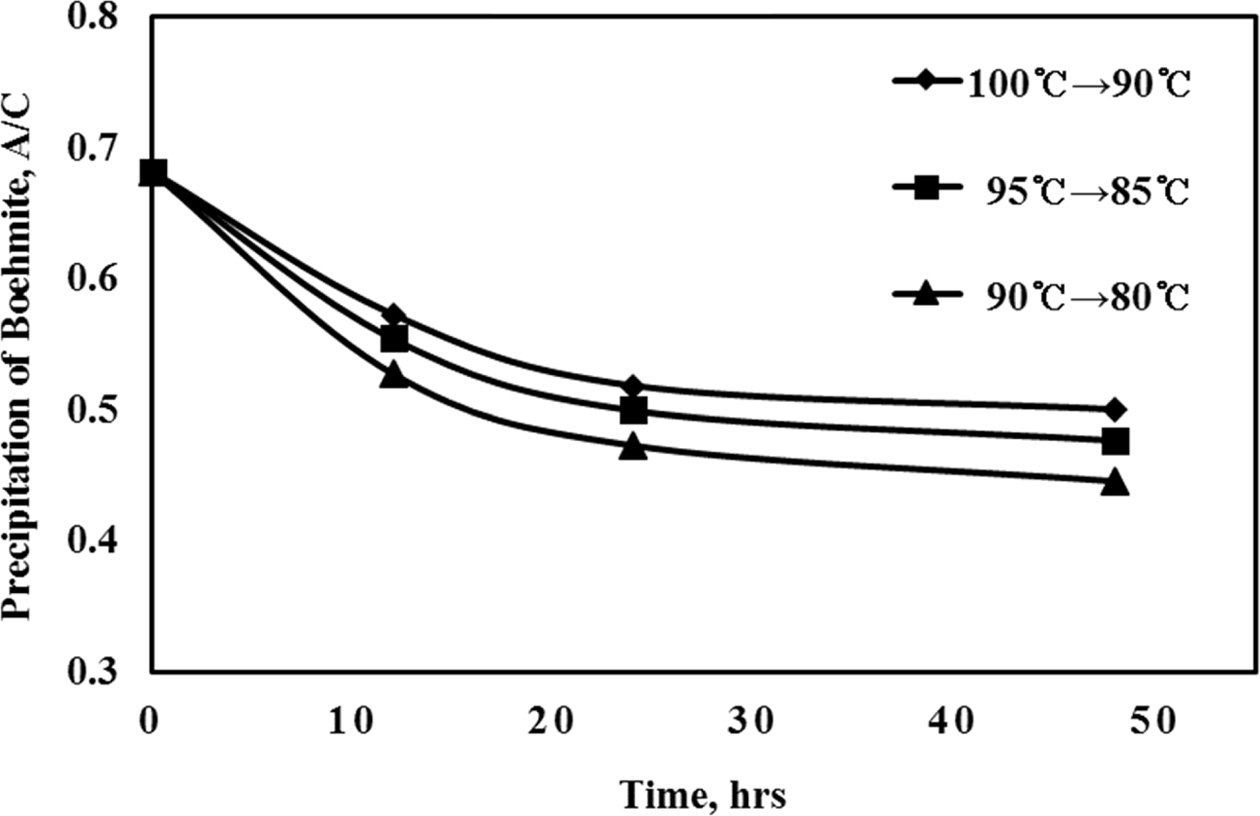
In the Bayer process, the precipitation rate of
gibbsite/ATH increases depending on the difference between the initial
precipitation temperature and its final value afterwards after cooling. Based
on this, it was also applied to boehmite precipitation and the cooling
rate was 0.4 oC/hr from the starting temperature.
Conditions for this test series are tabulated in Table 3.
The experimental results (Fig. 6) show that the lower the
initial temperature is, the higher precipitation rate and yield, as expected
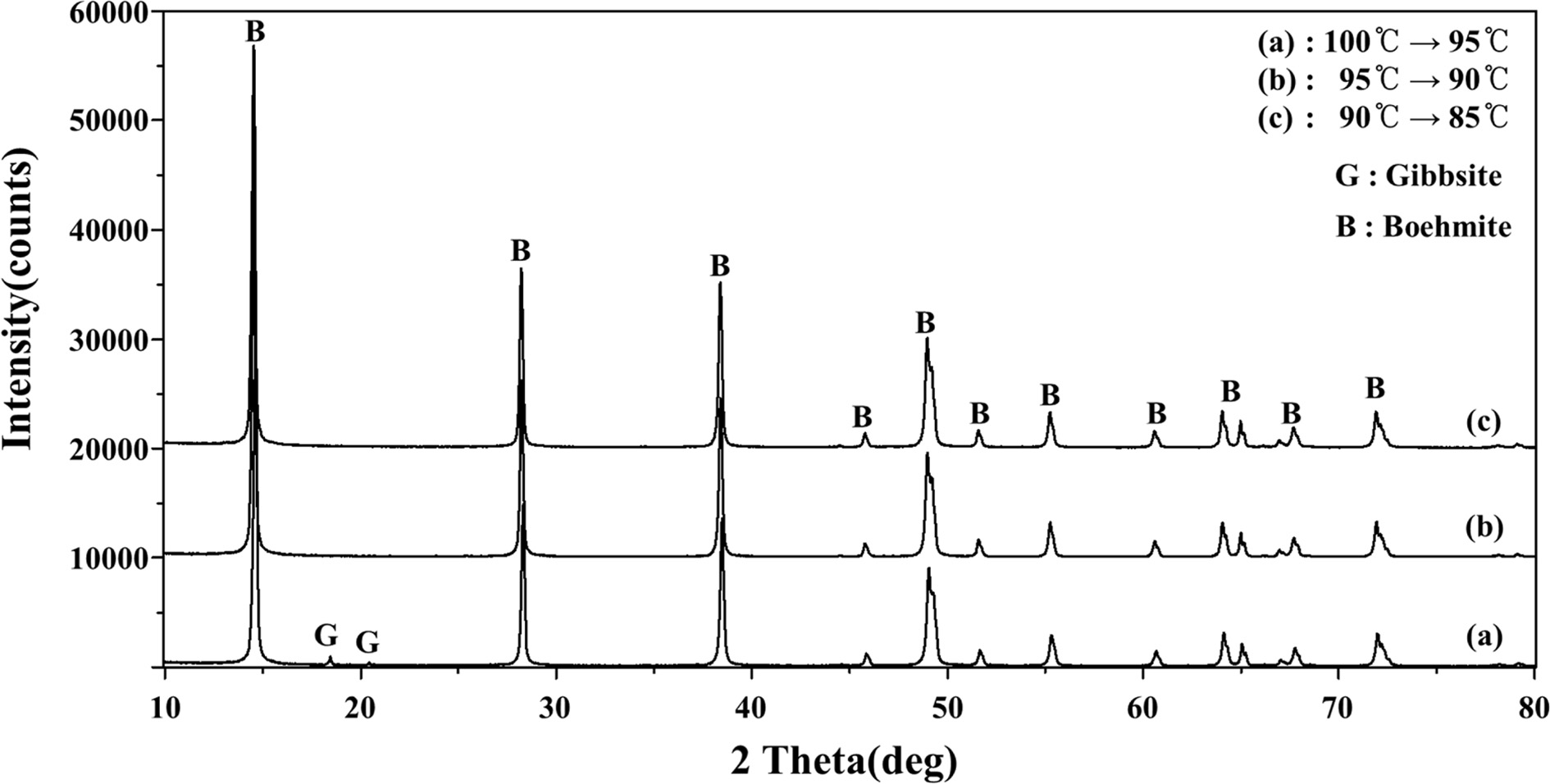
with lower final A/C ratio. It was also confirmed by XRD analysis (Fig. 7) that
gibbsite will be formed if the final temperature is less than 85 oC.
The effect of other parameters such as A/C ratios, seed
ratio, etc was also studied and results are as follows.
Precipitation
at different A/C ratios
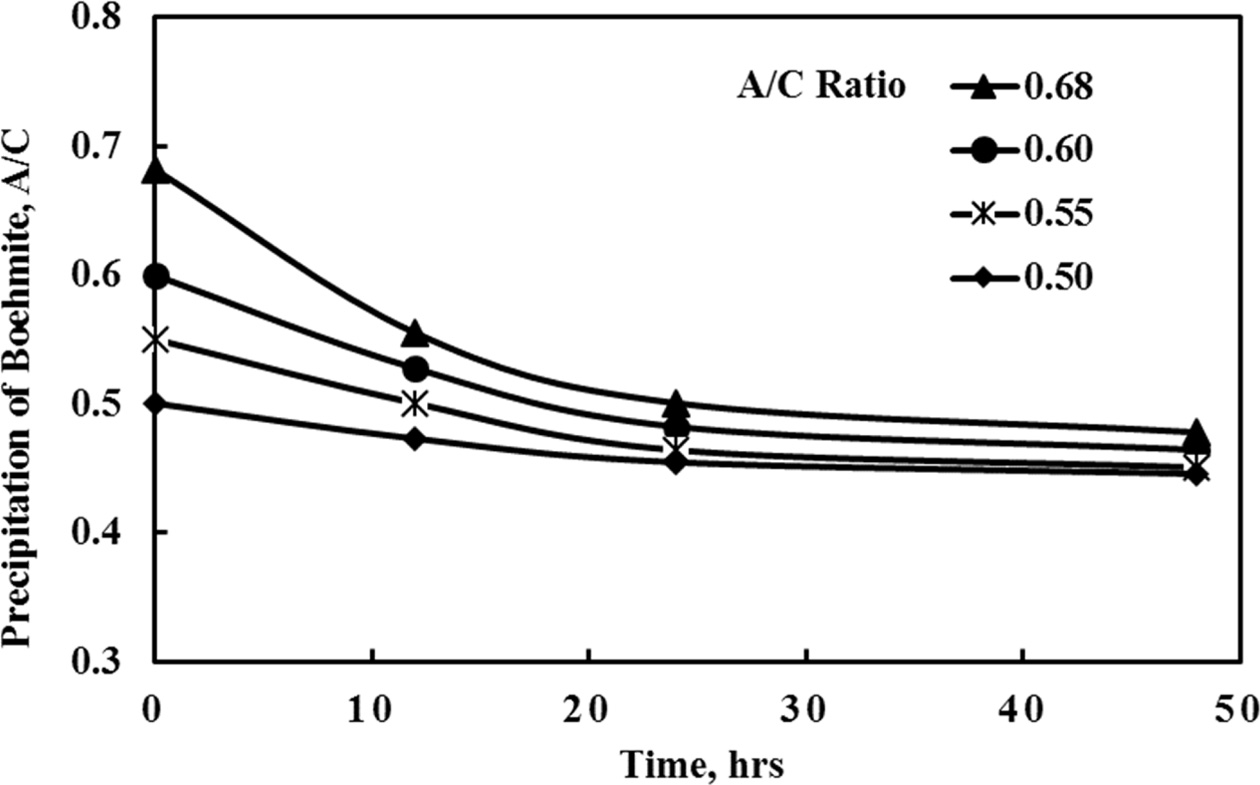
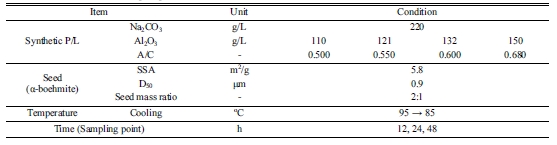
Tests were conducted to evaluate the effect of varying A/C
ratios during boehmite precipitation according to conditions shown in Table 4.
As expected at a higher A/C ratio the precipitation rate
and yield are higher. At all conditions the steady state would be reached
slowly merging to an A/C ratio of ~ 0.45 after 48 h (Fig. 8).
It is best therefore to use a liquor having an initial A/C
ratio of 0.68 to achieve maximum product yield. This value therefore was chosen
for all tests.
Effect
of seed mass ratio (AlOOH mass/Al2O3 mass in liquor)
Since the boehmite precipitation process requires a higher
precipitation temperature compared top gibbsite, a relatively high seed mass
ratio is required to increase the initial precipitation rate. The effect of
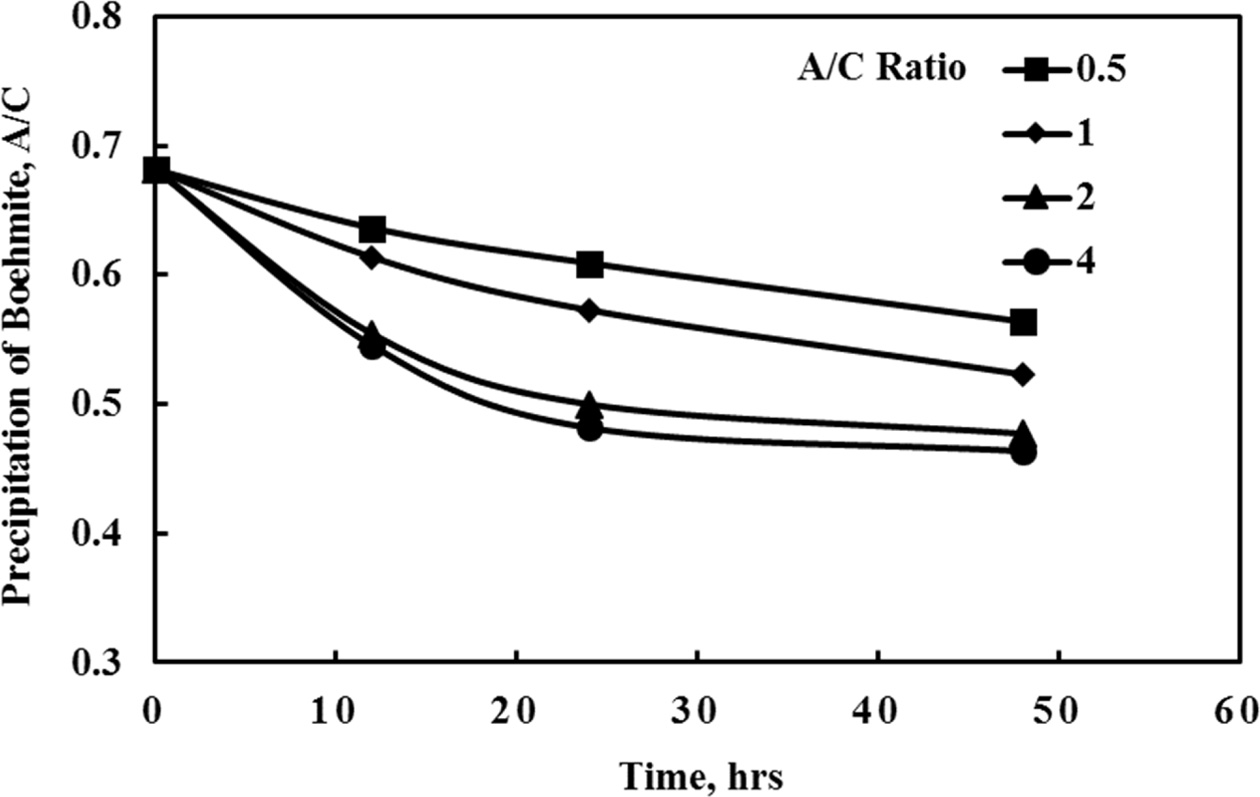
seed mass ratio is shown in Fig. 9, showing a higher precipitation rate at a
higher seed mass ratio in the range 0.5 ~ 2:1. Above this 2:1 ratio,
there is not much of a change of A/C ratio with respect to the seed mass ratio.
The 2:1 seed mass ratio was therefore chosen as optimum conditions as shown in
previous results.
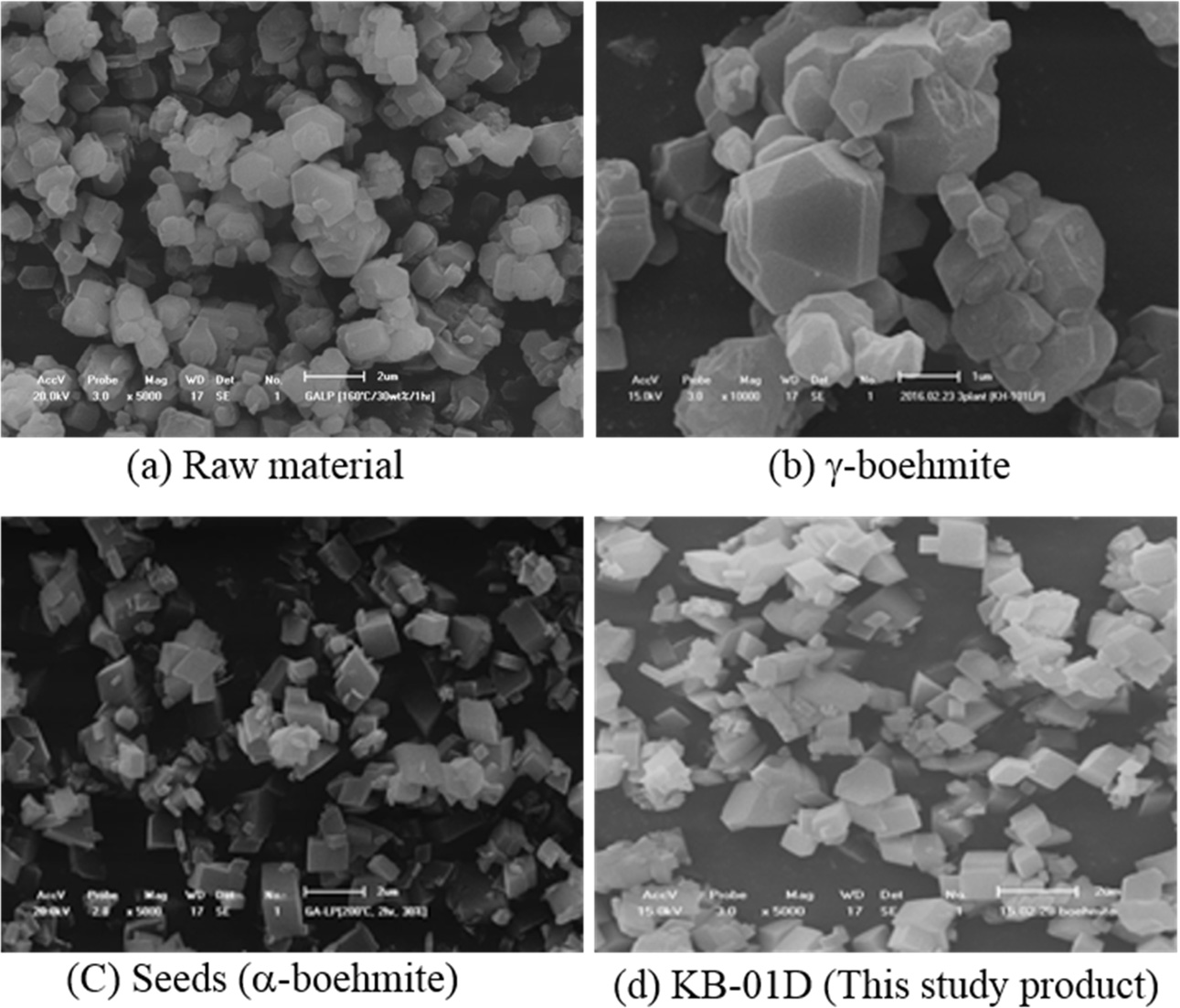
Under SEM typical materials through different stages of
the process are shown in Fig. 10. It is clear that the raw material (gibbsite)
was transformed to g-boehmite after heating to 420 oC, when the
calcined crystallites tended to sinter to hexagonal agglomerates
> 2 μm in sizes. After hydrothermal treatment at 190 ~ 200 oC
these agglomerates were broken and the a-boemite seeds became cubic in form
with reduced sizes to mostly < 2 μm. This mainly cubic shape is
maintained after precipitation into the final boemite product.
Adopting the optimum conditions for precipitating boemite, the products obtained are very compatible to typical commercial
products such as Nabaltec APYRAL AOH-30 and SR-100 MES
produced by TOR MINERALS – USA (Table 5). The boemite
produced from this study meets the specifications for particle size with D50
in between 1 ~ 2 μm, surface area 3 ~ 6 m2/g.
The purity of boehmite from this study is better than these commercial
products.
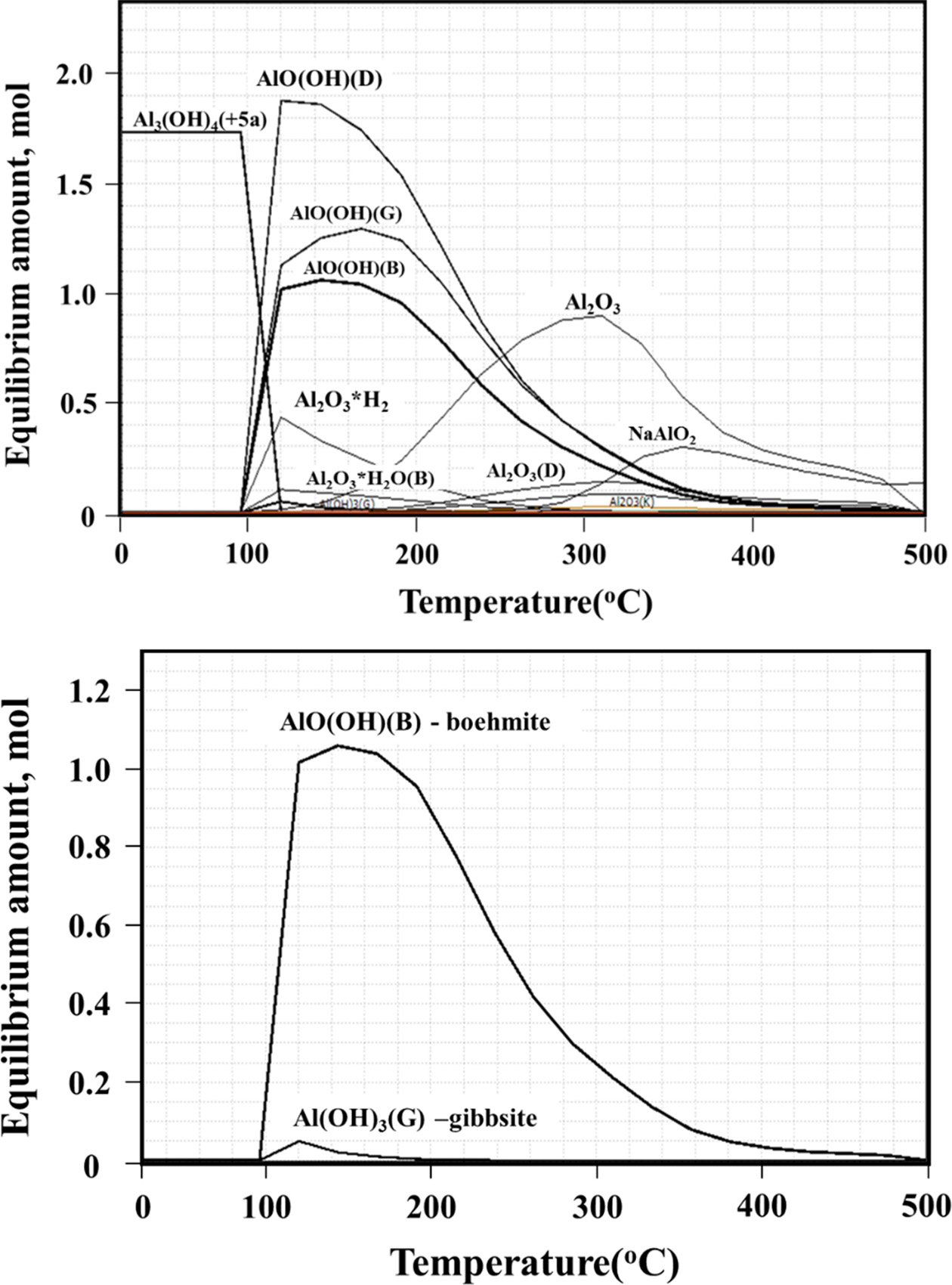
|
Fig. 2 HSC simulation of boehmite precipitation at different temperatures. |
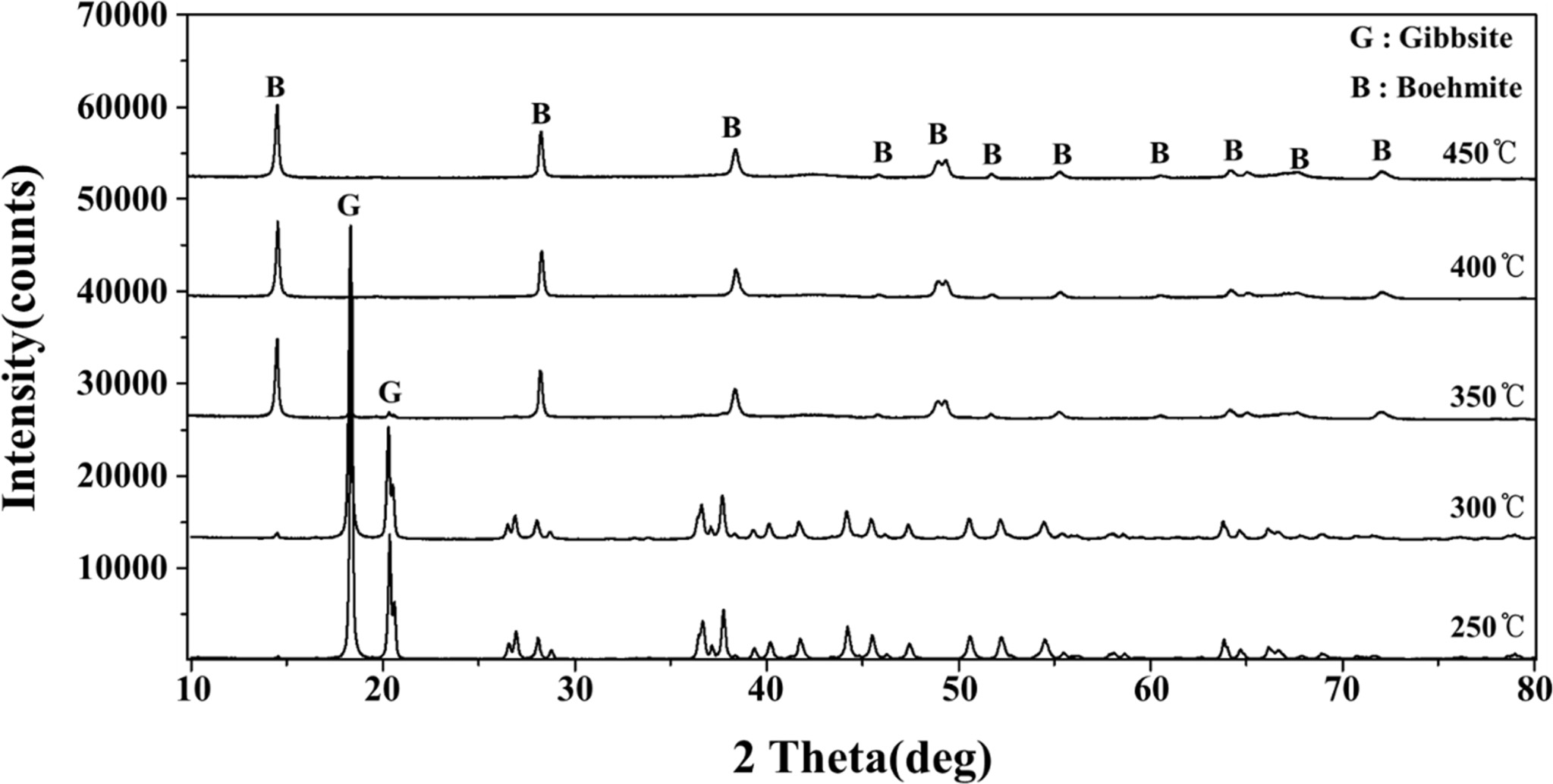
|
Fig. 3 XRD patterns of KC superfine plant gibbsite/ATH after calcining at different temperatures in the range 250 ~ 450 oC. |
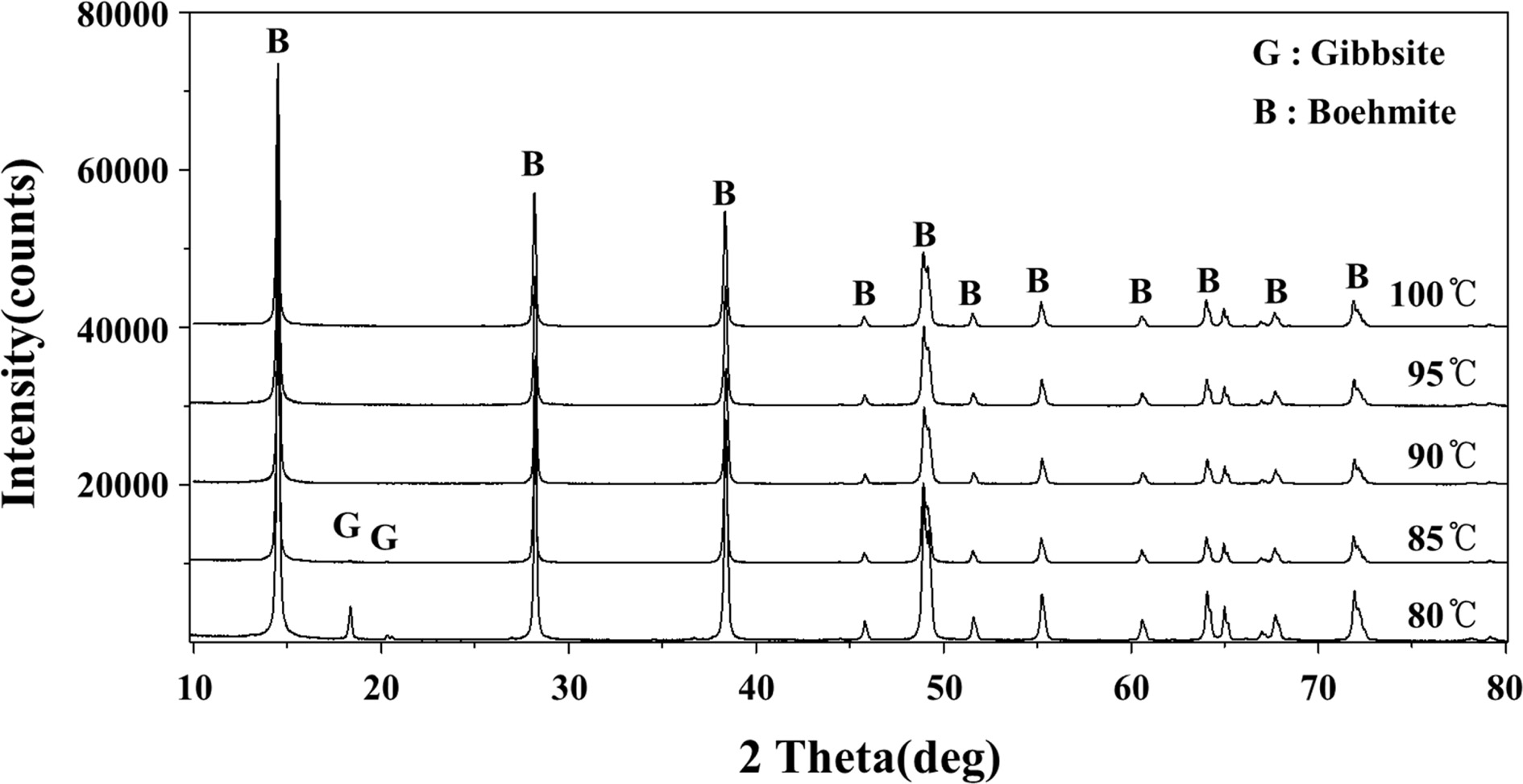
|
Fig. 4 XRD patterns of boehmite products according to different precipitation (isothermal) temperatures, showing contamination of gibbsite when precipitation was at 80 oC. |
|
Fig. 5 Change of boehmite precipitation A/C ratio with set temperature |
|
Fig. 6 Change of boehmite precipitation A/C at different cooling regimes |
|
Fig. 7 XRD patterns of boehmite product according to different cooling regimes showing boemite can only be formed at a final temperature > 85 oC. |
|
Fig. 8 Change of boehmite precipitation A/C ratio at different initial conditions. |
|
Fig. 9 Change of boehmite precipitation A/C ratio according to different seed ratio. |
|
Fig. 10 SEM analysis showing morphology of the different materials throughout the synthesis of α-boehmite. |
|
Table 2 Conditions for determining optimum temperatures for making boemite product |
|
Table 5 Comparison of the boehmite product produced from this study to other commercial products. |
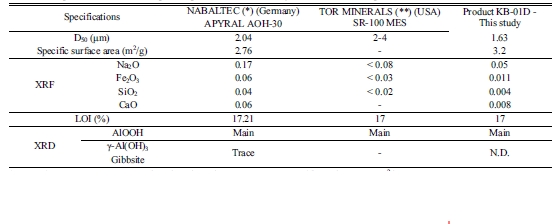
*Note : (*) APYRAL AOH-30 product data sheet shows D50 1.8 μm, Specific surface area 3 m2/g |
This study was conducted to investigate the production
of 1 ~ 2 μm particle sizes required commercially as a fire
retardant. Boehmite seeds (mean particle size 0.30 μm) were first produced by
pyrolysis of a plant superfine gibbsite
(< 2 μm) at 420 oC, which was then subsequently
treated by a hydrothermal process at 190 ~ 200 oC.
These seeds were then used in a precipitation step at optimum conditions
determined from this study.
Optimum conditions for precipitation are : caustic (NaOH)
concentration of 220 g/L measured as Na2CO3,
alumina/caustic (A/C) ratio of 0.68, seed mass ratio (boemite/Al2O3
in the liquor) of 2:1 and precipitation temperature of 85 to 95 oC
and in 48 h. Following these conditions the process would yield a high quality
crystalline boehmite of 1 ~ 2 μm in sizes, 2 ~ 4 m2/g
surface area. The boemite produced from this process are
compatible to other high quality commercial products.
The authors acknowledge the support of Daejoo-KC’s
laboratories for providing analyses for this study.
- 1. B. Dash, B.C. Tripathy, I.N. Bhattacharya, S.C. Das, C.R. Mishra, and B.K. Mishra, Hydrometallurgy 95 (2009) 297-301.
-

- 2. S. Ghanizadeh, X. Bao, B. Vaidhyanathan, and J. Binner, Ceramic International 40 (2014) 1311-1319.
-

- 3. F. Karouisa, M. Boualleg, M. Digne, and P. Alphonse, Advanced Powder Technology 27 (2016) 1814-1820.
-

- 4. E. Konigsberger, L. Konigsberger, and D. Ikievski, Hydrometallurgy 110 (2011) 33-39.
-

- 5. G. Li, Y. Liu, D. Liu, L. Liu, and C. Liu, Materials Research Bulletin (2010) 1487-1491.
-

- 6. G. Liu, Z. Li, X. Li, T. Qi, Z. Peng, and Q. Zhou, International Journal of Minerals, Metallurgy and Materials 24[8] (2017) 954-963.
-

- 7. D. Mishra, S. Anard, P.K. Panda, and R.P. Das, Material Letters 42 [1-2] (2000)38-45.
-

- 8. Outotec HSC Chemistry software, available from : �https://www.outotec.com/products/digital-solutions/hsc-chemistry/?gclid=CjwKCAiAkrTjBRAoEiwAXpf9CStfCuWGa-E64 DLVhHCW4YQL-YYZQ6VqjJLL-pehH9EltjWLF5ESVBo CH74QAvD_BwE
- 9. P.K. Panda, V.A. Jalel, and S. Usha Devi, Journal of Material Science 41 (2006) 8386-9389.
-

- 10. D. Panias and I. Paspaliaris, Erzmetall 56[2] (2003) 75-81.
- 11. D. Panias and A. Krestou, Poeder Technology 175 (2007) 163-173.
-

- 12. S.P. Santos, A.C.V. Coelho, S.H. Santos, and P.K. Kiyohara, Materials Research 12[4] (2009) 437-335.
-

- 13. P.S. Santos, R.F. Neves, and H.S. Santos, Colloid and Polymer Science 271[2] (1993) 197-200.
-

- 14. C. Skoufadis, D. Panias, and I. Paspaliaris, Hydrometallurgy 68 (2003) 57-68.
-

- 15. <!--[endif]-->W.L. Suchanek, Journal of the American Ceramic Society 93 (2010) 399-412.
-

- 16. J. Yang and R. Frost, Journal of Inorganic Chemistry (2008) Article ID 602198.
-

- 17. Z. Wang, J. Zhang, R. Xu, and Z. Guo, in Light Metals 2002, edited by Carlos E.Suariez (The Minerals, Metals and Materials Society, 2002) p107-112.
-

- 19. Z. Wang, R. Xu, L. Yang, and Z. Guo, in Light Metals 2012, edited by Carlos E.Suariez (The Minerals, Metals and Materials Society, 2012) p120-125.
 This Article
This Article
-
2020; 21(1): 50-56
Published on Feb 28, 2020
- 10.36410/jcpr.2020.21.1.50
- Received on Jul 19, 2019
- Revised on Nov 15, 2019
- Accepted on Nov 22, 2019
 Services
Services
- Abstract
introduction
experimental
results and discussion
conclusions
acknowledgement
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Myong Jun Kim
-
Department of Energy & Resources Engineering, Chonnam National University, Gwangju, Korea
Tel : +82 62 530 1727 Fax: +82 62 530 1729 - E-mail: junkim@jnu.ac.kr






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.