- Characterization of morphology and hardness of electroless Ni-P-PTFE composite coatings
Young-Min Byouna,*, Sun-Kyo Seob, Jin-doo Yoonc and Sang-Jo Nac
aMetal & Machinery Team, Korea Conformity Laboratories (KCL), 199 Gasan digital 1-ro, Geumcheon-gu, Seoul 08503, Korea
bT&C Co., LTD, 21 Oido-ro, Siheung-si, Gyeonggi-do 15014, Korea
cTDongjin Metal Co., LTD, 459 Gongdan-ro, Seongsan-gu, Changwon-si 51555, Korea
Electroless nickel coatings are widely used in the chemical, mechanical, and electronic industries because of their excellent wear and abrasion resistance. In this study, the effect of polytetrafluoroethylene (PTFE) particles on composite coatings was investigated. To improve mold lubrication, Ni-P-PTFE composite coatings with various PTFE content were studied. The results showed that the incorporation of PTFE particles increased when the PTFE content increased from 5 g/L to 20 g/L; particularly when the content of PTFE was 20 g/L, the incorporation was found to be the highest at 12.23 wt.%. On the other hand, when the content of PTFE was 30 g/L or more, the incorporation amount decreased. The increase and decrease in the amount of incorporation are higher due to the precipitation effect when the content of the particles is proper. However, when the concentration of particles was higher than a certain value, the incorporation amount decreased because of inter-particle interference and inter-particle collisions. Tests were conducted to evaluate the mechanical and friction properties. The observed friction characteristics revealed that the electroless Ni-P-PTFE composite coatings had relatively low friction coefficients.
Keywords: PTFE, Electroless deposition, Ni-P, Hardness, Composite, Corrosion, Friction, Wear
Recently, the demand for various functionalities have increased as a result of high functionalization and high-speed industrialization. Therefore, surface treatment technologies such as composite coating have attracted attention [1-3].
Composite coatings have long been known as a method of coating. Hard particles, such as Al2O3, Cr2O3, SiC, and polytetrafluoroethylene (PTFE), are dispersed in a coating solution and then electroless-deposited onto a surface to provide a composite of these ions. Such a treatment improves the coating characteristics, such as abrasion resistance, heat resistance, corrosion resistance, and lubricity [4-6].
Electroless-deposited Ni–P coatings have various applications in industry because of their unique properties such as corrosion resistance and high hardness [8]. Furthermore, it is known that the incorporation of particles into Ni–P matrix enhances corrosion and wear resistances, depending on the particle type [7].
Recently, various studies reported that the Ni–P coatings can be improved by the incorporation of nano- and micro-sized particles, which reinforce Ni–P matrix to form functional composite coatings [9-10].
In particular, polytetrafluoroethylene (PTFE) has attracted considerable attention because of its self-lubricating properties, low coefficient of friction(COF), high temperature resistance, and corrosion resistance [11-12].
In the field of surface treatment, the research of PTFE in composite coatings is mainly conducted with the use of Ni–P coatings. Zhao studied the effects of surfactant and PTFE addition on the speed and PTFE content of the Ni-P-PTFE composite coating. They reported that the adhesion strength of Ni-P-PTFE composite coating gradually increased by the PTFE content, which significantly improved the coating characteristics [13].
In addition, Ramalho and Miranda studied friction and wear properties of Ni–P and Ni-P-PTFE composite coatings in sliding contact with hard chrome steel and confirmed that wear resistance was improved by adding PTFE particles [5]. Mafi and Dehghanian reported that Ni-P-PTFE composite coatings were prepared by various types of surfactants, and the corrosion resistance of Ni-P-PTFE composite coatings was greatly improved by adding cetyl trimethyl ammonium bromide (CTAB) and poly-vinylpyrrolidone (PVP) surfactants [14].
It is known that incorporation in the composite coating with added particles are influenced by physical properties, dispersion, particle size, pH, stirring speed, and current density of the dispersed particles. In the case of a large particle size, there is a possibility that other parts may be damaged by particles when in contact with other parts. To solve this problem, it is necessary to study the composite coating [15].
In this study, electroless Ni-P-PTFE composite coatings of different PTFE concentrations were deposited to examine their mechanical properties. The results are discussed in this paper.
Ni-P-PTFE composite coatings were electroless-deposited on Fe hullcell substrates of size 50 mm × 50 mm × 0.4 mm. Prior to electroless deposition, the Fe hullcell substrates were mechanically polished down to 2000-grit SiC carbide paper, followed by the electrolytic degreasing with alkaline solution for 5 minutes after pickling using a 5 wt% hydrochloric acid aqueous solution for 3 min. Then, the specimens were rinsed in distilled water before depositing the Ni-P-PTFE composite coatings.
The composition of Ni-P-PTFE bath was 30 g/L nickel sulfate, 25 g/L sodium hypophosphite, 20 g/L sodium citrate, and 2 g/L thiourea; in addition, 5, 10, 20, and 30 g/L of PTFE (Sigma Aldrich, assay 60 wt % dispersion in H2O) particles with an average diameter of 50~100 μm were dispersed in the solution. The pH of Ni-P-PTFE bath was adjusted electrometrically to 5.0 using ammonia. All the solutions were prepared using analytical grade chemicals and distilled water.
The preparation of Ni-P-PTFE composite coating was agitated with the magnetic stirrer for 24 h; ultrasonic treatment was applied for 60 min to prevent sedimentation.
Electroless deposition was carried out for 60 min with a stirring rate of 250 rpm at a temperature of 90 ± 2 ℃. The composition of Ni-P-PTFE bath and procedure conditions are provided in Table 1.
The surface and cross-sectional morphologies of the deposited coatings were observed using field emission scanning electron microscopy (FE-SEM, Hitachi, S-4200) and energy-dispersive X-ray spectroscopy (EDXS).
The crystalline structure and constituent phase of the composite coatings were measured using high-resolution X-ray diffraction (XRD, X’Pert-RRO MRD, Phillips) with Cu Kα radiation with a 2θ angle ranging from 10o to 80o. The hardness values of composite coatings were measured using nano-indentation (PICODENTOR HM500, Helmut Fischer); the mean values and standard deviations were found.
The electrochemical tests were carried out in a 5.0 wt.% NaCl solution using a potentiostat (Bio-Logic SAS, model SP-150) to investigate the corrosion properties. A three-electrode system was employed in the electrochemical tests: the working electrode exposed a surface area of approximately 1 cm2; a saturated calomel electrode (SCE) (saturated KCl) and a carbon rod were used as the reference and auxiliary electrodes, respectively. Potentiodynamic polarization measurements were conducted after 5 min of immersion in a 5.0 wt.% NaCl solution at room temperature. To ensure the accuracy of the results, the measurements were repeated three times for each specimen. From the polarization curves, the corrosion behaviors of the electrodeposited coatings were compared and discussed. The friction coefficients were estimated using a ball-on-disc tribometer (JLTB-02 tribometer, J&L); the measurements were performed at a constant applied lad of 25 N with a linear speed of 100 mm/s using an STB2 ball (diameter of 6 mm) in dry condition.
|
Table 1 Composition and procedure conditions of Ni-P-PTFE composite electrolyte |
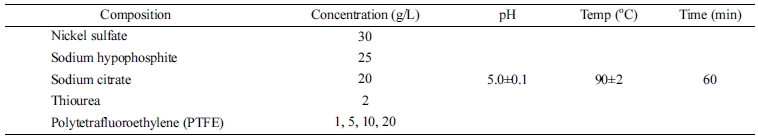
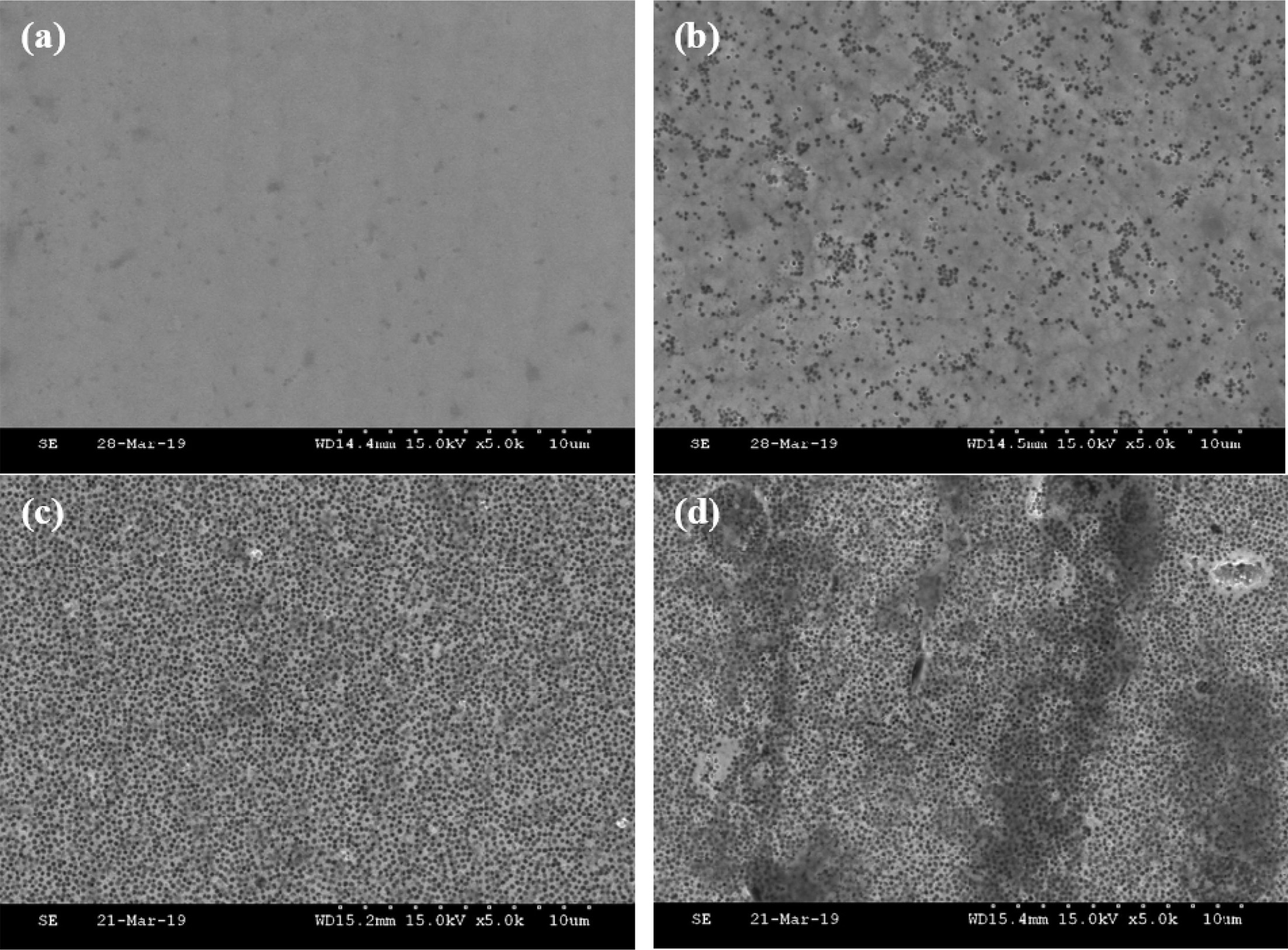
Fig. 1 shows the surface shapes of the N-P-PTFE composite coatings with different PTFE contents. In general, the surface morphology of electroless Ni–P coatings is represented by a nodular structure, while the surface morphology of Ni-P-PTFE composite coatings is not a nodular structure. By observation at high magnification black spots had a uniformly distributed smooth surface uniformly at a diameter of 100 nm. These black spots are probably related to hydrogen evolution during the deposition process [16].
This implies that the PTFE particles were embedded inside the Ni–P matrix. As the amount of PTFE content increased, there was a difference in the incorporation and distribution of PTFE particle.
At 5 g/L, a smooth surface was observed because of the low PTFE content, while at 10, 20, and 30 g/L, the black spots number gradually increased. Particularly, at 30 g/L, it was found that black spots were more aggregated. It can be attributed to the interstitial collisions caused by the increase of the PTFE content, which results in the unevenness of the texture [17-18].
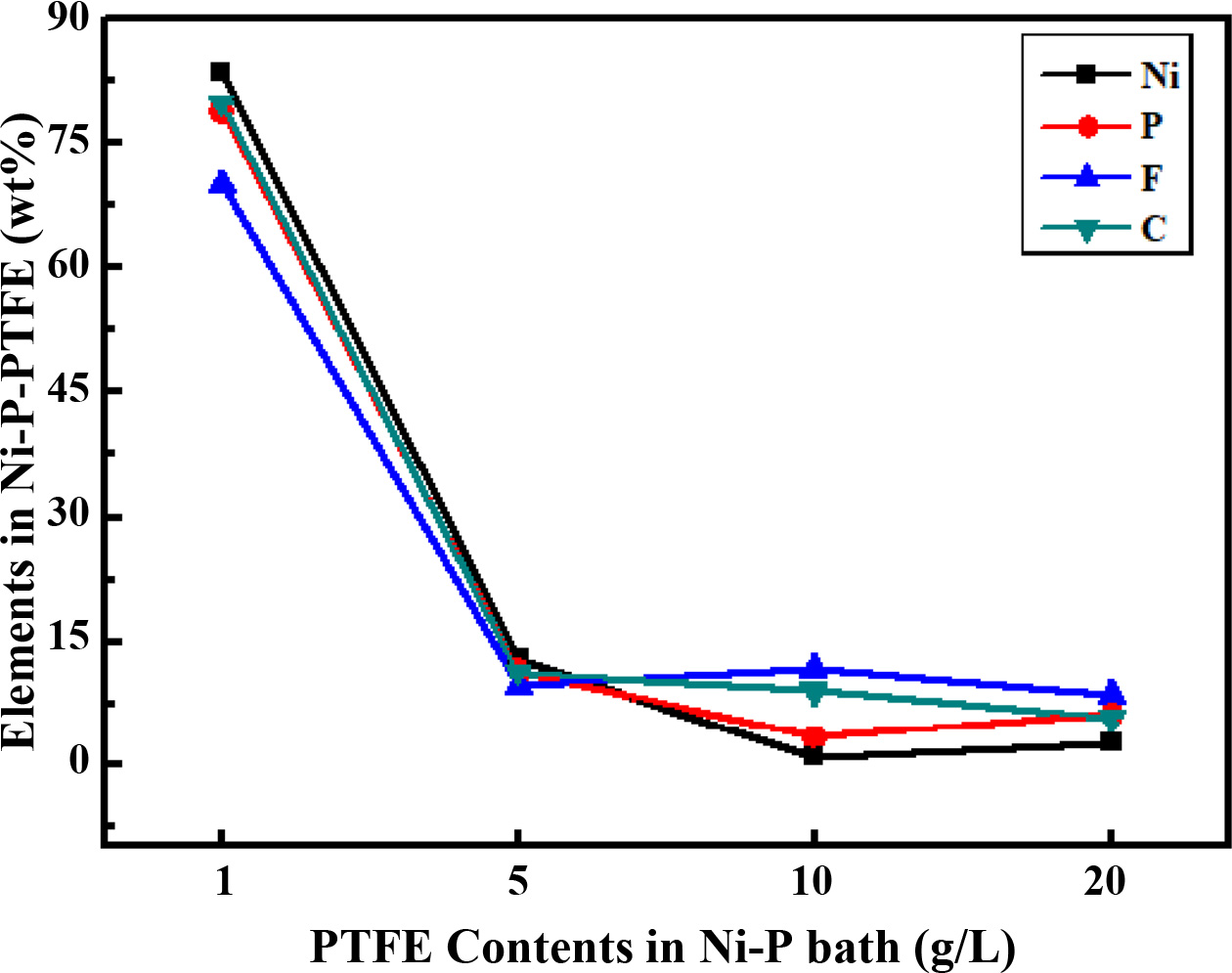
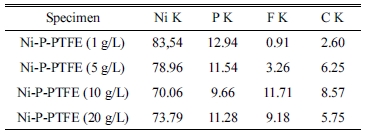
Table 2 and Fig. 2 present the EDS analyses of the electrodeposited Ni-P-PTFE composite coatings with different PTFE concentrations. Nickel, phosphorus, fluorine, and carbon were detected on the surface of Ni-P-PTFE composite coatings.
When the PTFE content increased from 5 to 20 g/L, the content of F increased from 0.91 wt.% to 11.71 wt.%. However, when the PTFE content was 30 g / L, it decreased to 9.18 wt.%.
These changes in the amount of incorporation can be explained by the Guglielmi’s three-step absorption model an increase in the electric field strength and coulomb force to an increase in the incorporation rate of the PTFE particles [19].
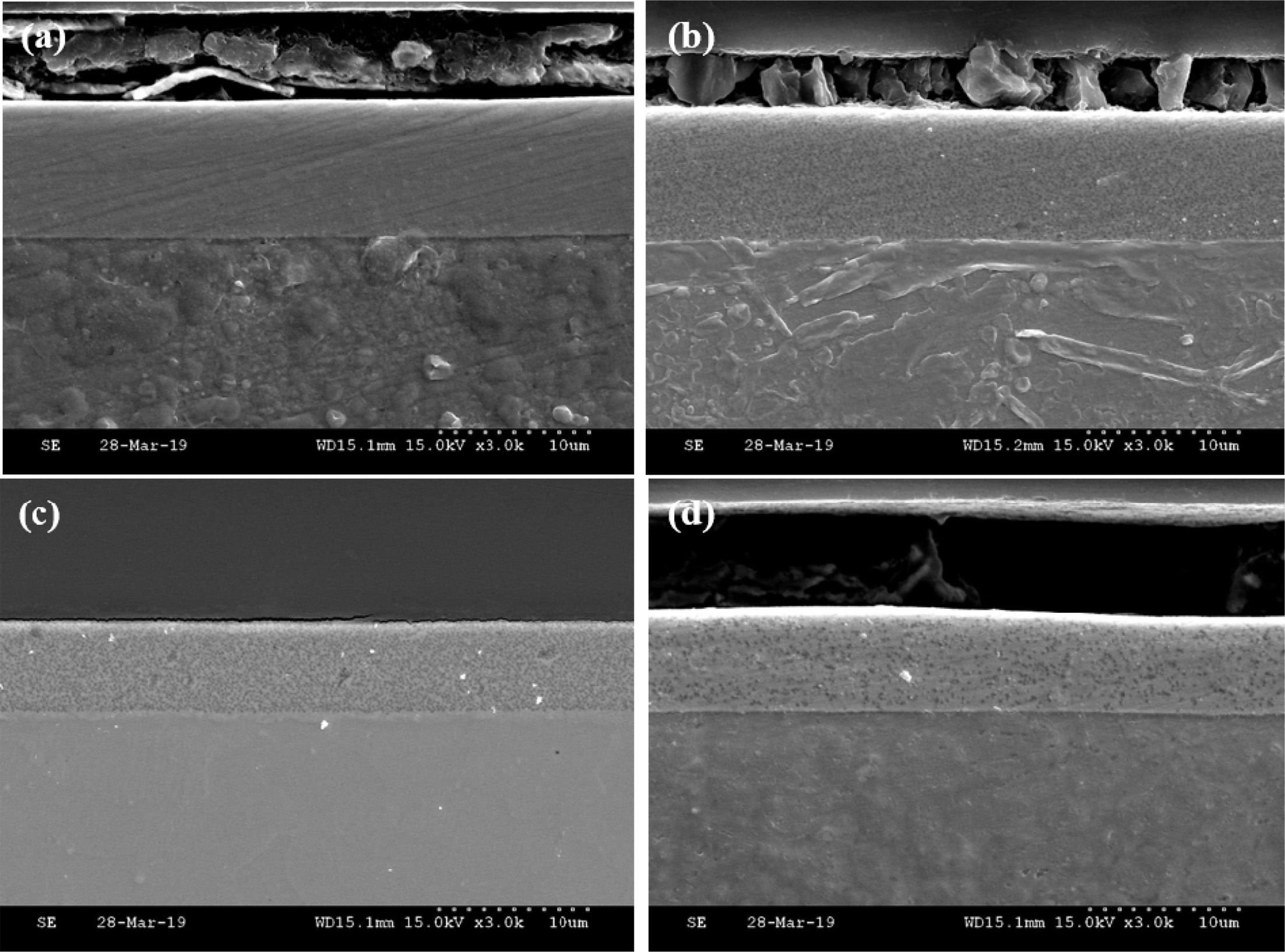
Fig. 3 shows the cross-section of Ni-P-PTFE composite coatings with different contents of PTFE particles. When the content of PTFE was 5, 10, 20, and 30 g/L, the electroless-deposited thicknesses were 9.08, 8.64, 5.86, and 6.86 μm, respectively. It was found that the highest electroless-deposited thickness was formed at 5 g/L. In the EDS analysis, the fluorine content was lowest at 5 g/L of PTFE. The thickness was high because it was not affected by PTFE. On the other hand, when the PTFE content was 10, 20, and 30 g/L, the thickness of Ni-P-PTFE composite coating was decreased. The PTFE was chemically inert and had a soft phase, and it was confirmed that the thickness of Ni-P-PTFE composite coating was low because of the presence of the fluorine content.
This indicates that PTFE is chemically inert and has a soft phase. When the content of PTFE increased, the coating thickness reduced by lowering the mechanical strength of the substrate [20].
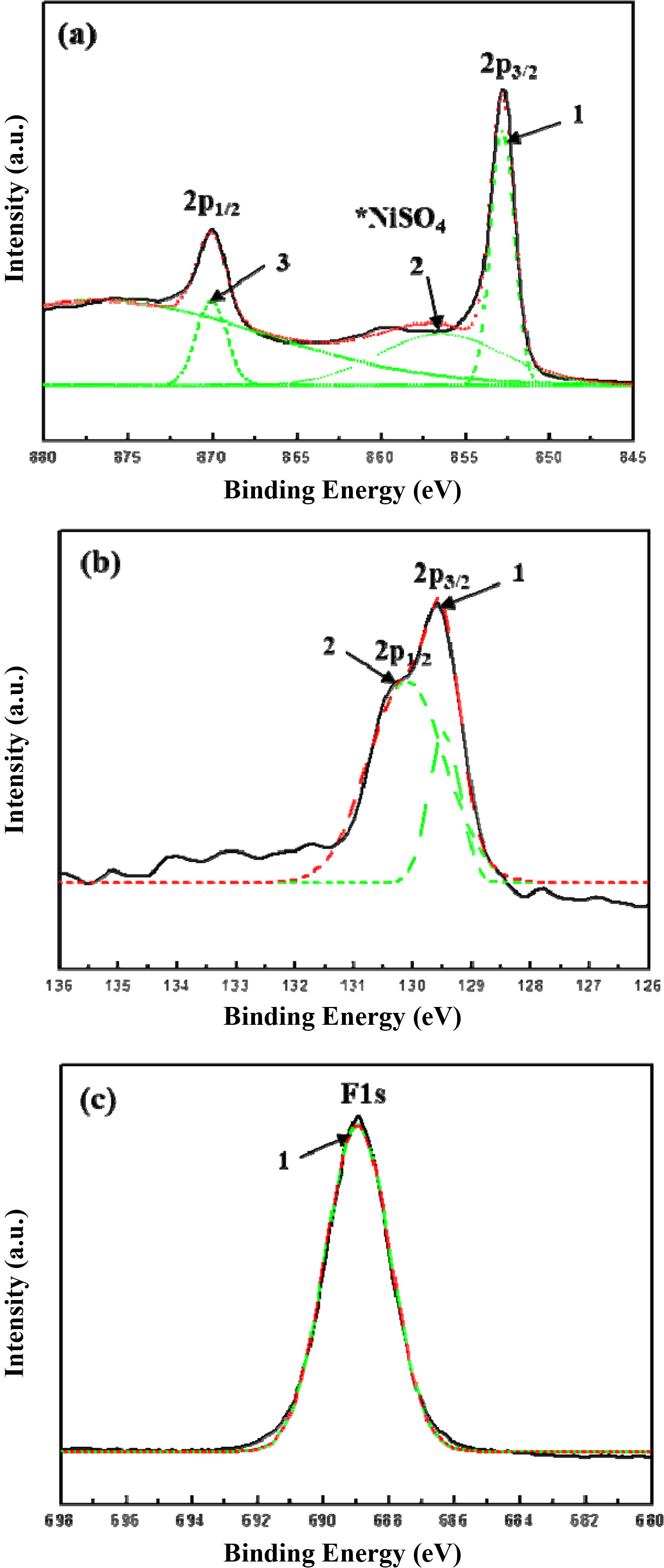
Fig. 4 shows the XPS spectra results. These results indicate that Ni, P, and F of PTFE were combined in the Ni-P-PTFE composite coating. Figs. 3(a), (b), and (c) show the XPS spectra of Ni2p, P2p, and F1s bands, respectively. The bonding energy for the Ni2p band was found to be BE = 852.8 and BE = 856.4 eV, which is a salt of Nickel at 2p3/2 and 2p1/2 peak, was confirmed by BE 856.4 eV for NiSO4. The P2p band showed BE = 129.4 eV at 2p3/2 peak and BE = 130.1 eV at 2p1/2 peak. The F1s band showed BE = 688.94 eV [21].
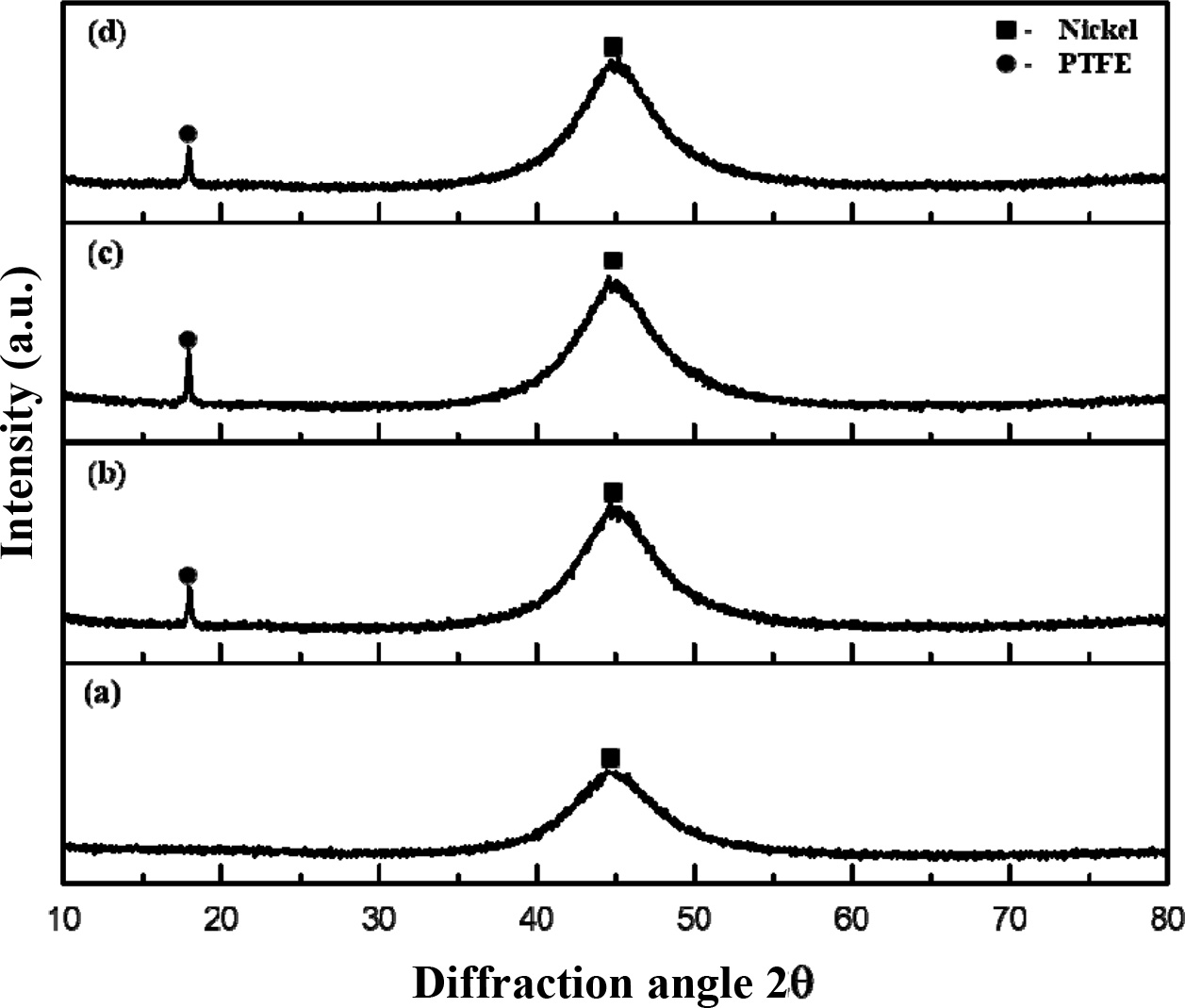
Fig. 5 shows the XRD patterns of Ni-P-PTFE composite coatings with different PTFE contents. It is known that the amorphous Ni–P layer has a peak at 44.9o and forms amorphous structure, which exhibits excellent corrosion resistance [22]. The Ni-P-PTFE composite coating was similar to the N–P coating pattern. It means that the PTFE particles have little effect on the Ni–P matrix.
When the content of PTFE is 5 g/L, the PTFE peak is not present because of the negligibly small amount of PTFE. On the other hand, when the PTFE content is 10, 20, or 30 g/L, the XRD pattern has a diffraction peak at 18.1o. It indicates that PTFE particles are embedded in the Ni–P matrix of the Ni-P-PTFE composite coating.
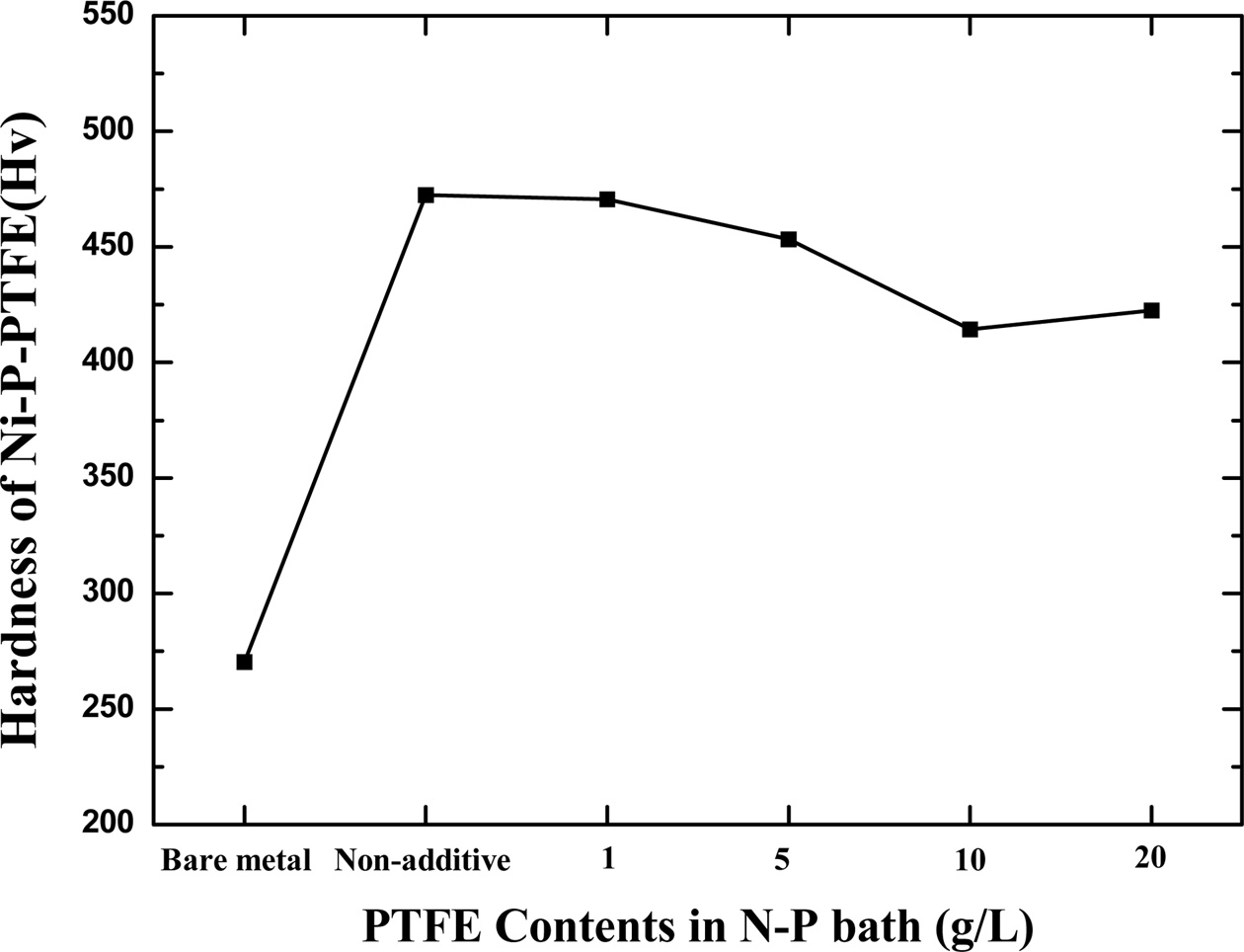
To analyze the surface hardness of the electroless Ni-P-PTFE composite coatings, nano-indentation (PICODENTOR HM500, Helmut Fischer) analyzer was used; the results are shown in Fig. 6. When the PTFE content was 5, 10, 20, and 30 g / L, the hardness was 472.5, 470.6, 453.4, 414.3, and 422.6, respectively. The highest hardness was observed when the content of PTFE in the Ni-P-PTFE composite coating was 5 g/L. When the PTFE content was 10, 20, and 30 g/L the hardness value decreased. As the PTFE concentration increases and begins to play an important role in the adhesive strength, the properties of the PTFE and surfactant, and significant amount of surfactant accumulates in the Ni-P-PTFE composite, which reduces the hardness of the Ni-P-PTFE composite with 20 g/L PTFE content [20].
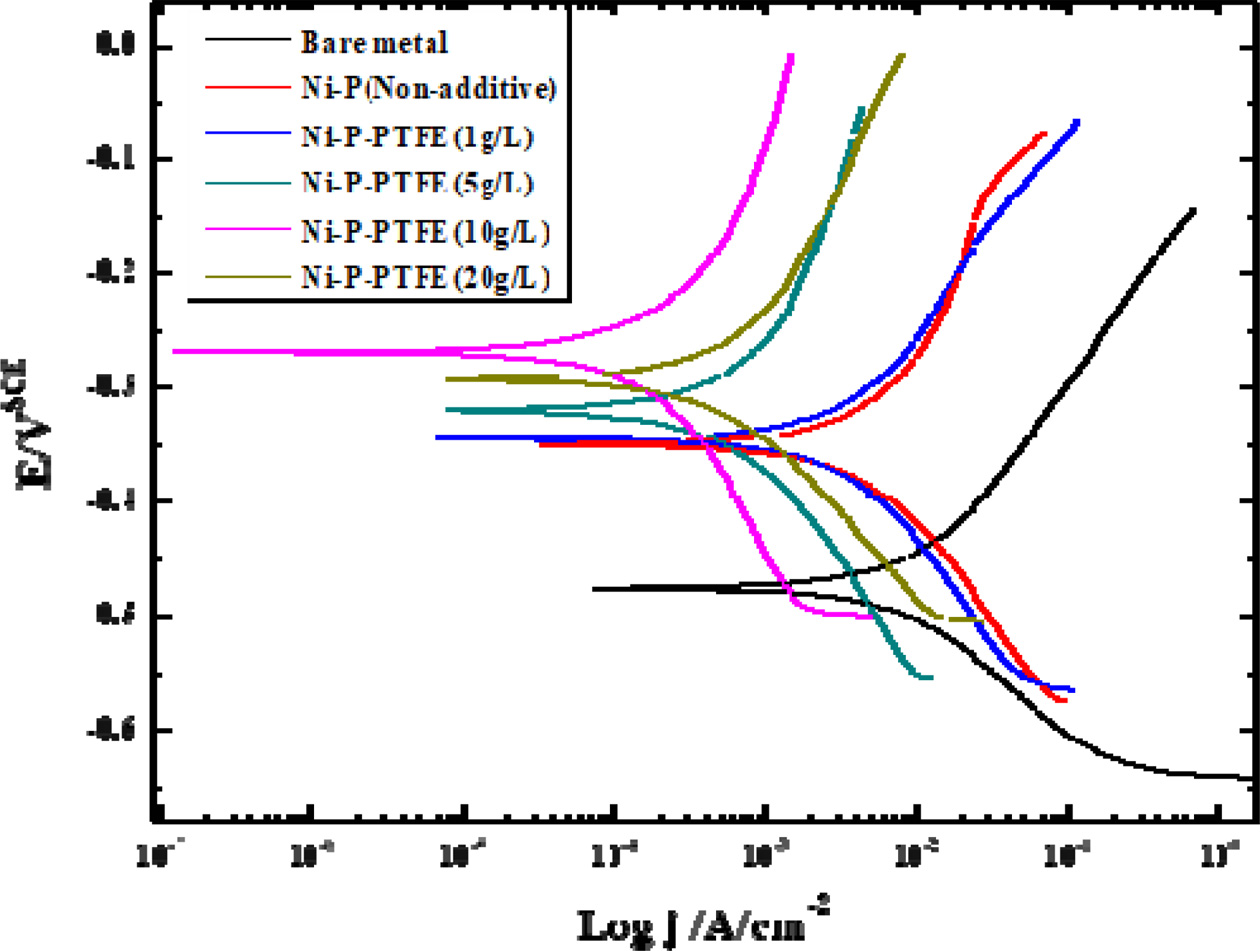
The potentiodynamic polarization curves for the Fe hullcell substrate, Ni–P coating, and Ni-P-PTFE composite coatings with different PTFE concentrations in a 5.0-wt.% NaCl solution at room temperature are shown in Fig. 7.
In general, the corrosion resistance of Ni–P coatings improves as the P content increases [25]. Mukherjee et al. reported that high amorphous Ni–P coatings with high P content have very high corrosion resistance [26]. In this study, the Ni–P and Ni-P-PTFE composite coatings had amorphous structure, and the amount of P decreased with the increasing PTFE content. However, the composite coatings containing PTFE showed the highest corrosion resistance, although the P content was low. The possible reason is that the i of the PTFE particles are uniform in surface and less defective. It also improves the corrosion resistance because PTFE particles are inert and have very low electrical conductivity.
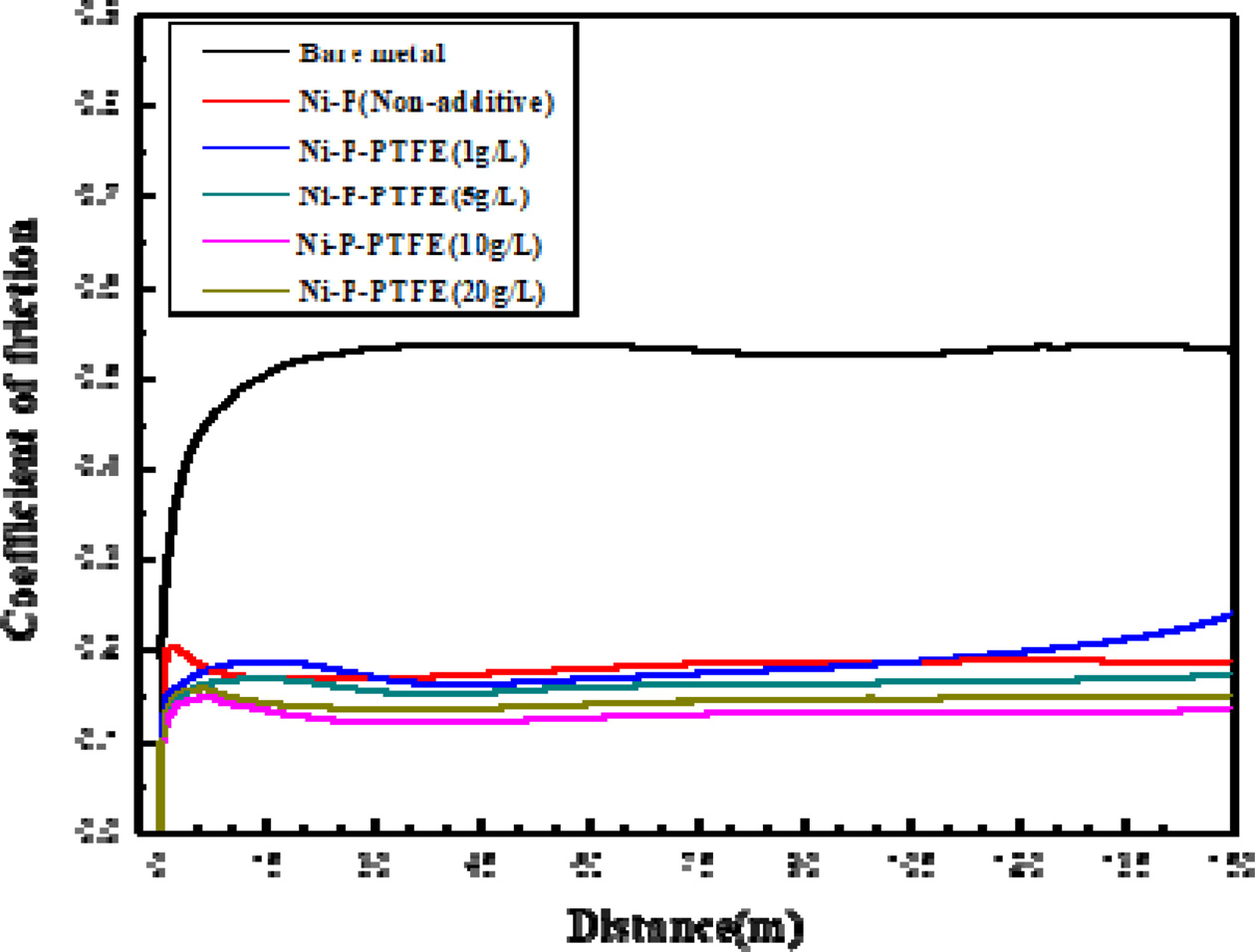
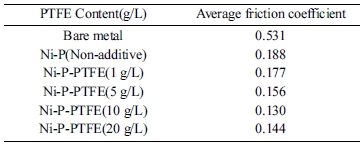
Table 3 and Fig. 8 show the tribology test results of bare metal, Ni–P coating, and Ni-P-PTFE composite coatings; friction coefficients and friction curves are presented.
It was found that Ni-P-PTFE composite coatings have lower wear coefficient than bare metal and Ni–P coating. On the other hand, the tribology test demonstrated that the friction coefficient decreases as the PTFE content increases; 20 g/L was found to be the optimal PTFE content.
It can be attributed to the fact that the low friction coefficient affects the wear loss caused by the influence of the material strength; therefore, in the Ni-P-PTFE composite coatings, wear resistance is superior to that of the Ni–P matrix, and wear and tear are relatively difficult. It suggests that Ni-P-PTFE composite coatings are practical and promising, as they can reduce wear loss.
|
Fig. 1 Surface morphology images of Ni-P-PTFE composite coatings at different PTFE concentrations; (a) 1 g/L, (b) 5 g/L, (c) 10 g/L, and (d) 20 g/L. |
|
Fig. 2 EDS analysis of the Ni-P-PTFE composite coatings with different PTFE concentrations. |
|
Fig. 3 Cross-section morphology images of Ni-P-PTFE composite coatings with different PTFE concentrations; (a) 1 g/L, (b) 5 g/L, (c) 10 g/L, and (d) 20 g/L. |
|
Fig. 4 XPS spectra of (a) Ni2p, (b) P2p, and (c) F1s for Ni-P-PTFE composite coating. |
|
Fig. 5 XRD analysis of Ni-P-PTFE composite coatings with different PTFE concentrations; (a) 5 g/L, (b) 10 g/L, (c) 20 g/L, and (d) 30 g/L. |
|
Fig. 6 Hardness of Ni-P-PTFE composite coatings as a function of PTFE concentration. |
|
Fig. 7 Potentiodynamic polarization curves of Ni-P-PTFE composite coatings with different PTFE concentrations. |
|
Fig. 8 Friction coefficients of Ni-P-PTFE composite coatings with different PTFE concentrations depending on the sliding distance. |
|
Table 2 Chemical composition (wt%) by the EDS analysis on Ni-P-PTFE composite coatings with different PTFE concentrations. |
|
Table 3 Average values of friction coefficient of Ni-P-PTFE composite coatings with different PTFE concentrations. |
In this study, we examined the possibility to improve the corrosion resistance and friction coefficient of Ni-P-PTFE composite coatings with different PTFE concentrations. The physical properties were investigated, and the following characteristics were confirmed:
1. Ni-P-PTFE coatings showed that PTFE particles have been incorporated into the amorphous Ni-P matrix by SEM and XRD. The SEM of Ni-P-PTFE composite coatings observations revealed that PTFE particles have been embedded inside of the Ni-P matrix. On the other hand, when the amount of PTFE contents was minimum, them were not incorporated.
2. The amorphous structure of Ni-P-PTFE composite coatings has a mixed diffraction pattern of the amorphous and crystalline phases of PTFE. The diffraction pattern of PTFE particles was observed.
3. Tribology test showed that the mechanical properties of Ni-P-PTFE composite coatings were improved by PTFE addition.
4. Corrosion resistance of Ni-P-PTFE composite coatings was observed to be superior to that of Fe hullcell substrate showing better resistance.
5. It was suggested that the Ni-P-PTFE composite coatings manufactured by electroless co-deposition method are practical and promising.
This work was supported by the Technological Innovation R&D Program (S2488272) funded by the Small and Medium Business Administration (SMBA, KOREA).
 This Article
This Article
-
2019; 20(5): 556-562
Published on Oct 31, 2019
- 10.36410/jcpr.2019.20.5.556
- Received on Apr 30, 2019
- Revised on Jul 31, 2019
- Accepted on Aug 2, 2019
 Services
Services
Shared
 Correspondence to
Correspondence to
- Young-Min Byoun
-
Metal & Machinery Team, Korea Conformity Laboratories (KCL), 199 Gasan digital 1-ro, Geumcheon-gu, Seoul 08503, Korea
Tel : +82-10-8652-0279
Fax: +82-2-856-5636 - E-mail: 82bym@kcl.re.kr






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.