- Electrochemical properties of Ni-Mn hydroxide and carbon cryogel composite electrodes for supercapacitors
Deuk Yong Leea and Young-Jei Ohb,c,*
aDepartment of Biomedical Engineering, Daelim University, Anyang 13916, Korea
bOpto-electronic Materials & Devices Research Center, Korea Institute of Science and Technology, Seoul 02792, Korea
cDepartment of Nano Material Science and Engineering, Korea University of Science and Technology, Daejeon 34113, Korea
The Ni-Mn hydroxide/carbon
cryogel (20 to 80 wt%) composite electrodes were synthesized by freeze-drying
to improve electrochemical properties of the Ni-Mn hydroxide. XRD results
revealed that the composite electrode showed a distorted spinel-like structure
similar to the Ni-Mn hydroxide plus the graphitized carbon. The crystallinity
of carbon cryogel in composite electrode was improved dramatically with
increasing the carbon cryogel content. The presence of the carbon cryogel
played a significant role in inhibiting the crystal growth of Ni-Mn hydroxide
on the surface of carbon cryogel. Electrochemical properties of the composite
electrodes, such as capacitance, power density, and energy density, were observed
to be always higher than those of the Ni-Mn electrodes. The highest specific
capacitance of 221 F/g was observed for the electrodes containing 20 wt% of
carbon cryogel at a scan rate of 5 mV/s and an electrode loading amount of 15
mg/cm2 due to easy transport of the ions into pores. However, the
highest energy density of 10.8 Wh/kg and power density of 927 W/kg were
observed for the composite electrodes containing 80 wt% of carbon cryogel.
Experimental results suggested that tunable electrochemical properties of the
composite electrodes can be adjusted by varying the carbon cryogel content.
Keywords: Ni-Mn hydroxide, Carbon cryogel, Capacitance, Power density, Energy density, Supercapacitor
Lithium secondary battery and electrochemical
supercapacitor can be used as a portable power source as an energy storage
system using electrochemical principle [1-7]. Although the secondary battery
has excellent energy density (the amount of energy that can be accumulated per
unit weight or volume), there is still room for improvement in terms of usage
time, charging time, and amount of energy available. Although
the electrochemical supercapacitor has a smaller energy density than the
secondary battery, it exhibits superior characteristics to the secondary
battery in terms of use time, charging time, and output density [6]. Manganese
and nickel electrode materials display considerable electrochemical activity
not only in low discharge rates applied in conventional secondary batteries but
also under the usual harsh conditions for the electrochemical supercapacitors
[8-11]. Ni-Mn oxide and hydroxide-based electrodes reveal a significant
reversible electrochemical activity in the alkaline
electrolytes and an extended operating voltage window (~1.8 V)
[8]. Ni-Mn ilmenite-based electrode materials demonstrate a low fade
rate and high specific electrochemical capacity values at high
discharge rates (up to 70 mAh/g at I = 70 mA/cm2) that
makes feasible their application in high rate batteries and
electrochemical supercapacitors. These metal oxides are widely
used as active electrode with polypyrrole, polyaniline, and polythiophene for
supercapacitors due to the availability of dual metal cations [8, 12-16]. Carbon-based
materials such as activated carbon, carbon black,
carbon aerogels, graphene, carbon fiber materials, porous
nanocarbon, carbon nanotubes, and multiwalled carbon
nanotubes have been widely used in the electrode materials
for supercapacitors [6, 12-20]. Carbon aerogels are
considered as a promising candidate material for the application of
supercapacitors because of high porosity, low electrical resistivity, and high
surface area [6]. However, the cost of supercritical drying limits a widespread
use. Nowadays, carbon cryogels are emerging as the
material of choice for the electrode materials of supercapacitors due to the
simplicity because the use of activated carbon or carbon nanotube mixed with
the metal oxide powder and the polymer binder may cause the complexity of the
process and the deterioration of the electrical conductivity [1-7, 12-20].
The purpose of this study is
to solve the problems of the complexity of the process and the deterioration of
the electrical conductivity of the composite electrode as a result of the use
of the mixed carbon with the metal oxide powder and the polymer binder. In
order to improve the power density, the energy density, and the capacitance of
electrochemical capacitors, we have studied electrodes by adding the
freeze-dried carbon cryogel, which has excellent specific surface area and
electrical conductivity, to the coprecipitated Ni-Mn hydroxide possessing high
specific capacitance and energy density. Carbon cryogels are synthesized by
sol-gel polycondensation of resorcinol with formaldehyde, followed by a freeze
drying and subsequent pyrolysis in an inert atmosphere [1-7]. Electrochemical
properties of the carbon cryogel
electrode mixed with Ni-Mn metal oxide are then evaluated.
Carbon
cryogel
Carbon cryogel possessing a large specific surface area
and pore size was prepared to compensate for the drawbacks (low specific
surface area and electrical conductivity) of Ni-Mn hydroxide, which is shown in
Fig. 1. The aqueous polycondensation of resorcinol (R) with formaldehyde (F)
were prepared from resorcinol (Sigma-Aldrich), formaldehyde
(Sigma-Aldrich, 37 wt% solution in water), sodium
carbonate (Na2CO3, Sigma-Aldrich), and distilled water.
After stirring for 1 h, the solution was gelled by curing for 4 days at
50 oC. This gel was washed by immersing the gel in t-butanol
for 1 day. The as-washed gels were frozen for 1 h at -30 oC and
then dried for 3 days to obtain the RF cryogel [6, 21, 22]. After aging
the RF cryogel for 4 h at 250 oC, carbon
cryogel was prepared by pyrolysis of the cryogel for 4 h at
1,000 oC, as depicted in Fig. 1. Details of the experimental
process is described elsewhere [6]
Ni-Mn
hydroxide/carbon cryogel composite electrode
The composite electrode was prepared by mixing Ni-Mn
aqueous solution and carbon cryogel (0~80 wt%), as displayed in Fig. 2. Ni-Mn
hydroxides were coprecipitated from aqueous solutions followed by
freeze drying. The freeze-dried powders were isothermally processed for 2 h at 400 oC. The electrode paste
was prepared by mixing them with
polytetrafluoroethylene (PTFE) binder.
The paste was pressed into the nickel foam substrate and then dried for 12 h at
100 oC [6, 8].
Electrochemical
properties
The electrochemical properties of the composite electrodes
were examined at room temperature with a three-electrode apparatus
consisting of a working electrode, a Pt counter electrode, and an Hg/HgO
reference electrode in KOH solution with
different concentrations [6].
Electrochemical properties of carbon
cryogel as electrode material in supercapacitor was evaluated using cyclic voltammetry (CV), a
galvanostatic charge-discharge test,
and electrochemical impedance spectroscopy (EIS). The CV measurements were
performed at various scan rates in the range of 5 mV/s and 50 mV/s. Galvanostatic
charge-discharge behavior was performed under various constant current
densities in a potential between 0 and 1.0 V. Impedance analysis was done with
10 mV amplitude in the frequency range of 100 kHz to 10 mHz using a
two-electrode system [13]. Prior to the experiment, carbon cryogel electrodes
were immersed in electrolyte for 24 h to diffuse the aqueous electrolyte
solution into the pores of carbon cryogel [6, 8, 14-20].
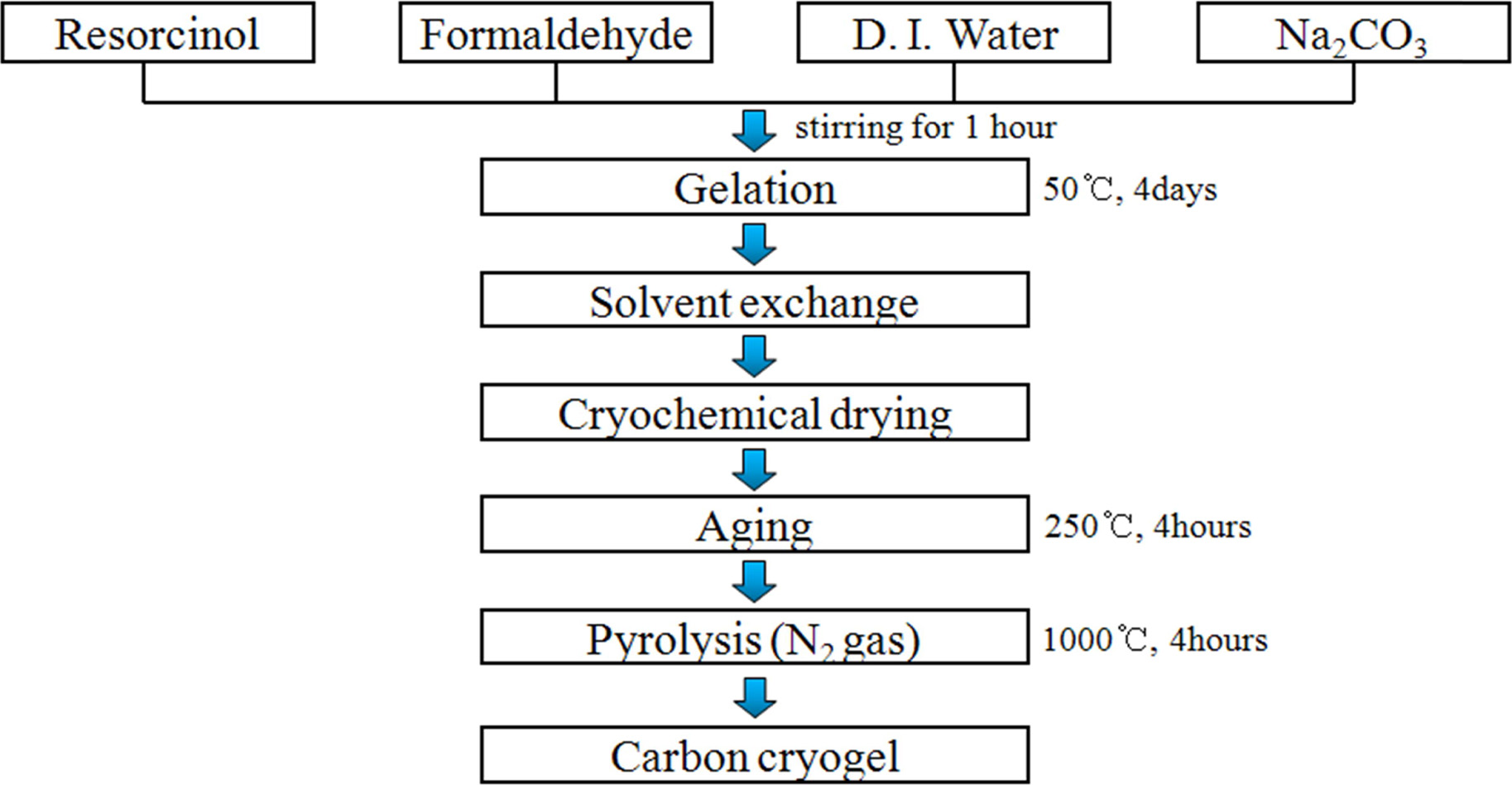
|
Fig. 1 Fabrication procedure of carbon cryogel. |
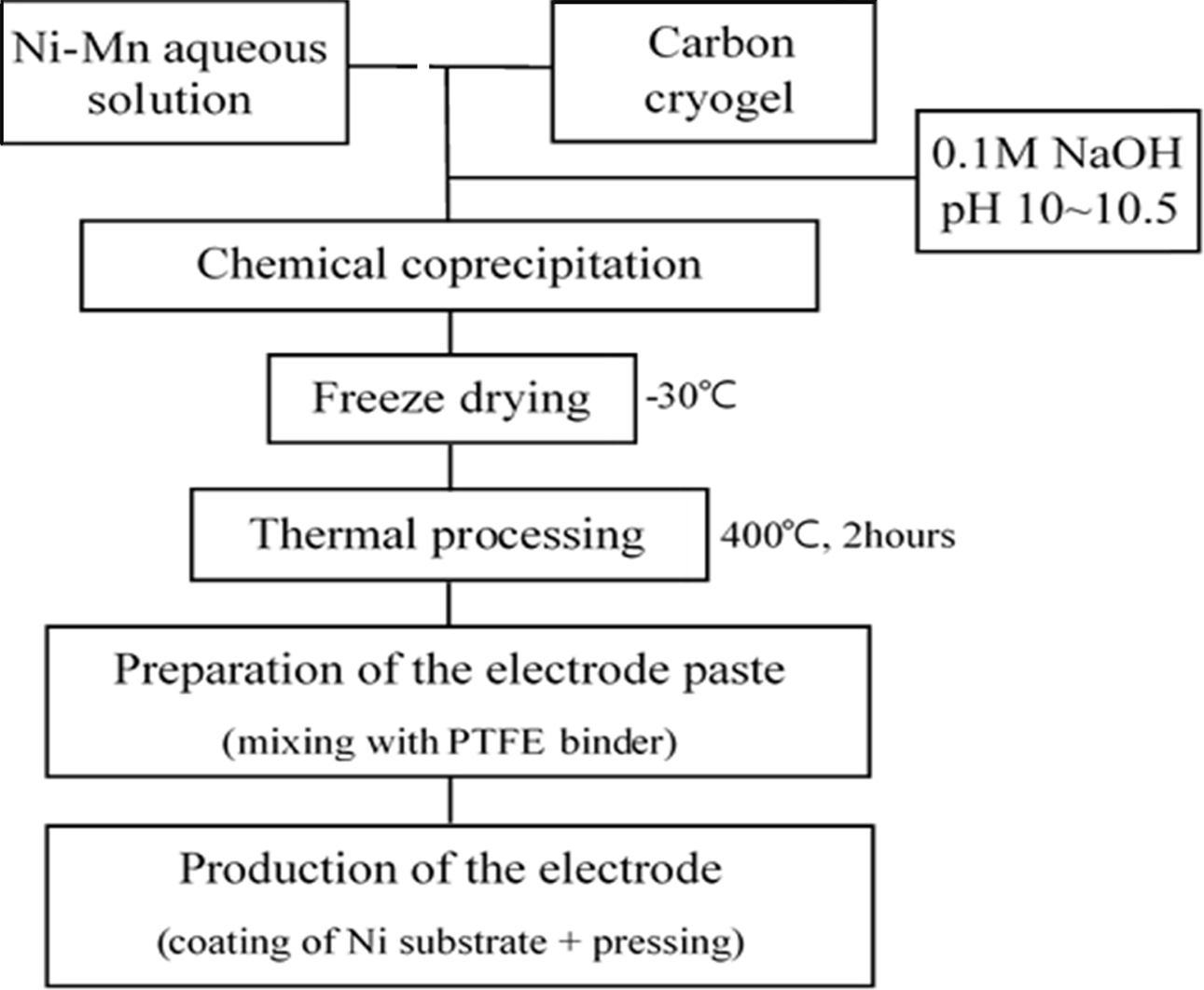
|
Fig. 2 Manufacturing procedure of Ni-Mn hydroxide+carbon cryogel composite electrode. |
Our previous studies revealed that carbon cryogels had a high
surface area of 1264 m2/g, large mesopore volumes of 0.63 cm3/g,
and average pore diameter of 20 Å [6]. XRD peaks of the carbon
cryogels were reported to be located at 2θ of 23.5o and 43.8o,
exhibiting the strong (002) and weak (101) diffraction
lines, respectively, implying that the carbon cryogels were composed of most
amorphous and small amount of graphitized carbons [6]. In the present study,
the effect of the amount of carbon cryogel on the electrochemical properties of
Ni-Mn and carbon cryogel composite electrodes was investigated. Fig. 3
exhibited that the composite electrode showed a distorted spinel-like structure
similar to the Ni-Mn hydroxide plus the carbon cryogel peaks located at 2θ
of 23o [8]. As the amount of carbon cryogel increased from 0 to 80
wt%, the peak intensity of graphitized carbon rose dramatically
without any second phases, implying that the carbon cryogel can be regarded as
graphitized carbon rather than carbon. In addition, the XRD peak of Ni-Mn was
slightly shifted to lower angle due to the formation of distorted spinel-like
structure [13].
Microstructure of the composite electrode as a function of
carbon cryogel concentration was examined as shown in
Fig. 4. As the carbon cryogel concentration increased,
Ni-Mn hydroxides were uniformly distributed on the
surface of carbon cryogel having higher specific surface area. And the particle
size of Ni-Mn hydroxide on the surface of carbon cryogel
decreased dramatically with increasing the content of
carbon cryogel from 20% to 80%. Although physical mechanisms involved are not
yet completely understood, the presence of the carbon cryogel played a
significant role in inhibiting the crystal growth of Ni-Mn hydroxide.
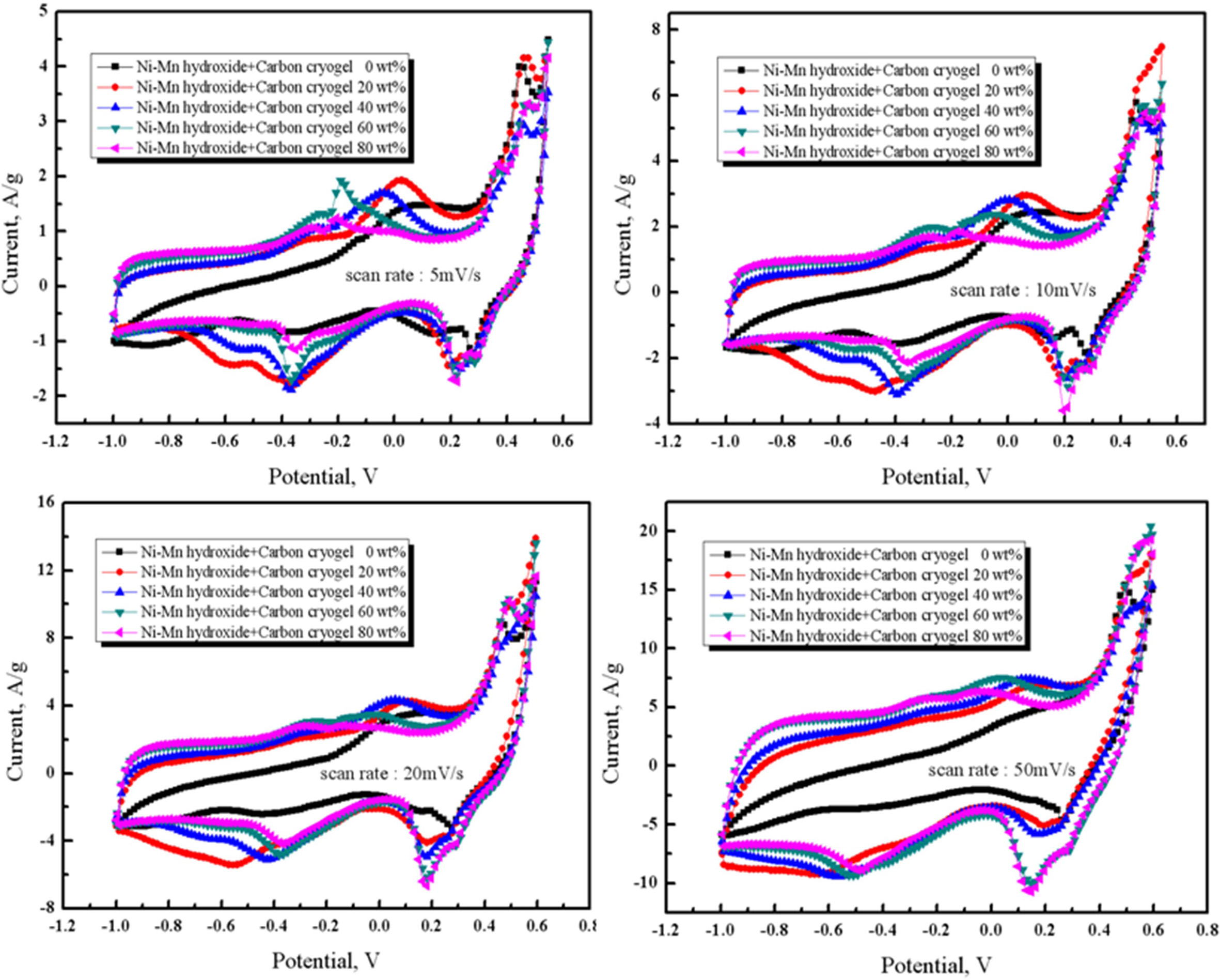
The CV curves were examined in 6M KOH electrolyte
to investigate the variation of capacitance with various scan rates in the
range of 5 mV/s to 50 mV/s, as shown depicted in Fig. 5. The specific
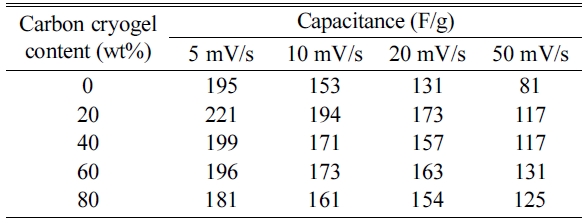
capacitances of the electrodes (C), as summarized in Table 1,
were estimated from the equation of C = {(Ia + |IC|) / 2W(dV/dt)},
where Ia, Ic, W,
and dV/dt are the current of anodic and cathodic voltammetric curves on
positive and negative sweeps, mass of the composite, and the sweep rate,
respectively [6, 23]. As can be seen in Fig. 5 and Table 1, the
capacitance started to decrease with increasing the scan rate
from 5 mV/s to 50 mV/s. Excellent capacitances were observed for
the composite electrode containing 20 wt% of carbon cryogel when the scan rates
were in the range of 5 mV/s and 20 mV/s. As the carbon cryogel concentration
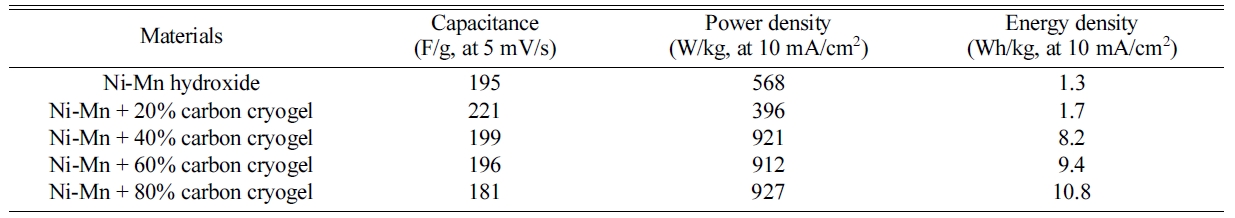
rose from 20 wt% to 80 wt%, the capacitance decreased from 221 F/g to 181 F/g
due to the decrease in the amount of Ni-Mn hydroxide. In
addition, the specific capacitance of the composite electrode
containing 20 wt% of carbon cryogel decreased from 221 F/g
to 117 F/g with increasing the scan rate from 5 mV/s to 50 mV/s. It is reported
that the ions can transport into pores more easily at low scan rate, however,
they had a difficulty in diffusing into pores when scan
rates were above 5 mV/s [6]. It is conceivable that the low
scan rate (5 mV/s) and the content of carbon cryogel (20 wt%) are mainly
attributed to higher capacitance of the composite electrodes.
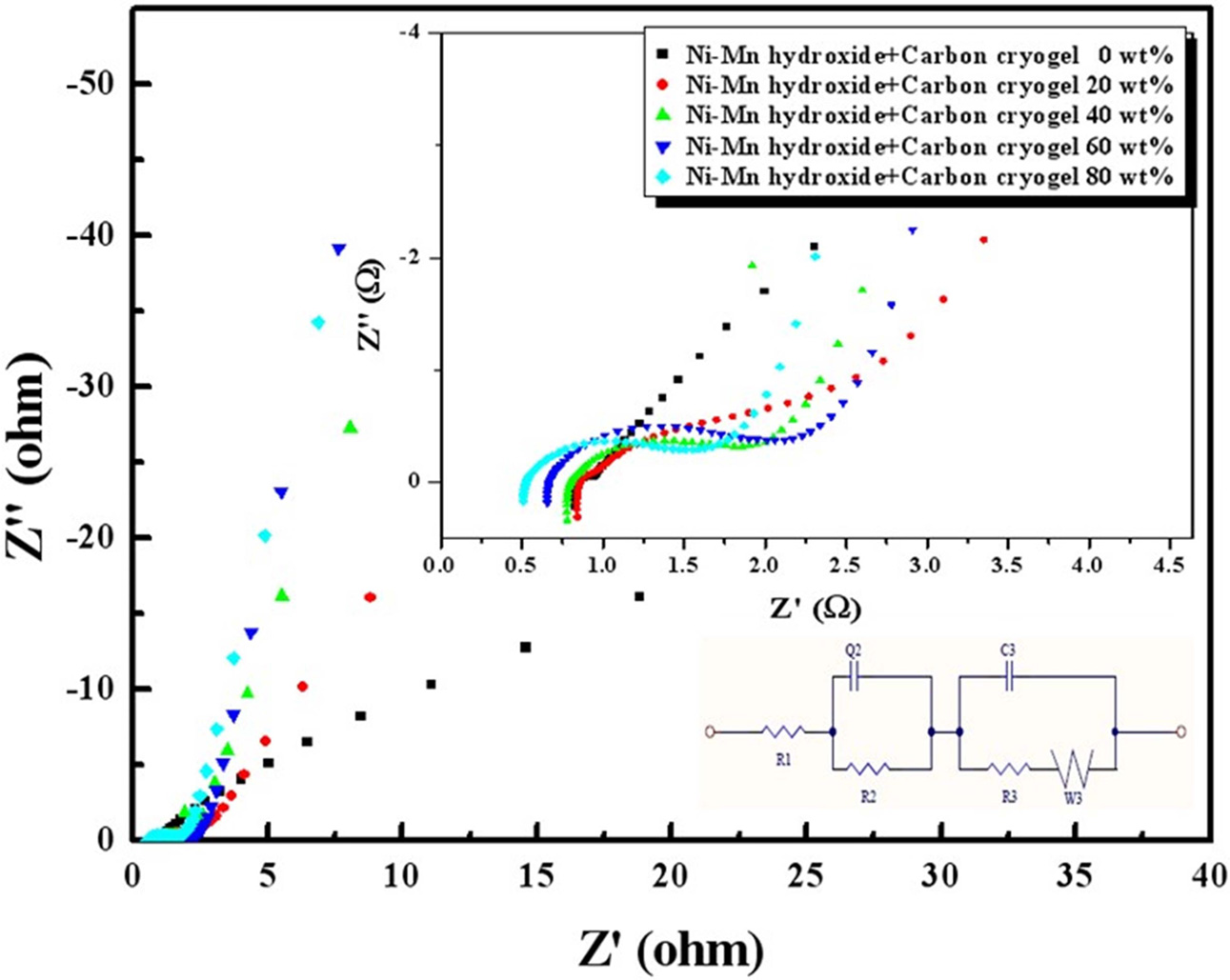
The imaginary part of the impedance is plotted as a function
of real part to compose the impedance spectrum (Nyquist plot), as displayed in
Fig. 6. The capacitance can be calculated from the imaginary part of the
impedance spectrum according to the equation of Z'' = 1/2πfC,
where f and Z” are the applied frequency and the imaginary
impedance, respectively [6, 12-20]. Electrochemical
impedance spectroscopy of the composite electrodes
in 6 M KOH electrolyte with different concentrations of carbon cryogel in the
range of 0 to 80 wt% revealed that the variation in carbon cryogel
concentration can be found in the impedance plot of the composite electrodes.
This phenomenon can be evaluated by an equivalent circuit model (Fig. 6). R1
is the electrolyte resistance. R2, R3, and Q2,
represent the contact resistance, the reaction resistance, and the capacitance
between carbon cryogel and Ni-Mn hydroxide particles,
respectively. C3 and W3 are the capacitance of double
layer on the surface and the Warburg impedance, respectively
[13]. R1 decreased gradually with increasing the content
of carbon cryogel probably due to the use of carbon aerogel and Ni-Mn oxide
instead of activated carbon, as displayed in Fig. 6. In the low frequency region,
the impedance plot of these capacitors increases, implying
that it is purely capacitive. The intersection with the real axis and the first
semicircle indicates the internal resistance of the capacitor,
which is an equivalent series resistance (ESR) of the
electrolyte in contact with the current collector and composite electrode. The higher
the content of carbon cryogel, the lower the ESR. The
combination of resistive and capacitive behaviors of the ions penetrating into
the electrode pores leads to a Warburg diffusion line and a capacitive line.
The stiffer slope in Fig. 6 implies that the ions can be penetrated into the
pores of the composite electrodes more easily with increasing the content of
carbon cryogel.
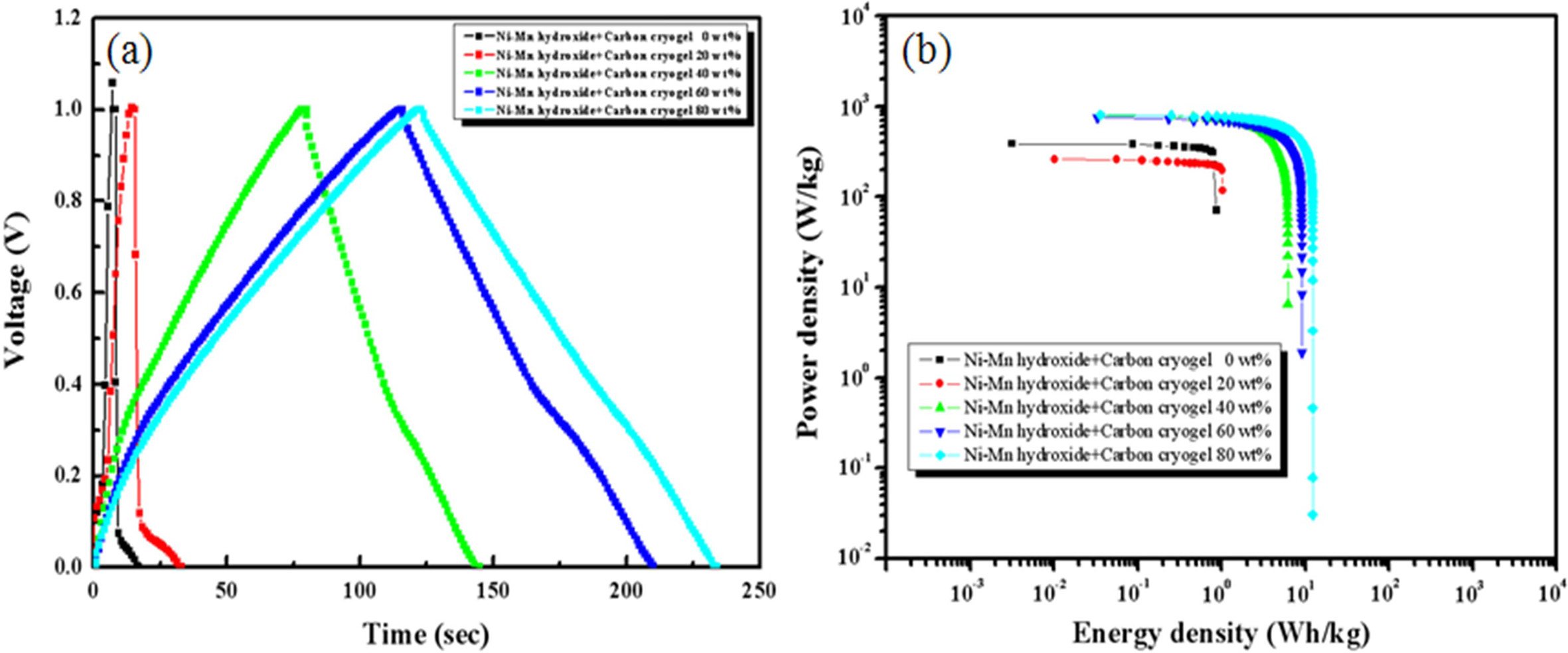
The charge-discharge curves and Ragone plot of the
composite electrodes measured in 6 M KOH at current density of 10 mA/cm2
are shown in Fig. 7(a). As the carbon cryogel concentration increases, the IR
drop occurring at the initial state of discharge decreases and the
discharge time becomes longer, suggesting that it can store more energy. The
carbon cryogel content is mainly attributed to excellent
charge-discharge characteristics and energy density. The energy density rose
from 1.7 Wh/kg to 10.8 Wh/kg with increasing the carbon cryogel content from 20
wt% to 80 wt%. The specific capacitance and power density are calculated using
the charging/discharging characteristics. As a result of Ragone plot (Fig. 7(b)
and Table 2), the power density of
the electrodes regardless of the carbon cryogel content reached a
plateau and then decreases dramatically at a certain value
of energy density. The composite electrodes with 80 wt%
of carbon cryogel exhibited much better power output of 927 W/kg. In addition,
capacitance, power density, and energy density of the composite electrodes can
be tailored through the adjustments of the experimental parameters (the content
of carbon cryogel, scan rate, and energy density) for
supercapacitors.
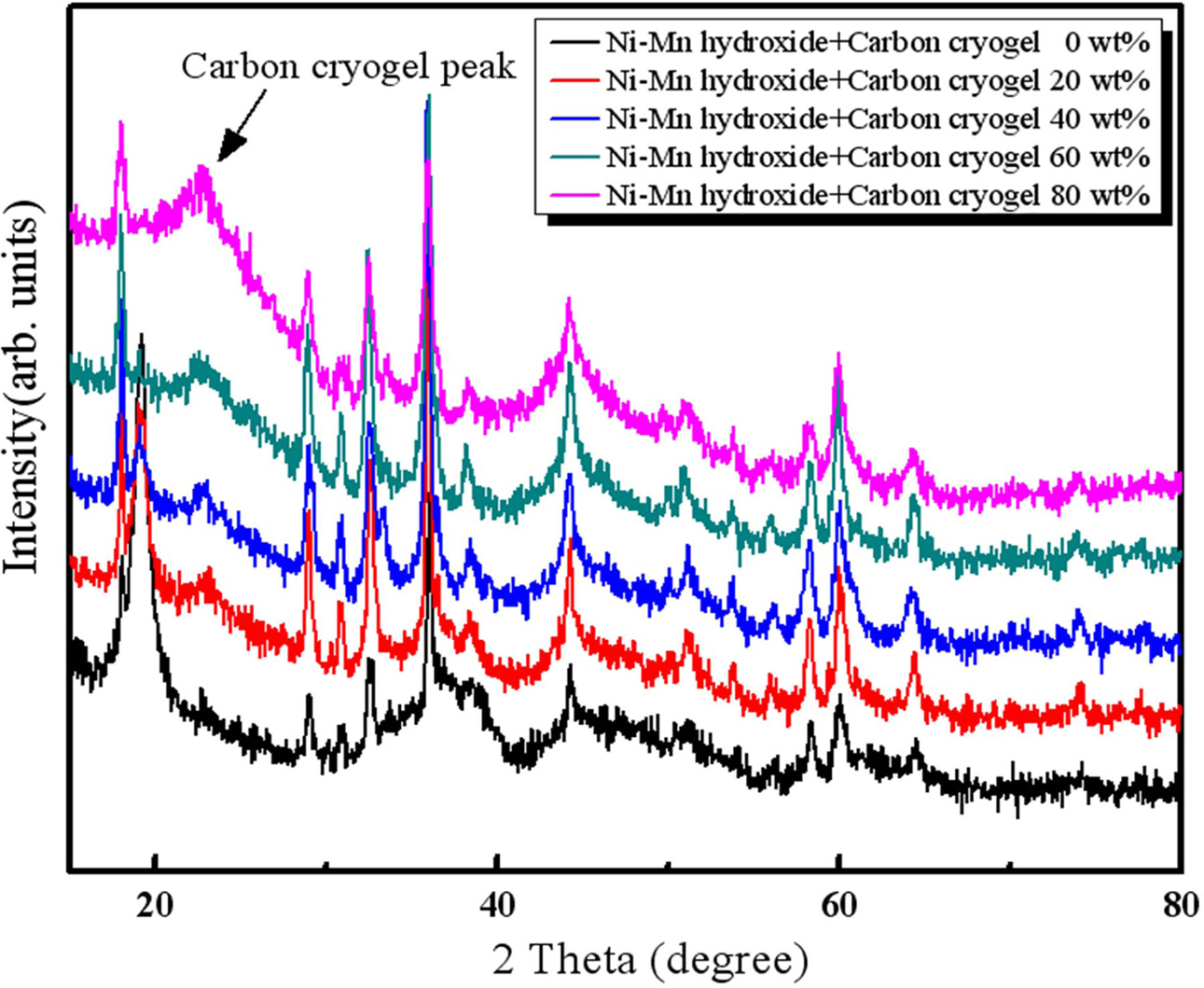
|
Fig. 3 XRD patterns of Ni-Mn hydroxide and carbon cryogel composite electrodes. |
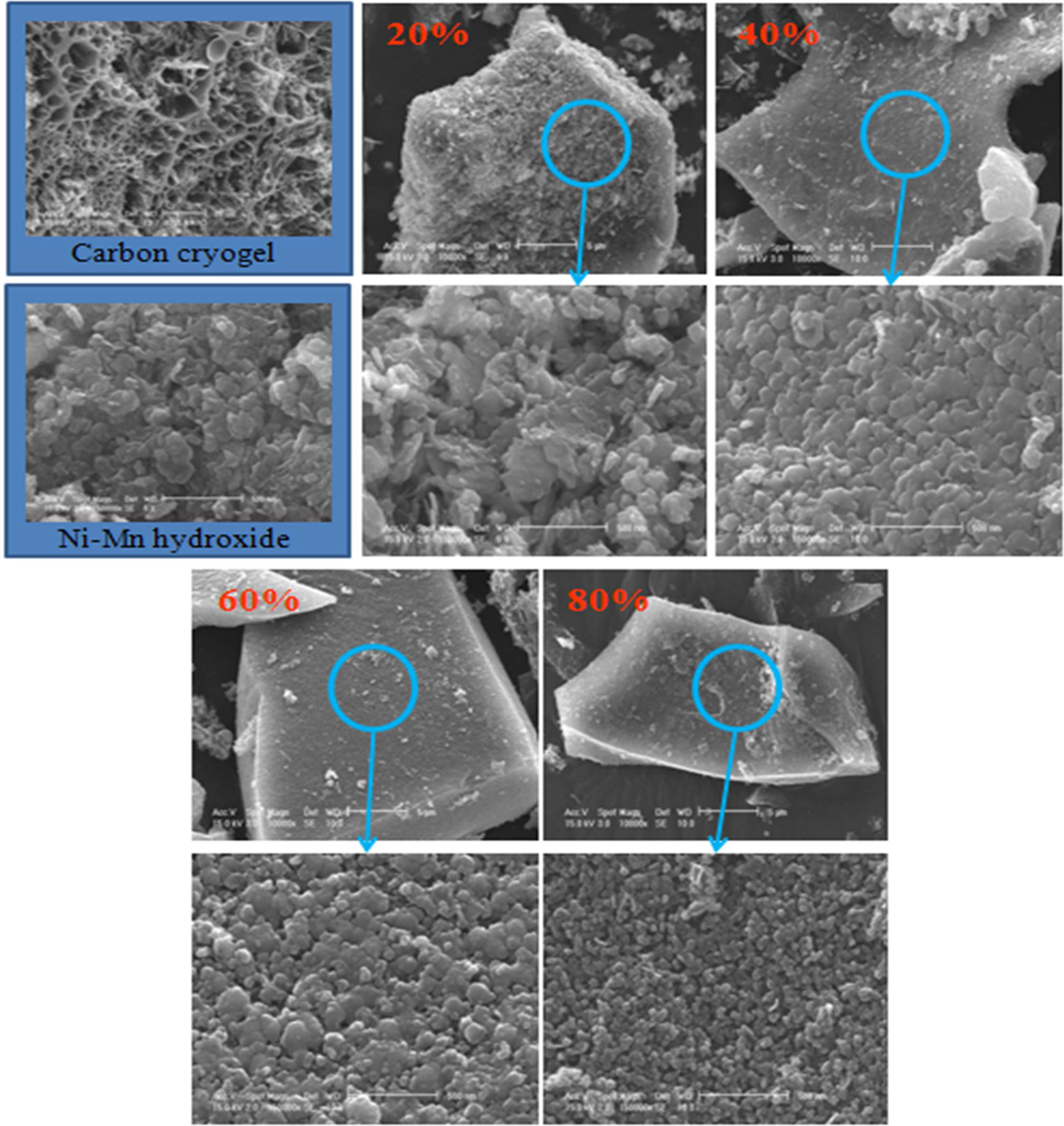
|
Fig. 4 SEM images of the surfaces of Ni-Mn hydroxide and carbon cryogel composite electrodes. Note that the particle size decreased with increasing the content of carbon cryogel from 20 wt% to 80 wt%. |
|
Fig. 5 Cyclic voltammogram of the Ni-Mn hydroxide and carbon cryogel composite electrodes with various scan rates in 6M KOH electrolyte. |
|
Fig. 6 Electrochemical impedance spectroscopy of carbon cryogel electrode in KOH electrolyte with different carbon cryogel concentration. |
|
Fig. 7 (a) Charge-discharge curves and (b) Ragone plot of Ni-Mn hydroxide and carbon cryogel composite electrodes. |
|
Table 1 Capacitance of the Ni-Mn hydroxide and carbon cryogel composite electrodes with various scan rates in 6 M KOH electrolyte. |
|
Table 2 Capacitance, power density, and energy density of Ni-Mn hydroxide and carbon cryogel composite electrodes. |
To improve electrochemical properties of Ni-Mn hydroxide,
20 to 80 wt% of carbon cryogels with high specific surface area and electrical
conductivity were added to Ni-Mn hydroxide electrode. XRD results of the
composite electrodes revealed that the composite electrode
showed a distorted spinel-like structure similar to the Ni-Mn
hydroxide plus the graphitized carbon. The electrolyte resistance (R1)
decreased gradually with increasing the content of carbon cryogel.
Although the power density and the energy density of the composite
electrodes increased with increasing the carbon cryogel concentration, the
highest capacitance was observed for the electrodes containing 20 wt% of carbon
cryogel due to the reduction in Ni-Mn hydroxides. The Ni-Mn hydroxide and carbon
cryogel materials demonstrated sufficient electrochemical activity and high
capacitance of 221 F/g at an electrode loading amount of 15
mg/cm2, implying that the composite electrodes are suitable
for supercapacitors.
- 1. J.R. Miller and P. Simon, Science 321[5889] (2008) 651-652.
-

- 2. G. Wang, L. Zhang, and J. Zhang, Chem. Soc. Rev. 41[2] (2012) 797-828.
-

- 3. A.M. Saleem, V. Desmaris, and P. Enoksson, J. Nanomater. 2016 (2016) 1537269.
-

- 4. O.A. Shlyakhtin, S.H. Choi, Y.S. Yoon, and Y.-J. Oh, Electrochim. Acta 50[2-3] (2004) 511-516.
-

- 5. O.A. Shlyakhtin and Y.-J. Oh, J. Electroceram. 23 (2009) 452-461.
-

- 6. M. Song, S. Nahm, and Y.-J. Oh, J. Korean. Ceram. Soc. 45[11] (2008) 662-666.
-

- 7. N.M. Shinde, J.M. Yun., R. S. Mane, S. Marthur, and K.H. Kim, J. Korean. Ceram. Soc. 55[5] (2018) 407-418.
-

- 8. O.A. Shlyakhtin, A.M. Skundin, Y.S. Yoon, and Y.-J. Oh, Mater. Lett. 63[1] (2009) 109-112.
-

- 9. J. Cao, S. Yuan, H. Yin, Y. Zhu, C. Li, M. Fan, and H. Chen, J. Sol-gel Sci. Technol. 85 (2018) 629-637.
-

- 10. M.-S. Jeong, B.-K. Ju, Y.-J. Oh, and J.-K. Lee, Korean J. Mater. Res. 21[6] (2011) 309-313.
-

- 11. O.A. Shlyakhtin, A.M. Skudin, S.J. Yoon, and Y.-J. Oh, Mater. Lett. 63[1] (2009) 109-112.
-

- 12. J.L. Figueiredo, J. Sol-gel Sci. Technol. 89 (2019) 12-20.
-

- 13. H.S. Min, S. Kim, W.-K. Choi, Y.-J. Oh, and J.K. Lee, Korean J. Mater. Res. 19 (2009) 544-549.
-

- 14. J.Y. Hwang, M. Li, M.F. El-Kady, and R.B. Kaner, Adv. Funct. Mater. 27 (2017) 1605745.
-

- 15. S. Ramesh, D. Vikraman, K. Karuppasamy, H.M. Yadav, A. Sivasamy, H. Kim, J. Kim, and H. Kim, J. Alloy Comp. 794 (2019) 186-194.
-

- 16. J. Theerthagiri, G. Durai, K. Karuppasamy, P. Arunachalam, V. Elakkiya, P. Kuppusami, T. Maiyalagan, and H. Kim, J. Ind. Eng. Chem. 67 (2018) 12-27.
-

- 17. J.Y. Hwang, M.F. El-Kady, Y. Wang, L. Wang, Y. Shao, K. Marsh, J.M. Ko, and R.B. Kaner, Nano Energy 18 (2015) 57-70.
-

- 18. S. Chun, B. Evanko, X. Wang, D. Vonlanthen, X. Ji, G.D. Stucky, and S.W. Boettcher, Nat. Commun. 6 (2015) 7818.
-

- 19. Y. Shao, M.F. El-Kady, J. Sun, Y. Li, Q. Zhang, M. Zhu, H. Wang, B. Dunn, and R.B. Kaner, Chem. Rev. 118 (2018) 9233-9280.
-

- 20. M.F. El-Kady and R.B. Kaner, ACS Nano 8 (2014) 8725-8729.
-

- 21. Y. Kim, S. Son, C. Chun, J. Kim, D.Y. Lee, H.J. Choi, and T. Kim, Biomed. Eng. Lett. 6 (2016) 287-295.
-

- 22. O.A. Shlyakhtin, Y.S. Yoon, S.H. Choi, and Y.-J. Oh, Electrochim. Acta 50 (2004) 505-509.
-

- 23. X. Wang, X. Wang, W. Huang, P.J. Sebastian, and S. Gamboa, J. Power Sources 140 (2005) 211-215.
-

 This Article
This Article
-
2019; 20(6): 649-654
Published on Dec 31, 2019
- 10.36410/jcpr.2019.20.6.649
- Received on Jul 12, 2019
- Revised on Oct 1, 2019
- Accepted on Oct 7, 2019
 Services
Services
Shared
 Correspondence to
Correspondence to
- Young-Jei Oh
-
bOpto-electronic Materials & Devices Research Center, Korea Institute of Science and Technology, Seoul 02792, Korea
cDepartment of Nano Material Science and Engineering, Korea University of Science and Technology, Daejeon 34113, Korea
Tel : +82-2-958-5553 Fax: +82-2-958-5554 - E-mail: youngjei@kist.re.kr






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.