- Modifications of structural and optical properties of copper oxide thin films by thermal annealing
In-Sub Han, Ji-Soo Park and Il-Kyu Park*
Department of Materials Science and Engineering, Seoul National University of Science and Technology, Seoul 01811, Korea
The structural modification of
copper oxide thin films was investigated by controlling the thermal annealing
atmosphere and temperature, which in turn affected their optical and electrical
properties. Copper oxide thin films were deposited by spray pyrolysis
deposition at 300 oC to give a uniform surface consisting of
submicron-size grains with cubic Cu2O crystalline structure. As the
Cu2O thin films were thermally annealed at less than 700 oC,
they were transformed into the CuO phase. However, a mixed phase of CuO and Cu2O
was observed at temperatures above 800 oC. As the thermal
annealing temperature was increased from 400 to 700 oC, the
optical bandgap energy of the copper oxide thin films was decreased from 2.54
to 1.91 eV and the electrical charge carrier concentration was decreased
gradually due to the improved crystalline quality. In this way, the crystalline
structure of the copper oxide and its corresponding optical and electrical
properties could be controlled by thermal annealing.
Keywords: CuO, Cu2O, Spray pyrolysis deposition, Thermal annealing
Copper oxide semiconductors have received strong attention
for application to optical, electrical, and chemical devices
such as solar cells, light-emitting diodes, transparent
heaters, thin film transistors, and photocatalysts [1-7].
Copper oxide forms two stable crystalline phases: CuO
and Cu2O [5-7]. Cu2O has a direct energy bandgap
between 2.1 and 2.6 eV [8-10]. The many advantages offered by Cu2O
thin films include a long minority carrier diffusion length, a high hole
concentration of 1016-1019 cm-3,
and a high hole mobility of 50-100 cm2/V·sec at
room temperature [8-10]. Therefore, they have been applied
to the light absorbing layer or hole transport layer of solar cells
and of photoelectrochemical cells for
water decomposition [8-10]. The other crystalline phase, CuO,
exhibits n-type semiconductor properties with a
bandgap energy of 1.3-2.1 eV and offers chemical stability,
non-toxicity, and low cost processing [11-14]. These characteristics make these
oxides a promising material system for optoelectronic applications.
Recent research on the fabrication of copper oxide thin
films has included hydrothermal processing, electro- deposition, sputtering
deposition, atomic layer deposition, and
ultrasonic-assisted spray pyrolysis deposition (SPD) [8-13].
SPD has received strong attention because it provides
comparatively low cost processing and offers a larger area for mass production
compared to other vacuum technologies [12, 13]. In principle, SPD is a
hybrid method of a wet process and chemical vapor deposition. Metal compounds
dissolved in a source solution are transferred in the form of a spray and are
thermally decomposed on the substrate surface to nucleate and grow the thin
film [12, 13]. The structural and optical properties of the copper oxide
thin films in the SPD system are influenced by the source flow rate, growth
atmosphere, and substrate temperature [12]. For copper oxide,
the formation of Cu2O with a cubic structure and CuO with
a monoclinic structure is sensitive to the deposition temperature and
atmosphere, which makes it difficult to control these two crystalline phases
[12]. In addition, the effect of post thermal annealing on the structural,
electrical, and optical properties of the copper oxide thin
films has not been fully elucidated. In this paper, we investigated the effect
of thermal annealing atmosphere and temperature on the structural properties of
the copper oxide thin films deposited by SPD and their corresponding effects on
the films’ optical and electrical properties.
Fabrication
Copper oxide thin films were deposited on glass substrates
using ultrasonic-assisted SPD. The glass substrates were cleaned in acetone,
methanol, and deionized (DI) water for 5 min to remove organic pollutants and
then loaded into a commercial SPD system (Nano SPD-TV500, Ceon, Korea). The
source solution was prepared by dissolving 2 mM of copper acetylacetonate (Cu(acac)2, Sigma Aldrich), used
as a chelating agent to improve the Cu(acac)2 solubility, and 4 mM of ethylenediamine (C2H4(NH2)2), Sigma
Aldrich) in methanol. The solution was stirred at room temperature for 4 hrs
and finally changed to a clear and homogeneous solution. The copper oxide thin films were
deposited at 300 oC using an N2 carrier gas for 60,
120, and 180 min. The N2 flow rate was
maintained constant at 15 L/min. To investigate the effect of thermal
annealing on the optical and electrical properties of the copper oxide thin
films, rapid thermal annealing (RTA, ECOPIA RTP-1300) was performed at
temperatures between 400 and 800 oC in the N2 and O2 atmosphere. The thermal annealing
time was fixed at 5 min with a ramping rate of 25 oC/min.
The flow rate of the atmosphere gas was 10 L/min.
Characterization
The morphologies of the copper
oxide thin films were observed by scanning electron microscopy (SEM,
Hitachi S-4800). The crystalline structure of the
copper oxide thin films was measured by X-ray
diffraction (XRD, PANalytical Empyrean). The electrical
properties of the copper oxide thin films were characterized using
Hall effect measurement (HMS-3500, ECOPIA).
The transmittance and UV-visible absorption spectra of the copper oxide thin
films were measured by using a UV-Vis-NIR spectrophotometer (Cary 5000,
Agilent).
The copper oxide thin films were deposited at 300 oC
to fabricate the Cu2O crystalline structure. Previous results
reported for the SPD-growth of copper oxide thin films showed that the divalent
Cu2O was preferentially deposited rather than the CuO
phase at temperatures lower than 400 oC [12]. To investigate
the structural evolution during the deposition, the deposition time was varied
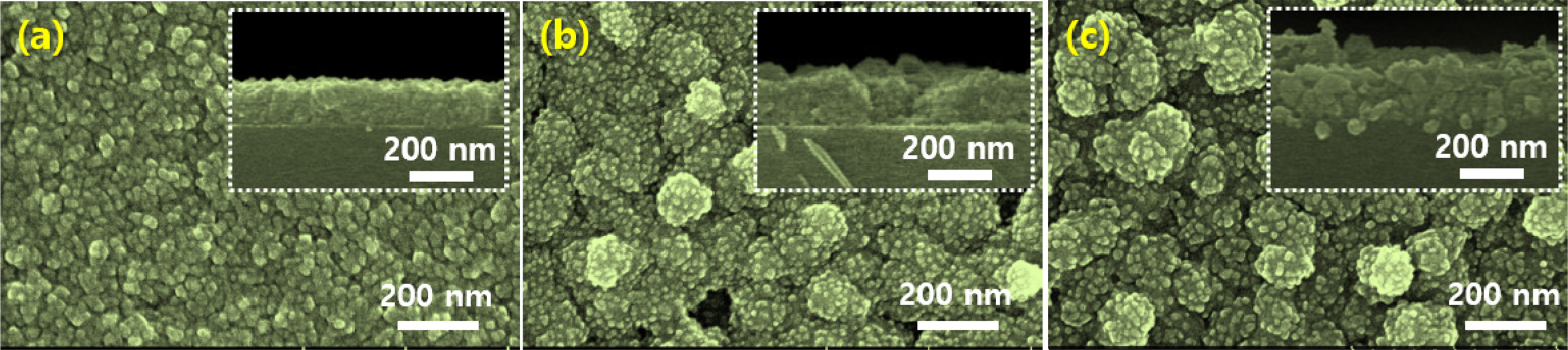
from 60 to 180 min. Figure 1 shows the surface and cross-sectional SEM images
of the copper oxide thin films deposited on the glass substrate with variation
of the deposition time. As shown in Fig. 1(a), surface of
the copper oxide thin films showed a uniform morphology
consisting of nano-crystalline grain. Because the growth
temperature was lower than 400 oC, the atomic mobility on the
substrate surface can be very low. This results in the formation of
nano-crystalline grains. As the growth time was increased from 60 to 120
and to 180 min, larger grain size consisting of nano-crystalline
particulates were observed. The limited surface atomic
diffusion enabled the small grain size to be maintained even as the deposition
time was increased, as shown in Figs. 1(b) and (c).
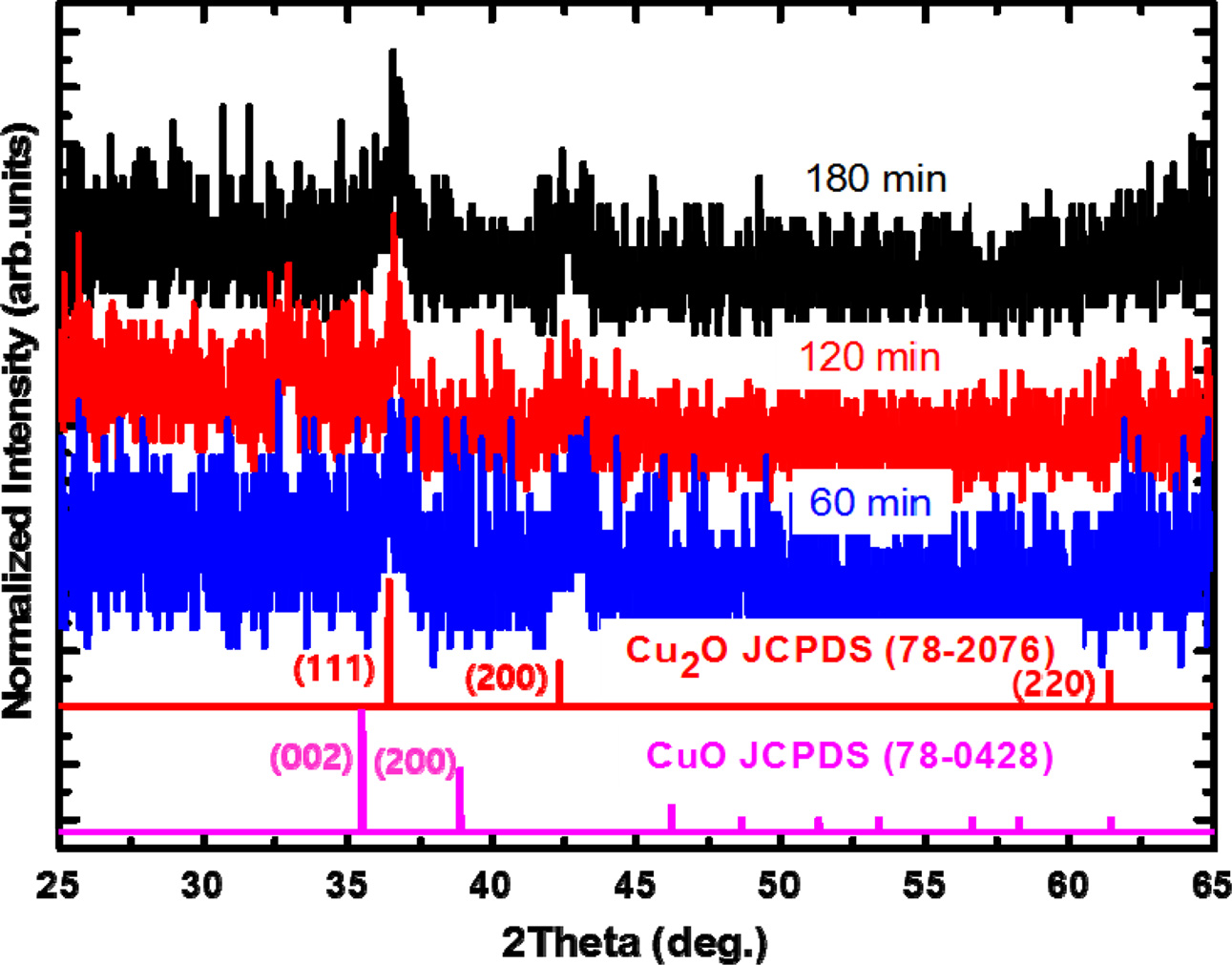
To investigate the crystalline structure of the
copper oxide thin films, XRD was measured for the samples with different
deposition times. As shown in Fig. 2, the XRD results for the samples mainly
showed two peaks at 36.44o and
42.32o, which correspond to the (111)
and (200) planes of the cubic Cu2O (space group: Pn-3m; a
= 0.428 nm, JCPDS 78-2076) crystal, respectively. Because the thin
films comprised small domains of Cu2O sized less than 20 nm, the
diffraction peaks were broad, which can be expected
from the Scherrer equation relating the XRD peak
broadness and the domain size. The absence of any additional
peaks in the diffraction results indicates that no unintentional chemical
species, such as CuO or Cu metallic compounds, were formed even with increasing
deposition time. The overall reactions for the formation of the nanostructures
can be summarized by the following reactions. The copper acetylacetonate
[Cu(acac)2; Cu(C5H7O2)2]
used as the copper source is dissolved in the aqueous solution via the following reactions [12, 15]:
Cu(acac)2 + H2O
→ Cu(acac)·(OH) + H(acac) (1)
Cu(acac)·(OH) → Cu(acac)+
+ OH- (2)
Cu(acac)+ + OH- → Cu(OH) + (acac) (3)
2Cu(OH) → Cu2O + H2O (4)
The chelating agent, ethylenediamine
(C2H4(NH2)2), increases the
solubility of the copper acetylacetonate by chelating the
Cu cations by two amine-based ligands. The agent also promotes the
uniform deposition of Cu2O thin films on the substrate by preventing
the random reaction between Cu cations and OH- anions even at low
temperature around 300 oC [16].
To investigate the effect of thermal annealing on
the structural and optical properties of the CuO2 thin films, the
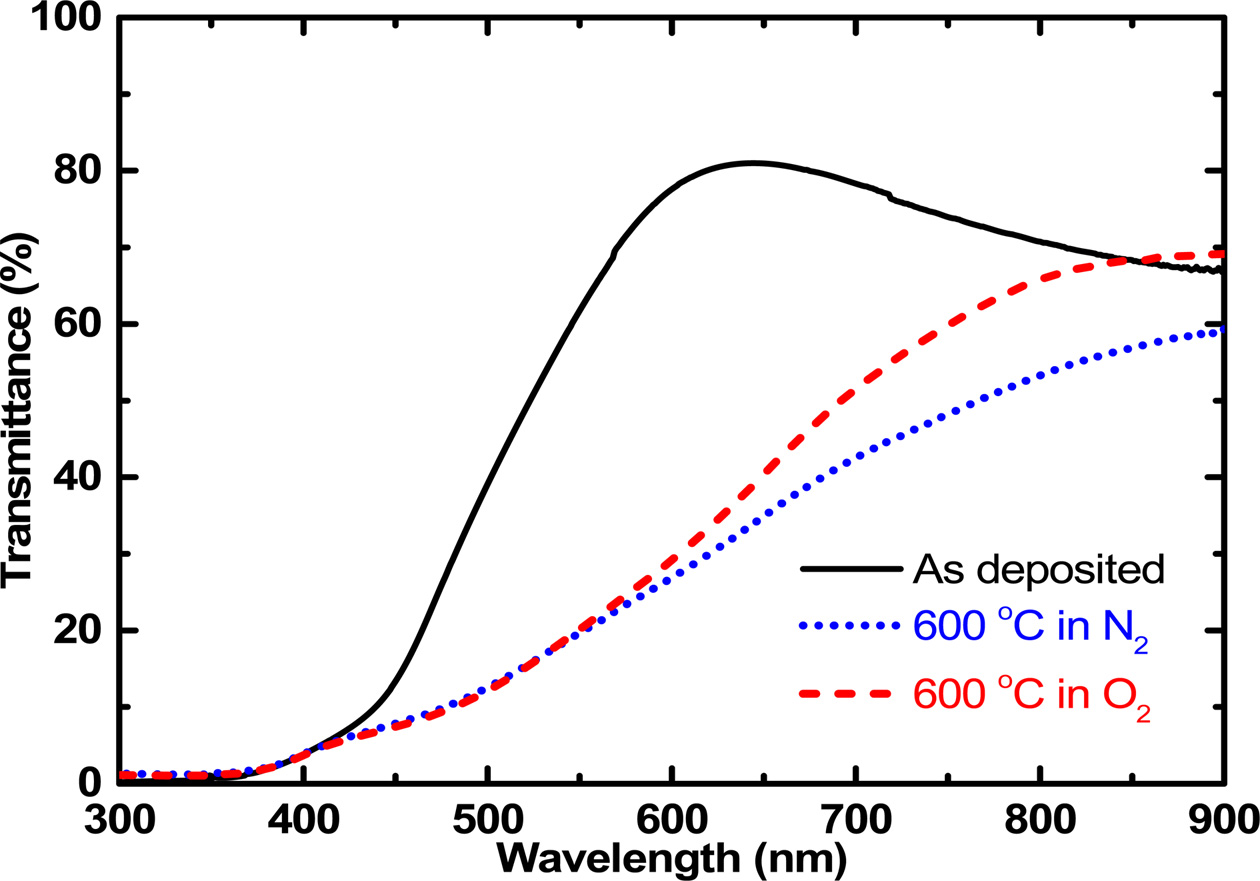
deposited thin films were annealed in the RTA system. Figure 3 shows the
optical transmittance results of the Cu2O thin films for the
as-deposited film and for that thermally annealed at 600 oC in N2 and O2 gas atmosphere. The as-deposited Cu2O
thin film showed a transmittance over 80% at the wavelength around 600 nm.
After the Cu2O thin film was thermally annealed at 600 oC in N2 and O2 gas atmosphere, the transmittance
decreased drastically in the visible spectral range and was maintained in the
infrared spectral range. In general, the increase of the light absorption in
the oxide thin film is originated from the phase transformation or increased
defect formation. In this case, the increase of absorption would be attributed
to the increased point defect during the annealing process. The optical
transmittance decreased further when the annealing atmosphere was in the N2
gas, which was attributed to the increased generation of oxygen vacancy-related
point defects in the N2 atmosphere because of high vapor pressure of
oxygen atoms. The oxygen vacancy-related point defects produce free charge
carriers to increase the absorption of light
[17]. This result indicates that thermal
annealing in O2 atmosphere is better for avoiding the generation of
oxygen vacancy-related point defects.
To investigate the dependence of the optical and structural
properties on the thermal annealing temperature, the Cu2O
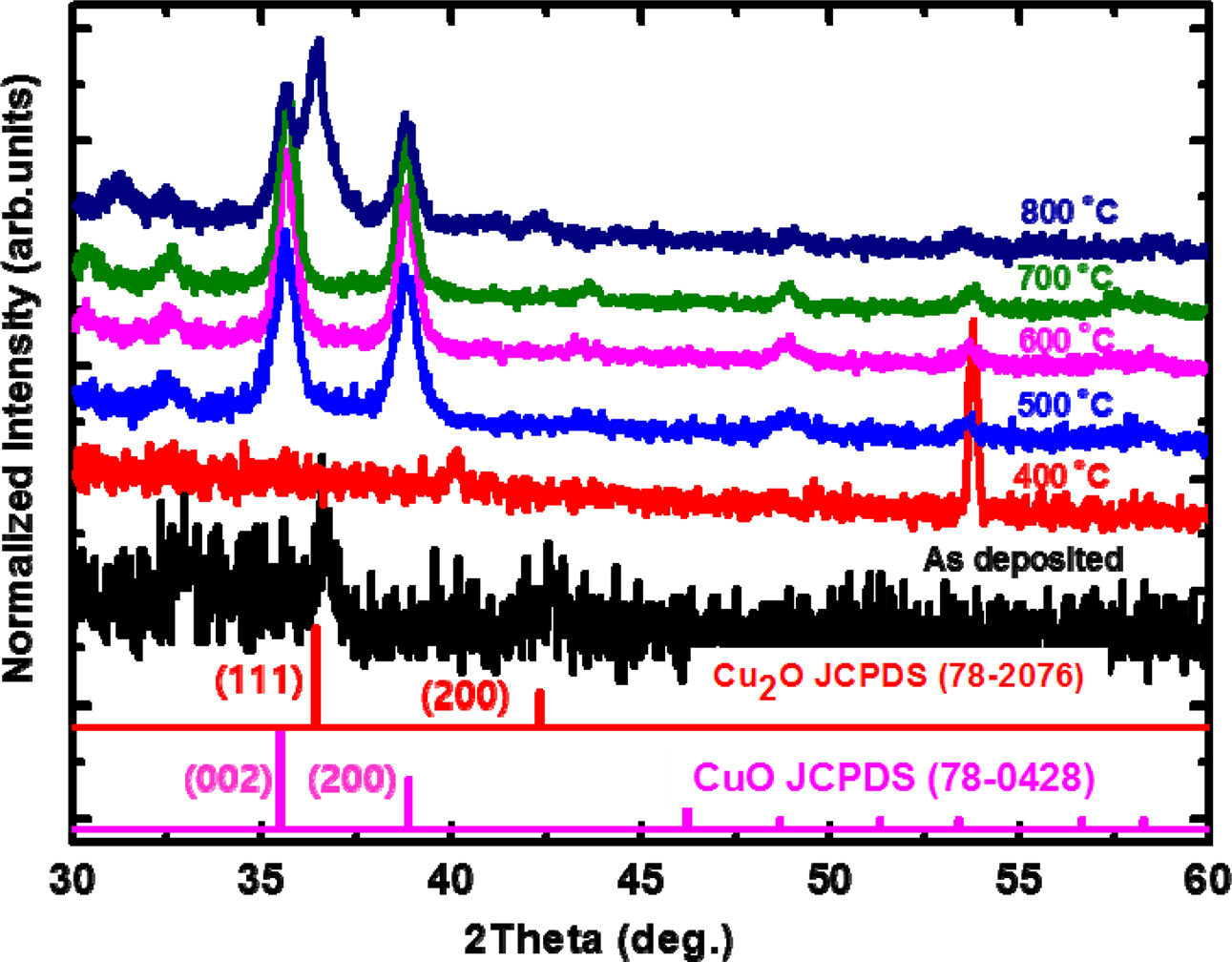
thin films were thermally annealed between 400 and 800 oC in O2 atmosphere. Figure 4 shows the temperature-dependent
structural evolution of the Cu2O thin films. At an
annealing temperature of 400 oC, the peaks corresponding to Cu2O
phase disappear. As the temperature
is increased from 500 to 700 oC, new peaks appear at 35.5o and 38.9o, which correspond to the (002) and (111) planes of the monoclinic CuO phase (space
group: C2/c; a = 0.32501 nm, b = 0.343 nm, c = 0.5131 nm, β = 99.549o,
JCPDS 78-0428) [18]. This was attributed to the ionization of the Cu
element into the Cu+ ion to form a Cu2O phase at
temperatures lower than 400 oC, but
into the divalent Cu2+ ions to form a CuO phase at temperatures higher than 500 oC. The full-width at half maximum of the peak became narrower as the
temperature was increased to 700 oC, which indicates the improved crystalline quality of the copper oxide thin
films. At temperatures higher than 800 oC, another peak corresponding to the (111) plane of the Cu2O
phase starts to appear, which leads to the formation of a mixed phase of CuO
and Cu2O. As the temperature is increased
further, the CuO phase becomes unstable and is transformed
into the Cu2O phase by evaporation of the oxygen atoms.
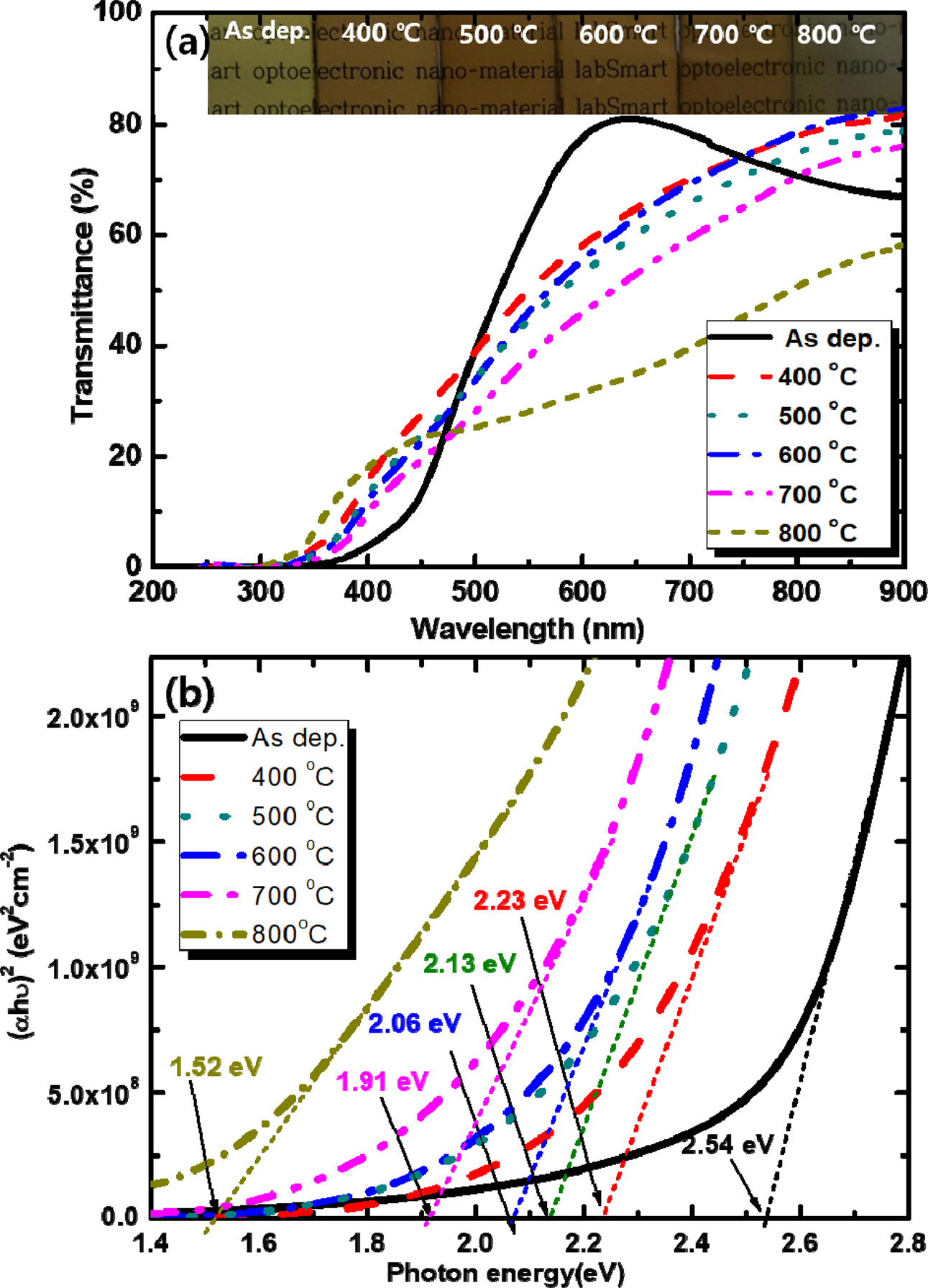
Figure 5 shows the dependence of the optical properties
of copper oxide thin films on temperature. As shown in the inset of Fig. 5(a),
the as-deposited Cu2O thin film shows a brown surface because the
energy bandgap of Cu2O is 2.1-2.6 eV. As the annealing temperature
is further increased up to 700 oC, the
sample becomes reddish. The transmittance decreases gradually as the annealing
temperature is increased from 400 to 700 oC. The bandgap energy of the samples was estimated by plotting
the photon absorption coefficient (α) versus the photon energy (hv),
as shown in Fig. 5(b). The optical bandgap energy was estimated by the Tauc and
Davis-Mott expression [19]:
(αhν)1/n = A(hν-Eg) (5)
In the equation, A and Eg are the
proportional constant and the bandgap energy, respectively,
and n is a characteristic constant that determines whether the
electronic transition is direct (n=0.5) or indirect (n=2) [19].
The plots were well fitted for n=0.5, indicating that the copper oxide
was a direct bandgap material. The bandgap energy of the as-deposited Cu2O
thin film was determined to be 2.54 eV, which is similar to the reported
bandgap energy of the bulk Cu2O [7-10]. As the annealing temperature
is increased from 400 to 800 oC, the
estimated optical bandgap energy decreases from 2.23 to 1.52 eV. The bandgap
energy of the CuO is 1.3~2.1 eV [10-12].
Therefore, these results indicate that the
as-deposited Cu2O phase changes to CuO phase as the annealing temperature is increased. This result is consistent with the XRD structural investigations.
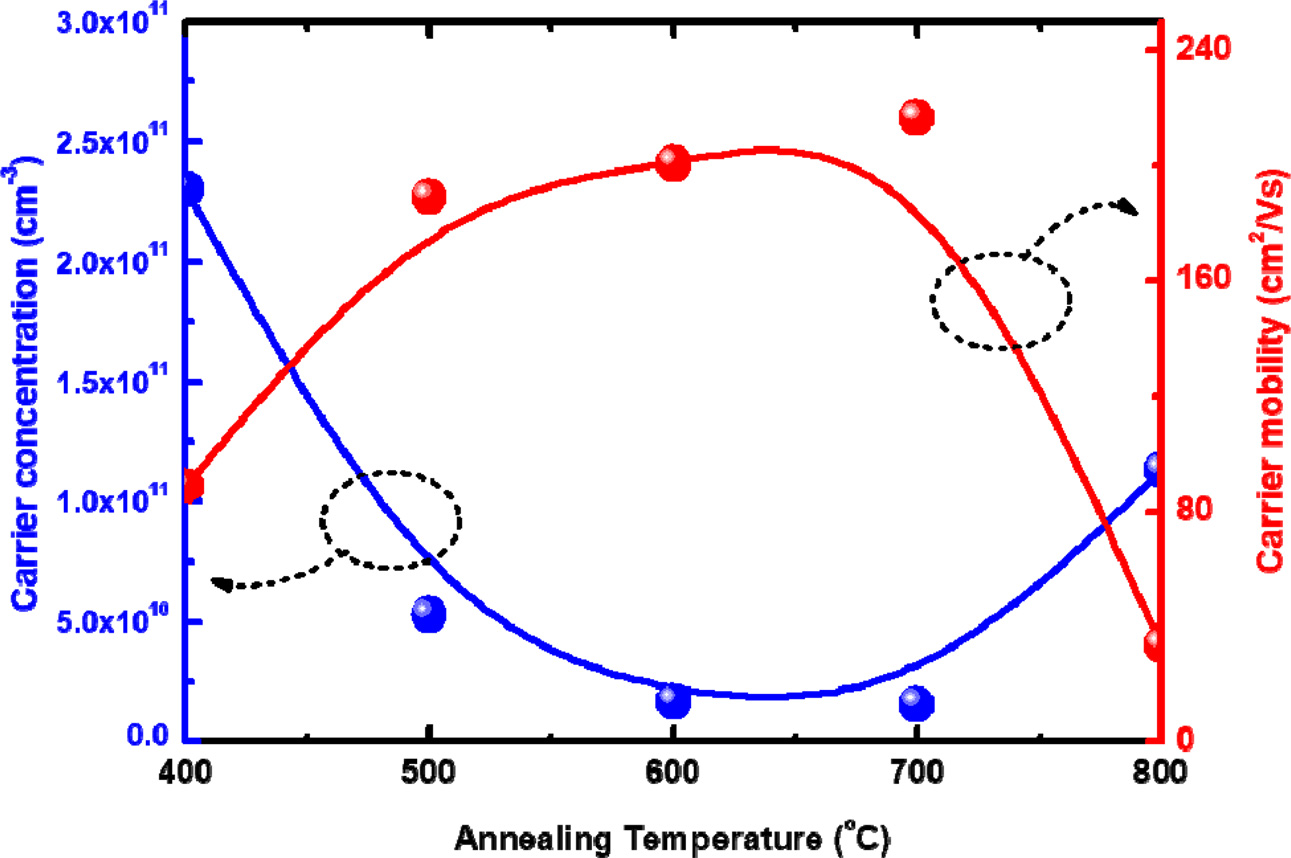
To investigate the effect of thermal annealing on the
electrical properties of Cu2O thin films, Hall effect measurement
was performed at room temperature. The copper oxide thin films showed highly
resistive n-type characteristics with only a small amount of charge carrier
concentration in the order of 1011 cm-3.
As shown in Fig. 6, the charge carrier concentration decreases
while the carrier mobility increases, as the annealing temperature
is increased up to 700 oC. This was attributed to the improved crystalline
quality of the CuO as the annealing temperature was increased. Because the copper oxide thin films were annealed in an oxygen
atmosphere, their crystalline quality was improved without evaporation of
oxygen from the thin film, as shown in the XRD results. As the thermal
annealing temperature is increased further up to 800 oC, the charge carrier
concentration increases but the mobility decreases, because the decomposition of the CuO crystalline phase above this
temperature leads to the formation of various point defects. Therefore, the
copper oxide phase can be modified by thermal annealing, which enables their
optical and electrical properties to be controlled.
These findings will support the development of improved copper oxide-based optoelectronic devices.
|
Fig. 1 Plane-view and cross-sectional-view SEM images of the copper oxide thin films with different deposition times: (a) 60 min, (b) 120 min, and (c) 180 min. |
|
Fig. 2 Normalized θ-2θ XRD patterns of the copper oxide thin films with different deposition times. |
|
Fig. 3 Transmittance spectra of the copper oxide thin films that were thermally annealed at 600 oC in N2 and O2 atmosphere. |
|
Fig. 4 Normalized θ-2θ XRD patterns of the copper oxide thin films according to the thermal annealing temperature in O2 atmosphere. |
|
Fig. 5 (a) UV-vis optical absorption spectra of the copper oxide thin films according to the thermal annealing temperature in O2 atmosphere. The inset shows the sample images observed by the naked eyes. (b) Specific absorption band edges calculated from Tauc-plot studies. Variation of (αhν)2 as a function of the photon energy (hν) to obtain the direct band gap of the copper oxide thin films. |
|
Fig. 6 Charge carrier concentration and carrier mobility of the copper oxide thin films according to the thermal annealing temperature. |
In conclusion, we investigated the structural evolution
of copper oxide thin films by controlling the thermal annealing, which in turn
affected their optical and electrical properties. The XRD and
SEM results showed that the as-deposited copper oxide thin films were
composed of small grains with Cu2O phase. As these Cu2O
thin films were thermally annealed between 500 and 700 oC, they
were transformed into the CuO phase. Thermal annealing at 800 oC
resulted in the formation of a mixed phase of CuO and Cu2O. As the
thermal annealing temperature was increased from 400 to 800 oC,
the optical bandgap energy of the copper oxide thin films
decreased from 2.54 to 1.52 eV. The electrical charge
carrier concentration of the copper oxide thin films was decreased gradually as
the thermal annealing temperature was increased from 400 to 700 oC
due to the improved crystalline quality. Therefore, the
crystalline structure of the copper oxide thin
films could be modified by thermal annealing, which enabled
their optical and electrical properties to be controlled. Considering the
optical and electrical properties of copper oxide thin films, the temperature
between 500 and 700 oC in the O2 gas atmosphere can
provide the optimal thermal annealing condition.
This study was supported by the Research program funded by
the Seoultech (Seoul National University of Science & Technology).
- 1. R. N. Briskman, Sol. Energy Mater. Sol. Cells 27 (1992) 361-368.
-

- 2. B. P. Rai, Sol. Cells 25 (1988) 265-272.
-

- 3. X. Deng, Q. Zhang, E. Zhou, C. Ji, J. Huang, M. Shao, M. Ding, and X. Xu, J. All. Comp. 649 (2015) 1124-1129.
-

- 4. R. Laskowski, P. Blaha, and K. Schwarz, Phys. Rev. B 67 (2003) 075102-075110.
-

- 5. J. T. Zhang, J. F. Liu, Q. Peng, X. Wang, and Y. D. Li, Chem. Mater. 18 (2006) 867-871.
-

- 6. H. Zhu, J. Wang, and G. Xu, Cryst. Growth Des. 9 (2009) 633-638.
-

- 7. A. S. Zoolfakar, R. A. Rani, A. J. Morfa, A. P. O’Mullane, and K. Kalantar-zadeh, J. Mater. Chem. C 2 (2014) 5247-5270.
-

- 8. R. Wick and S. D. Tilley, J. Phys. Chem. C 119 (2015) 26243-26257.
-

- 9. J. Luo, L. Steier, M.-K. Son, M. Schreier, M. T. Mayer, and M. Grätzel, Nano Lett. 16 (2016) 1848-1857.
-

- 10. Y. Yang, D. Xu, Q. Wu, and P. Diao, Sci. Rep. 6 (2016) 35158.
-

- 11. Md. A. Hossain, R. Al-Gaashani, H. Hamoudi, M. J. A. Marri, I. A. Hussein, A. Belaidi, B. A. Merzougui, F. H. Alharbi, and N. Tabet, Mat. Sci. Semicon. Proc. 63 (2017) 203-211.
-

- 12. I. S. Han and I. K. Park, Kor. J. Mater. Res. 28 (2018) 516-521.
-

- 13. C. Y. Kim, S. G. Cho, S. Park, and D. K. Choi, J. Ceram. Process. Res. 10 (2009) 851-854.
- 14. M. Y. Lee, S. H. Kim, and I. K. Park, Physica B 500 (2016) 4-8.
-

- 15. Y. C. Zhang, J. Y. Tang, G. L. Wang, M. Zhang, and X. Y. Hu, J. Crystal Growth 294 (2006) 278-282.
-

- 16. K. Chen, C. J. Chiang, and D. Ray, Mater. Lett. 95 (2013) 172-174.
-

- 17. L. Wang, Y. Yang, and T. J. Marks, Appl. Phys. Lett. 87 (2005) 161107.
-

- 18. I. Kaya, C. Cetin, H. Aydin, Z. Katircioġlu, B. Z. Bűyűkbekar, M. S. Yavuz, M. Uyaner, V. Kalem, and H. Akyildiz, J. Ceram. Process. Res. 16 (2015) 648-655.
- 19. J. Tauc, Opt. Acta Int. J. Opt. 17 (1974) 952-952.
 This Article
This Article
-
2019; 20(6): 660-664
Published on Dec 31, 2019
- 10.36410/jcpr.2019.20.6.660
- Received on Aug 5, 2019
- Revised on Sep 30, 2019
- Accepted on Oct 7, 2019
 Services
Services
- Abstract
introduction
experimental procedure
results and discussion
conclusions
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Il-Kyu Park
-
Department of Materials Science and Engineering, Seoul National University of Science and Technology, Seoul 01811, Korea
Tel : +82-2-970-6349 Fax: +82-2-973-6657 - E-mail: pik@seoultech.ac.kr






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.