- Effects of adding Y(NO3)3·6H2O on the phase change behavior and thermal conductivity of Aluminum Nitride ceramics
Jun Ki Chunga,*, Seongmin Jub and Tae Kwon Haa
aCenter for Industrial Technologies of Non-Ferrous Metals, Gangneung-wonju National University, Gangneung 25457, Korea
bSchool of Electrical Engineering and Computer Science, Gwangju Institute of Science and Technology, Gwangju 61005, Korea
The phase change behavior and
thermal conductivity of Aluminum nitride (AlN) ceramics with Y(NO3)3·6H2O
and Y2O3 as an additive were studied. Sintering was
performed in the temperature of 1,900 oC for up to 3 h under a N2
atmosphere to optimize the sintering conditions for each composition. The
microstructure and assemblage of the secondary phase have a significant effect
on the final thermal conductivity of the sintered AlN. The mechanical property
and thermal conductivity of the AlN composition of using the Y2O3
additive were improved by adding Y(NO3)3·6H2O,
which decreased the porosity. At 13.56 wt% Y(NO3)3·6H2O,
the AlN ceramic exhibited the highest strength of 375 MPa, the highest hardness
of 10.60 GPa, and the highest thermal conductivity of 200.2 W/m·K.
Keywords: AlN, Y(NO3)3·6H2O, Phase change behavior, Thermal conductivity, Mechanical property
Aluminum nitride (AlN) may be highly suitable as
substrates and packages for IC/LSI because of its high thermal conductivity
(theoretical value of 320 W/m·K), low dielectric constant (8.0 at 1 MHz), and
thermal expansion coefficient (4.8 × 10−6 K−1 at 20-500 ℃)
that is close to that of silicon [1-3]. However, because of its high
covalent bonding, it is difficult to sintering. Several sintering
additives, such as CaO, CaC2, C, CaO-Al2O3,
and Y2O3, were reported to be useful for the fabrication
of high densification and thermal conductivity AlN ceramics
[4-6]. Y2O3 is widely used as a sintering additive
because it forms binary eutectics at temperatures around of
~1,800 ℃ with native Al2O3, which is present
on the surface of AlN particles, resulting in a material with high density and
high thermal conductivity [7, 8]. A typical feature in ceramic processing
is the addition of a limited fraction of sintering additives, typically oxides,
to promote densification. However, an associated disadvantage
is that non-negligible alterations may occur of the bulk property for which the
ceramic phase has been originally selected. The thermal conductivity of poly
phase ceramics is strongly affected by internal phase geometry and other
microstructural details [9-11]. High thermal conductivity constitutes an
attractive property of AlN ceramics for high-power semiconductor
devices [12, 13]. However, the usual preparation of dense polycrystalline
AlN bodies involves the additive Y2O3 which, by virtue of
its good wet-ability of the AlN grain surface, enables ready densification by
pressureless sintering [4, 14, 15]. As substrates and packages, the
thermal conductivity of AlN ceramics is very important. Most studies look at
the influence of different sintering additives on the thermal conductivity of
AlN ceramics.
In this work, we studied to
compare the sintering behavior and crystalline phases change related with the
densification of AlN with Y2O3 and Y(NO3)3·6H2O
additives.
Commercially available AlN powders (Grade H, Tokuyama
Soda, Japan) was used as a starting material. First, the
Y(NO3)3·6H2O (99.99%, High Purity Chemicals,
Japan) or Y2O3 (99.9%, High Purity Chemicals, Japan) was
dissolved in ethanol, and the AlN powders were dispersed in the solution using
ultrasonic technology. The Y(NO3)3·6H2O and Y2O3
were added into a AlN suspension and then sonicated for 24
h to obtain a metal ion doped AlN suspension via surface adsorption.
After dried and calcinated (500 ℃), the mixed powders were
densified by hot pressing at 1,900 ℃ for 3 h under a pressure
of 20 MPa in flowing N2. The sintering additive
of Y(NO3)3·6H2O was added in the following
amounts: 10.1198, 11.8684, 13.5638, and 15.2594 wt% (equivalent to
6, 7, 8 and 9 wt% Y2O3 respectively), referred to here
after as A-6YP, A-7YP, A-8YP and A-9YP for the sintered samples. For
comparison, monolithic AlN was also prepared in the same process. The
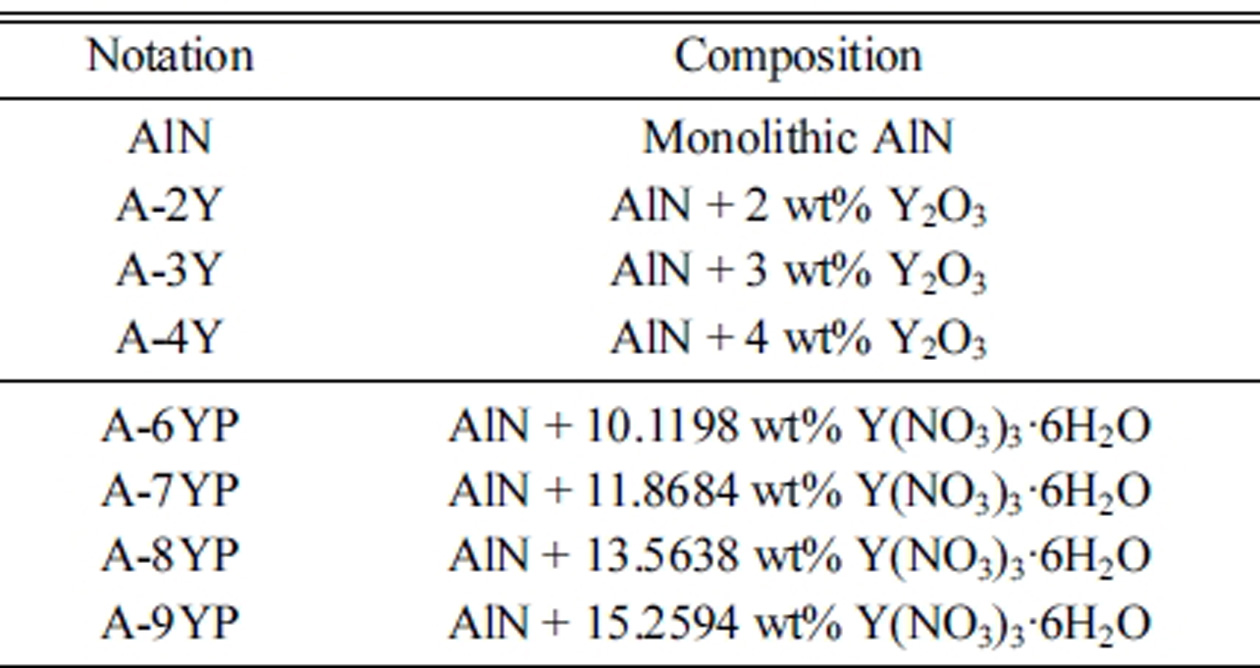
composition of powder mixture was indicated in Table 1.
Crystalline and 2nd
phases were identified with X-ray diffraction
pattern (D/MAX-2500V, RIGAKU) with Cu Kα
radiation (λ = 1.5406 Å). All the samples for mechanical test were prepared by diamond saw and
then grinded by diamond disc. Mechanical properties of specimen were evaluated
by four-point flexural strength (5882, Instron, USA) with a cross head speed of
0.5 mm/min and micro vickers hardness tester (HM124, Akashi, Japan) at a
load 9.8 N for 5 sec. The dimension of mechanical test specimen was
3 × 4 × 40 mm (width × length × height)
and the inner span and outer span were 10 and 30 mm, respectively. Fracture
surfaces of the pellets were observed by Field emission scanning electron microscopy (FE-SEM, S-4700, HITACHI, Japan). Thermal diffusivity measurement
(LFA 457, NETZSCH, Germany) at room temperature is measured by the laser flash
method. Thermal conductivity (K) is calculated using a heat capacity (Cp)
of AlN at room temperature where λ and d are the thermal
diffusivity and the density, respectively [16].
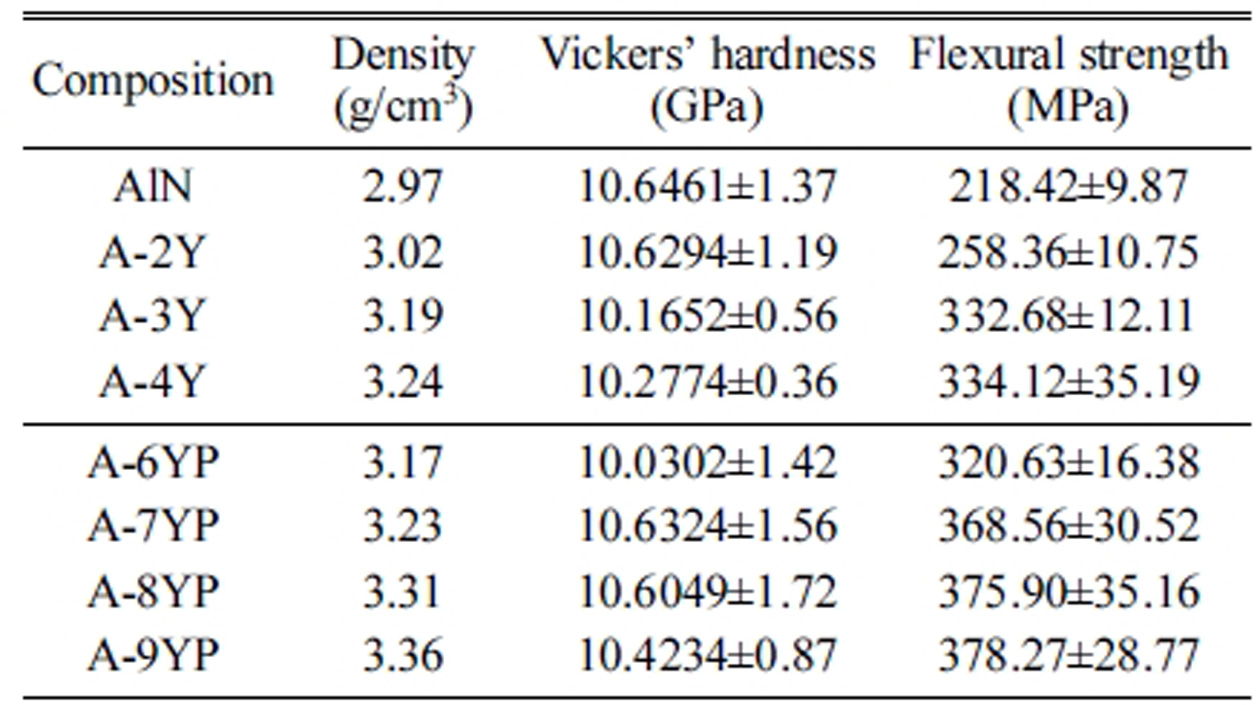
The densities and mechanical
properties of the sintered AlN ceramics are summarized in Table 2. The density
increases with the additive content. Compared with AlN when the additive is Y2O3,
better mechanical properties, such as Vickers’ hardness, flexural strength, are
obtained for sintered samples when the additive is ≤8 wt% Y(NO3)3·6H2O,
A-8YP exhibits the highest strength of 375 MPa, hardness of 10.60 GPa. This
high density is due to the formation of an aluminum oxy nitride (Al–O–N) liquid
by reaction between surface alumina and AlN. The addition of Y2O3
promotes more extensive liquid formation due to reaction to form a quaternary
liquid (Y–Al–O–N) at lower temperature.
Fig. 1 gives the X-ray powder
diffraction patterns for the all samples after sintering at 1,900 ℃
for three hours. Each composition shows strong peaks associated with AlN in
addition to minor peaks associated with the secondary grain boundary phase.
Fig. 1(a) showed peaks for AlN, YAlO3 (YAP) on
the 2 wt% of Y2O3. The YAP phases
disappeared with increasing Y2O3 content and then Y3Al5O12
(YAG) phases (3 wt% of Y2O3) Y4Al2O9
(YAM) phases (4 wt% of Y2O3) appeared.
But Fig. 1(b) showed peaks for AlN, YAP and YAG phases with increasing Y(NO3)3·6H2O
content. Because compared with AlN when the additive is Y2O3,
the improved dispersion properties are attributed to the increase in reactivity
between Al2O3 and Y(NO3)3·6H2O.
Al2O3 peaks were not detected
because the amount of residual Al2O3 is very small and
below the detection limit of the equipment. Moreover, Al2O3
occurs as a thin surface layer on AlN grains. Because of this surface layer and
residual spinel, thermal conductivity is very low for this sample, as can be
seen in Fig. 3. This means that the amount of added Y2O3
was insufficient to fully react with residual Al2O3 and
to purify the AlN and to complete the oxygen removal from both the lattice and
the surface.
From previous reports [17, 18], the Y2O3
decomposed from Y(NO3)3·6H2O reacts
with Al2O3, which is also present on the surface of AlN
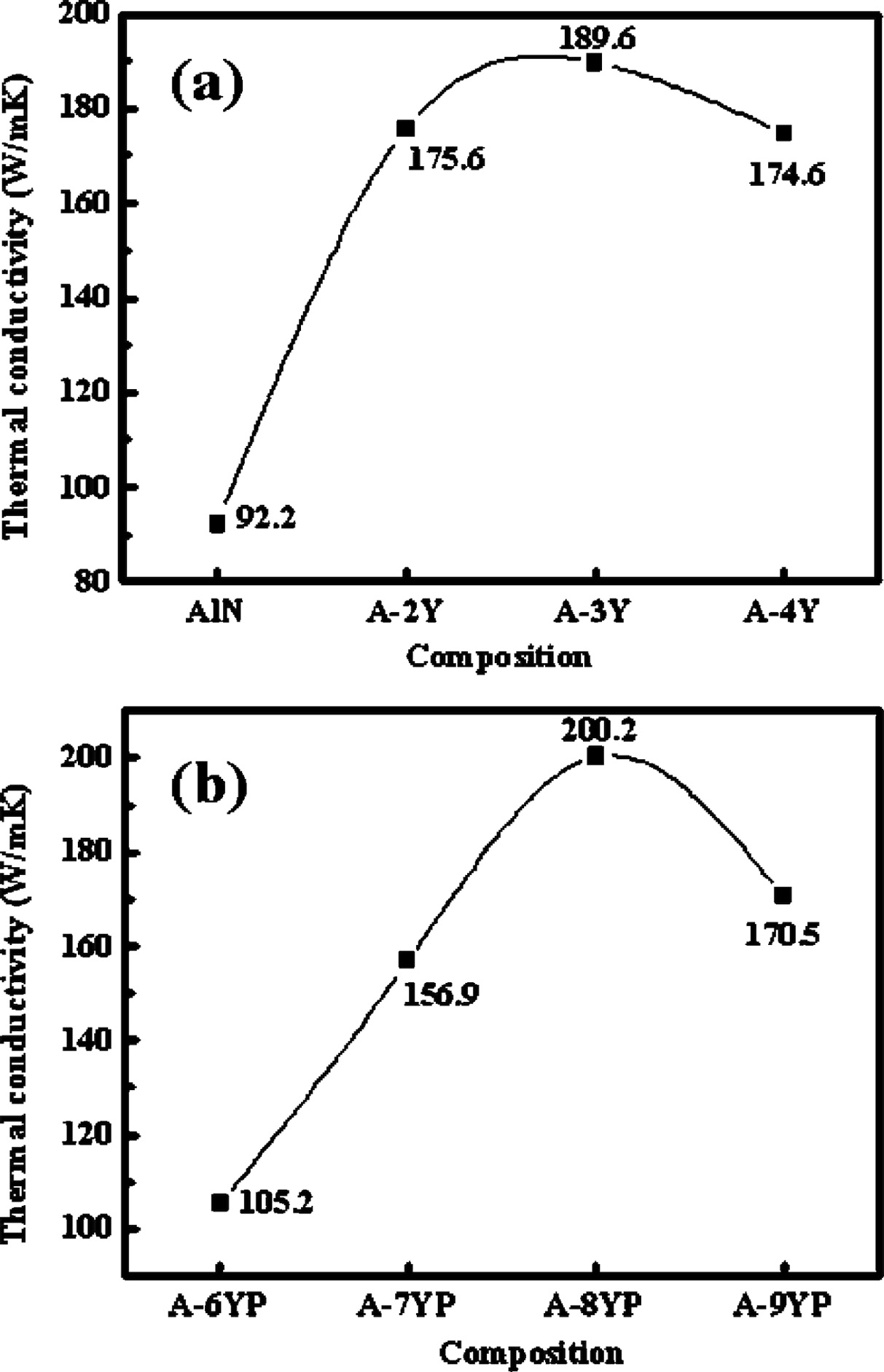
particles, to form yttrium aluminates: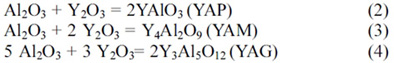
The formation of liquid phase (Y4Al2O9
and/or Y3Al5O12) during sintering process will
play an important role in the densification of the AlN ceramics.
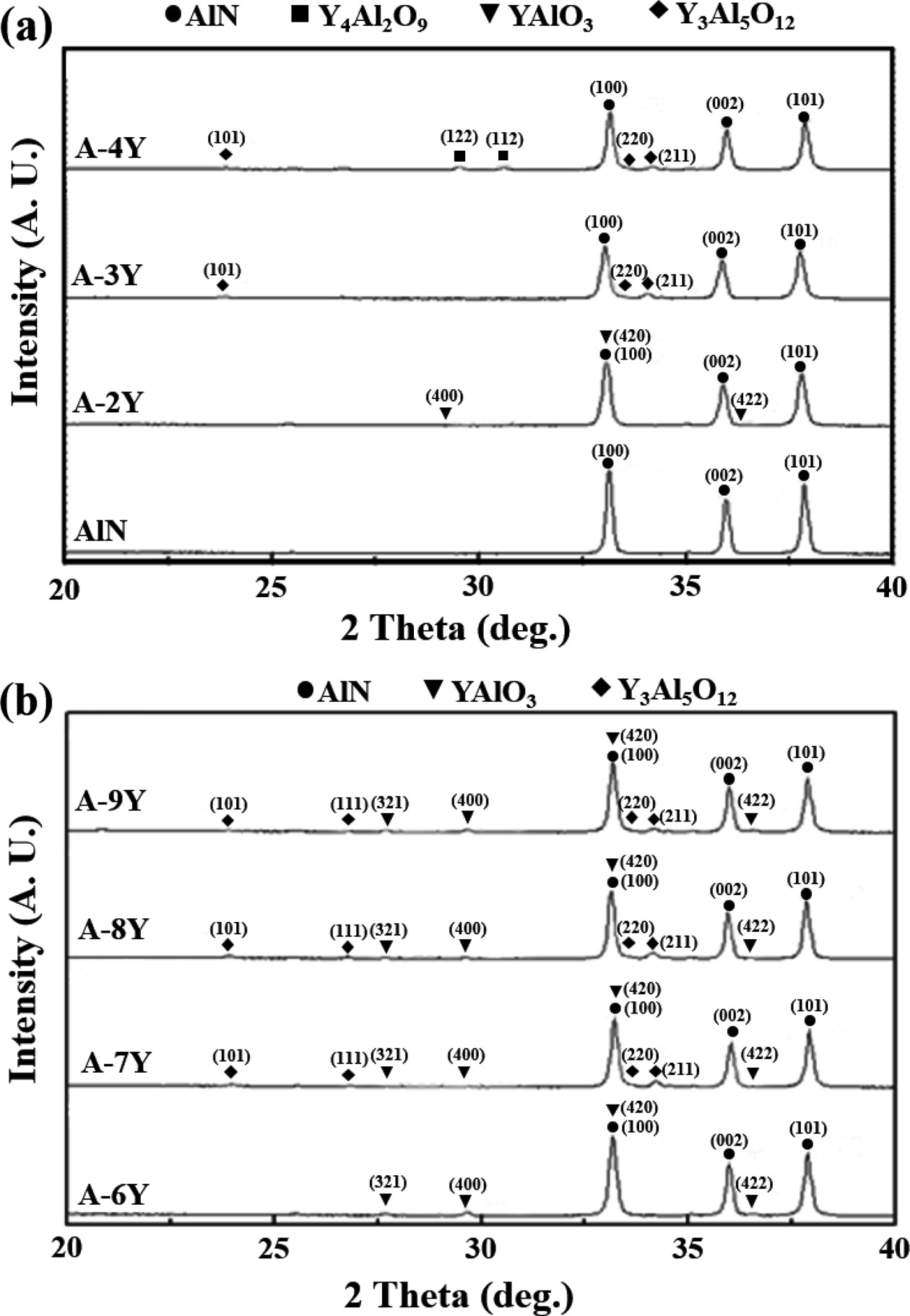
The FE-SEM microstructure of composition containing Y2O3
and Y(NO3)3·6H2O is shown in Fig. 2. The
secondary phase (white) shows up at the grain junctions only and not along
grain edges. Compared with AlN when the additive is Y2O3,
the addition of Y(NO3)3·6H2O appears to have a
further effect of changing the wetting behavior of the liquid phase with
respect to the AlN grains. Indeed, the dihedral angle of the secondary phase at
the grain junction is high. This has a significant effect on the thermal
conductivity (k).
Jackson et al. [19]
suggest that thermal conductivity rises to a maximum and then decreases due to
the increase in volume fraction of aluminates with further Y2O3
addition.
|
Fig. 1 X-ray powder diffraction patterns of AlN addition of Y2O3 and Y(NO3)3·6H2O. |
|
Fig. 2 Back-scattered electron SEM fractured surface images of AlN specimens sintered at 1,900 ℃ : (a) AlN, (b) A-3Y, (c) high magnification of (b), (d) A-6YP, (e) A-8YP, (f) high magnification of (e). |
|
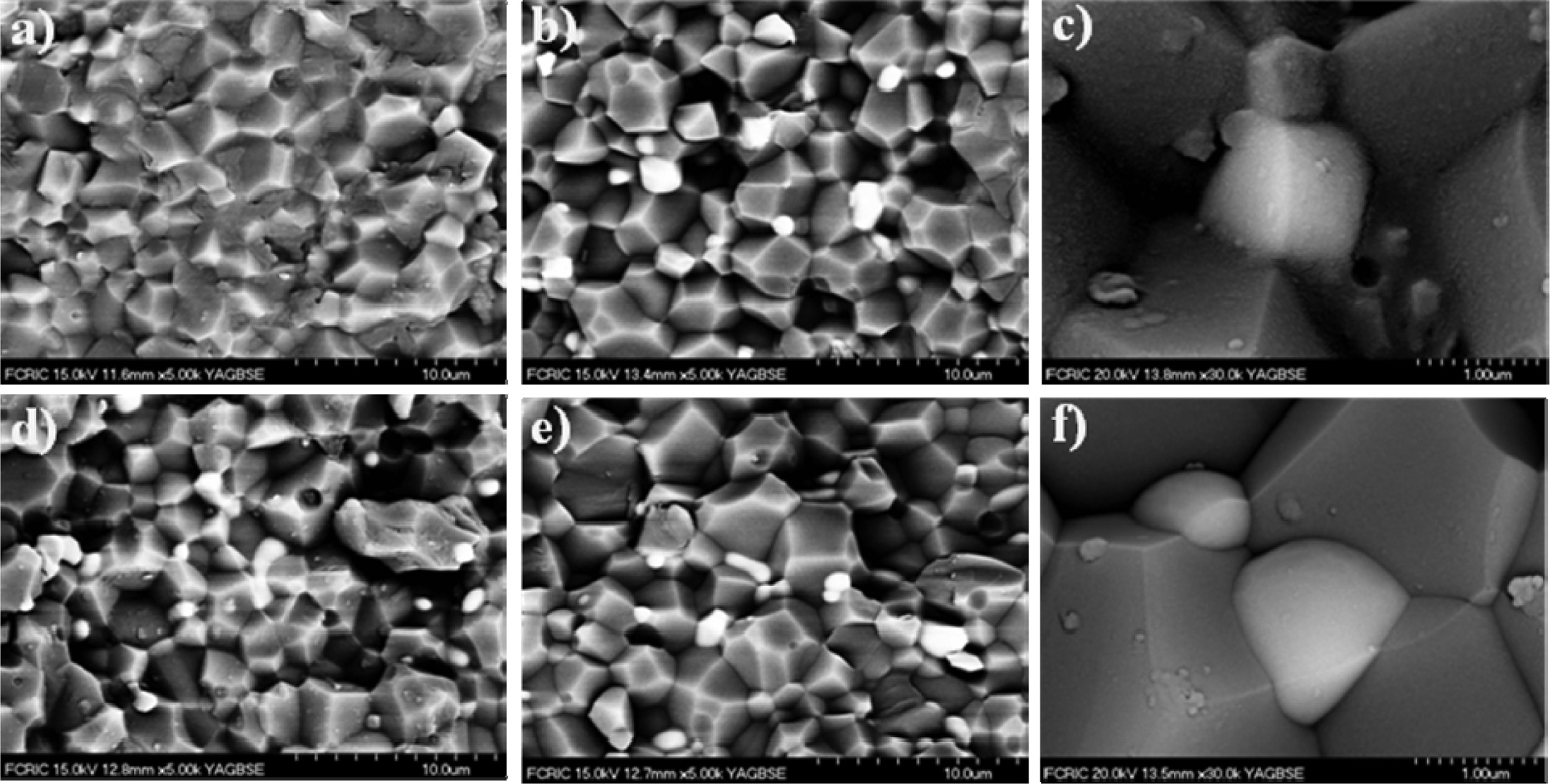
Fig. 3 Variation in thermal conductivity of AlN addition of Y2O3 and Y(NO3)3·6H2O. |
Fig. 3 shows the trend in thermal conductivity of the compositions.
The thermal conductivity of the aluminate phase
is low (<10 W/m·K), hence as the volume fraction increases,
a decrease in the overall thermal conductivity is
expected. The thermal conductivity of AlN sintered without sintering additives
has a significantly low value of 92.2 W/m·K. Therefore, the effect of the
additive has a major impact on the conductivity in two ways: (a) it removes
oxygen from particle surface and (b) a micro structural change occurs as the
aluminates de-wets the grain boundaries and segregates to the grain junctions
leading to AlN–AlN grain boundary contact. Also, this figure shows, that
compositions of using the Y(NO3)3·6H2O
additive have higher thermal conductivity than composition of using
the Y2O3 additive. Because of
the oxide layer was consumed to produce YAP, YAG as secondary phases.
Furthermore, it was shown by the experimental work of Medraj et al. [20]
that YAP wets the surface of AlN more than YAG or YAM if all
the experimental conditions are considered. Also,
the presence of YAP phase will prevent AlN–AlN surface contact. But higher YAP
content is associated with lower thermal conductivity.
Aluminum nitride (AlN) ceramics were prepared by
hot-pressing with Y(NO3)3·6H2O and Y2O3
as sintering additive. When increasing Y2O3 content, the
YAlO3 (YAP) phase disappeared and then Y3Al5O12
(YAG) phase (3 wt% of Y2O3) Y4Al2O9(YAM)
phase (4 wt% of Y2O3) appeared. But increasing Y(NO3)3·6H2O
content showed peaks for AlN, YAP and YAG phase. Because compared with AlN when
the additive is Y2O3, the improved dispersion properties
are attributed to the increase in reactivity between Al2O3
and Y(NO3)3·6H2O. The mechanical properties
and thermal conductivity are obtained for sintered samples when the additive is
≤13.5638 wt% Y(NO3)3·6H2O, A-8YP specimen
exhibits the strength of 375 MPa, hardness of 10.60 GPa and the highest
thermal conductivity of 200.2 W/m·K. The addition of Y(NO3)3·6H2O
appears to have a further effect of changing the wetting behavior of the liquid
phase with respect to the AlN grains. It was confirmed that AlN using Y(NO3)3·6H2O
showed relatively higher thermal conductivity and mechanical properties than
the Y2O3.
This work was financially supported by Ministry of Science
and ICT(MSIT) in Korean government and Korea Industrial Technology Association
(KOITA) as “A study on the programs to support collaborative research
among industry, academia and research institutes”
- 1. L.M. Sheppard, Am. Ceram. Soc. Bull. 69 (1990) 1801-1812.
- 2. G.A. Slack, J. Phys. Chem. Solids 34 (1973) 321-335.
-

- 3. R.R. Tummala, J. Am. Ceram. Soc. 74 (1991) 895-908.
-

- 4. Y. Kurokawa, K. Utsumi, and H. Takamizawa, J. Am. Ceram. Soc. 71 (1988) 588-594.
-

- 5. P.S. de Baranda, A.K. Knudsen, and E. Ruh, J. Am. Ceram. Soc. 76 (1993) 1751-1760.
-

- 6. E. Hagen, Y. Yu, T. Grande, R. Høier, and M.-A. Einarsrud, J. Am. Ceram. Soc. 85 (2002) 2971-2976.
-

- 7. L.A. Qiao, H.P. Zhou, H. Xue, and S.H. Wang, J. Eur. Ceram. Soc. 23 (2003) 61-67.
-

- 8. W.J. Tseng and C.J. Tsai, J. Mater. Process. Technol. 146 (2004) 289-293.
-

- 9. F.M. Xu, Z.J. Zhang, X.L. Shia, Y. Tana, and J.M. Yang, Journal of Alloys and Compounds 509 (2011) 8688-8691.
-

- 10. R.C. Progelhof, J.L. Throne, and R.R. Ruetsch, Polym. Eng. Sci. 16 (1976) 615-621.
-

- 11. H.J. Ott, Plastic Rubber Process. Appl. 1 (1981) 9-14.
- 12. W. Werdecker and F. Aldinger, IEEE Trans. C.H.M.T. 7 (1984) 399-404.
-

- 13. L.M. Sheppard, Am. Ceram. Soc. Bull. 69 (1990) 1801-1815.
- 14. Komeya, K., Inoue, H., and Tsuge, A., Yogyo-Kyokai-shi 89 (1981) 330-333.
-

- 15. Kuramoto, N., Taniguchi, H., Numata, Y., and Aso, I., Yogyo-Kyokai-shi 93 (1985) 517-521.
-

- 16. R. Koba, J. Harris, R. Youngman, M. Mallinger, and L.B. Max, Microwave RF (1997) 156-166.
- 17. A.V. Virkar, T.B. Jackson, and R.A. Cutler, J. Am. Ceram. Soc. 72 (1989) 2031-2042.
-

- 18. K. Watari, M. Kawamoto, K. Ishizaki, J. Mater. Sci. 26 (1991) 4727-4732.
-

- 19. T.B. Jackson, A.V. Virkar, K.L. More, R.B. Dinwidie, and R.A. Cutler, J. Am. Ceram. Soc. 80[6] (1997) 1421-1435.
-

- 20. M. Medraj, M. Entezarian, and R.A.L. Drew, Sintering 99 Conference Proceedings (2000) 307-312.
 This Article
This Article
-
2020; 21(1): 1-4
Published on Feb 28, 2020
- 10.36410/jcpr.2020.21.1.1
- Received on Dec 31, 2018
- Revised on Nov 18, 2019
- Accepted on Dec 9, 2019
 Services
Services
- Abstract
introduction
experimental
results and discussion
conclusions
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Jun Ki Chung
-
Center for Industrial Technologies of Non-Ferrous Metals, Gangneung-wonju National University, Gangneung 25457, Korea
Tel : +82-33-640-3018 Fax: +82-33-640-2245 - E-mail: junki@gwnu.ac.kr






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.