- Developing zircon-free opaque glossy frits special for double firing tiles in Na2O-MgO-CaO-Al2O3-ZnO-B2O3-SiO2-K2O System
Farzan Karimia,*, Saeid Baghshahib, Mohsen Nouri Khezrabadc and Nastaran Riahi-Noorid
aMaterial Engineering Dept., Science and Research Branch, Islamic Azad University, Tehran, Iran
bDepartment of Materials Science and Engineering, Faculty of Engineering, Imam Khomeini International University, Qazvin, Iran
cDepartment of Mining and Metallurgy Engineering., Yazd University, Yazd, Iran
dNiroo Research Institute, Tehran, Iran
In tile and ceramic industry, frits are used for producing opaque and glossy glazes. Commonly used frits are prepared by using opacifiers such as ZrO2 and TiO2, but the high price of these raw materials leads to a higher cost of the final product. In this research, an opaque frit containing in-situ synthesized Wollastonite (CaSiO3) and Diopside (CaMgSi2O6) was developed as a substitute for Zircon-containing frits. By choosing optimized proportions of oxides in the frit composition, sufficient crystallization occurred in MgO-CaO-Al2O3-ZnO-B2O3-SiO2-K2O-Na2O glass ceramic system. XRD, SEM, EDS, STA, and colorimetry (CIELab) tests were used to compare the prepared samples with a reference zircon containing one which is used in tile industry. The results showed that whiteness and glossiness indices of the prepared sample were 73.61 and 86.56, respectively, which are comparable with those of zircon-free reference sample with whiteness and glossiness indices of 77.05 and 89.22, respectively.
Keywords: Glossy opaque frit, Zircon, Diopside, Wollastonite, Whiteness and glossiness indices
Recently, ceramic tiles have undergone significant changes. In the 1990s, there was a large increase in demand for tiles with improved and advanced properties, such as high resistance to abrasion, higher hardness, lower closed porosity, and improved chemical resistance. For this reason, the possibility of using opaque glass-ceramic glazes for tile industry was investigated [1].
Whiteness and opacity are two important factors affecting the quality of opaque tiles. The difference between the refractive index of an opacifier and a glassy matrix leads to opacity.
Therefore, the greater the difference in refractive indices between the crystal phase and the matrix, the more will be the light scattering and hence, the opacity will be higher [2-3].
ZrSiO4 (Zircon) has been widely used as glaze opacifier in the ceramic glaze industry and several researches have reported the influence of frit composition on the solubility of zirconium compounds [4-8]. Zircon gives rise to opaque frits which are glossy, viscous and with a low fusibility [9]. However, Zircon is an expensive material and its price is mostly affected by market speculation, which results in higher cost of glaze production. Consequently, several researches have been carried out in order to develop zircon-free frits. For instance, some common crystalline phases in glass-ceramic glazes which have been used as a substitute for zircon are Anorthite [10, 11], Rutile and Anatase [12], Albite [13], Okermanite ferrous phase, dicalcium ferrite and wollastonite [14], Aluminosilicates [15], Wollastonite an Diopside [16-18].
Therefore, the present study also aimed to investigate the influence of altering the chemical composition of frits in order to obtain white opaque and zircon-free tile frits. This work has similarities with other reports [16-18] in which the authors studied the zircon-free frits in K2O-MgO-CaO-ZnO-Al2O3-B2O3-SiO2 glass ceramic system by forming diopside and wallastonite crystals in frit matrix. However, it should be noted that in mentioned reports, frit systems were developed for fast single firing wall/floor tiles, whereas our research has been based on double-firing wall tile system. The obvious differences in fast single firing and double-firing tile systems have been elaborated in literature [19] with an emphasis on the role of fluxes like Na2O in double-firing recipes. On the other hand, since the composition of our frit system was based on raw materials available on the Iranian domestic market, the glazes were developed in an octamerous glass ceramic system containing MgO-CaO-Al2O3-ZnO-B2O3-SiO2-K2O-Na2O.
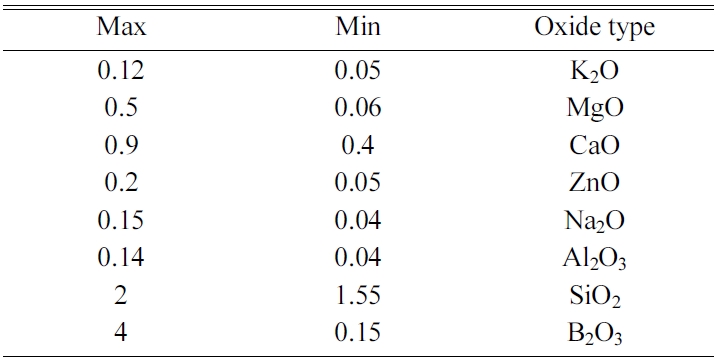
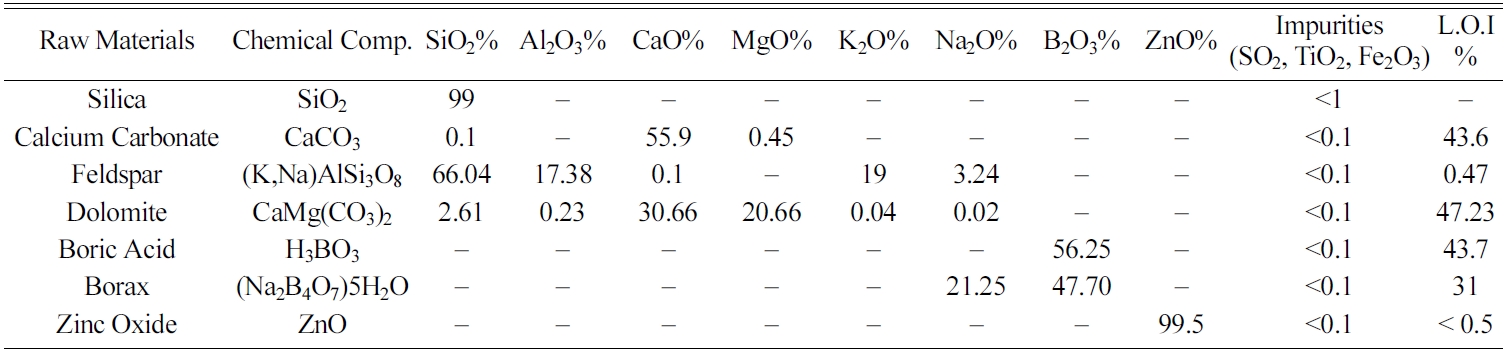
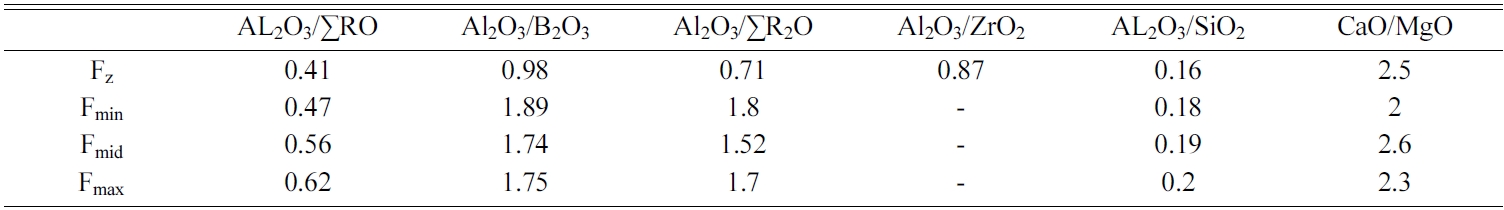
Different experiments were conducted to determine the ranges of seger formulas based on constituent oxides, as indexed in Table 1. On the other hand, Table 2 shows the raw materials used for preparing the frits which have been mostly supplied from Iranian domestic market, except for Borax and Boric acid which were supplied from Turkey (ETI Madan company). Table 3 shows the composition of tested zircon-free frits (Fmin, Fmax and Fmid) comparing with reference sample containing zircon (Fz). Also, important oxide ratios in tested frits versus reference sample have been listed in Table 4.
To synthesize the frits, raw materials (as presented in Table 2) required for 300 grams of frit samples were initially scaled followed by dry mixing in a lab mixer for 15 minutes. Afterwards, the mixtures were placed in an alumina crucible and placed in an electrical furnace. Heat treatment was done with a heating rate of 24 ℃/min to melt the frit after holding for one hour at 1450 ℃. The melt was then poured in cold water to obtain the desired frits.
To prepare the glazes, 100 g of the mentioned frits were scaled and mixed with 10 g Kaolin WBB, 45 g water, 1 g Carboxy Methyl Cellulose (CMC), and 1 g STPP as dispersant. Then the mixture was milled in a lab fast-mill with 400 rpm for 30 min. Density of glaze slurries (measured by a densimeter) was around 1.70 g/cm3 and their recorded viscosity was 40 seconds (Ford-cup 4mm viscometer). After applying the produced frits onto a tile, the glaze samples were fired at 1040 ℃ for 40 minutes.
After Firing, the samples were visually controlled in terms of surface quality indicating no apparent defects such as pinhole, blister, crawling, and so forth. Phase analysis was implemented by X-ray diffraction method (D8-advance, Bruker AXS Company). Differential thermal analysis (DTA) (model SPICO S800) was used to investigate crystallization behavior of samples versus temperature. Also, microstructural analysis was carried out by a field emission scanning microscope (FE-SEM) (MI RA3, TE-SCAN company) equipped with EDS elemental analysis.
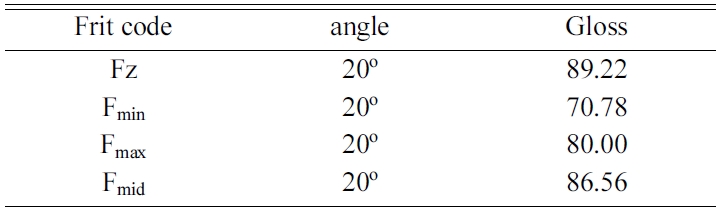
To evaluate the glossiness of final ceramic, glazes were examined by a gloss-meter device according to ASTM D523 Standard. Radiation angle of 20o was used for gloss measures over GU70. Also, whiteness percentage measurement was accomplished based on whiteness formula of blue light and photoelectric effect, using a tungsten halogen lamp.
It is worthy to note that developed frit was also tested in a tile factory to provide qualities in accordance with industrial applications. Fabricated glazes in both experimental or industrial stages, possessed the related industry standards including ISIRI-25, INSO-9169, ISO-10545, 13006, ASTM-E313, D523.
|
Table 3 Composition of tested zircon-free frits vs. reference sample containing zircon. |
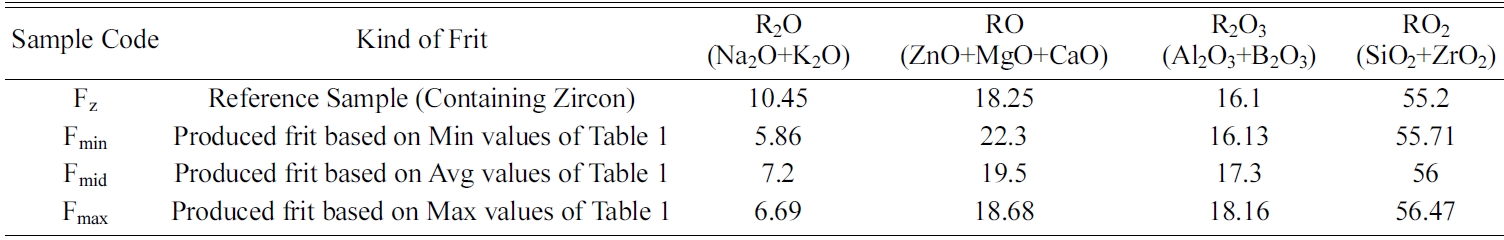
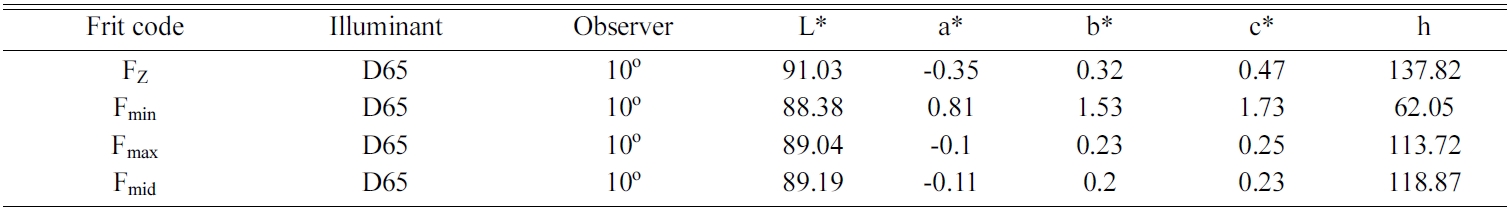
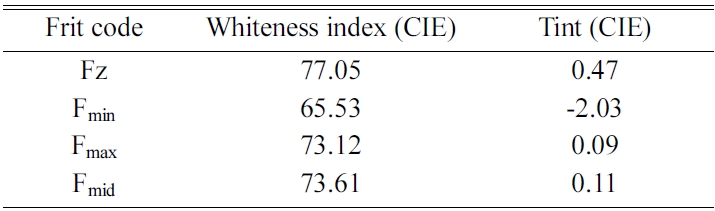
Table 5 shows the colorimetry test results for Fz and zircon-free glaze samples. In this table, L* indicates lightness, a* is the red/green coordinate, b* is the yellow/blue coordinate, c* is Chroma value and h* shows the Hue angle. Also, Tables 6 and 7, respectively, show the whiteness index and glossiness test results of the samples. It can be seen that whiteness and glossiness indices of the glaze containing zircon-free frit marked as Fmid is very close to those of reference sample containing zircon. For more evaluation, the samples were studied by DTA, XRD and SEM tests and the results were compared with reference sample containing zircon.
Fig. 1 shows Tg temperature of frits as well as their crystallization behavior tested by DTA method. Glass transition temperature (Tg) was 681 ℃ for Fz sample along with two exothermic peaks at 821 ℃ and 991 ℃. On the other hand, the glazes containing zircon-free frits (Fmin and Fmax) showed their Tg temperature around 648 ℃ as well as exothermic peaks at 842 ℃ and 936 ℃, whereas for Fmid frit, Tg and first exothermic peak shifted to lower temperatures (530 ℃ and 795 ℃ respectively). In order to identify the corresponding crystalline phases, the samples were characterized by XRD technique (Fig. 2).
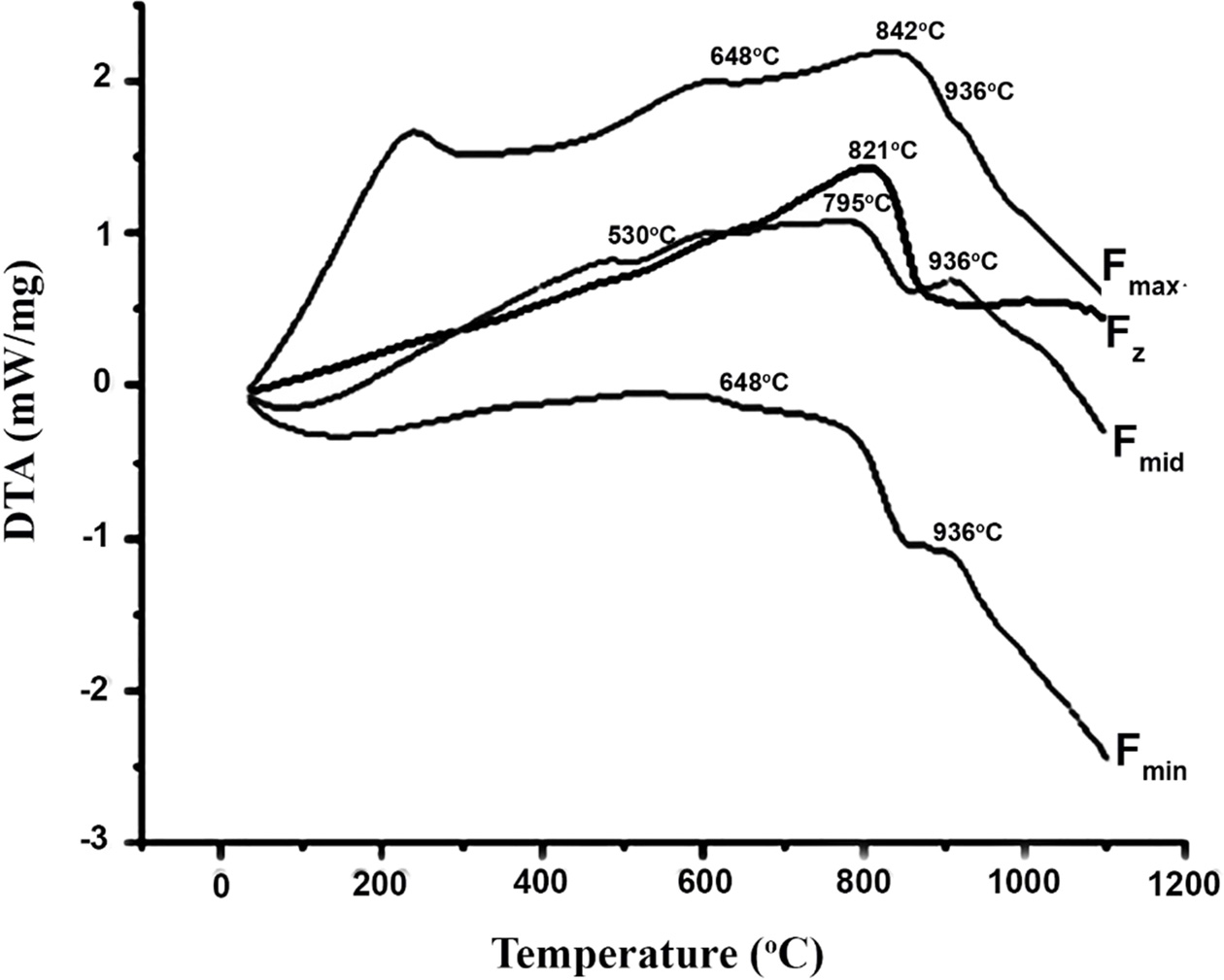
Referring to XRD pattern of Fz sample, it can be concluded that the DTA peak at 821 ℃ belongs to nucleation of wollastonite whereas the peak at 991 ℃ is related to zircon crystallization. On the other hand, XRD pattern of Fmin and Fmax sample proves that the crystallization events at 842 ℃ and 936 ℃ are correspondent to wollastonite and diopside respectively. However, in the sample containing Fmid frit, wollastonite crystallization has started in lower temperatures comparing to Fmin and Fmax. Also, XRD peaks for Fmid sample had significantly higher intensities which can be attributed to the lower Tg of this Frit, leading to more nucleation and crystallization of wollastonite and diopside. By referring to Table 3 and comparing the chemical composition of Fmid with Fmin and Fmax, the lower Tg of Fmid frit can be attributed to higher amounts of glass modifiers (Na2O+K2O) in this sample.
Figs. 3 and 4 show the SEM images of reference sample (Fz) and zircon-free one (Fmid) after heat treatment at 1040 ℃. The area marked as “Z” in Fig. 3 corresponds to zircon phase and “G” is amorphous glassy phase. On the other hand, the grey colored area marked as “D” in Fig. 4 belongs to Diopside phase whereas black-like region marked as “G” corresponds to amorphous glassy phase and needle-like area marked as “W” is wollastonite. Fig. 4 (right) shows the homogenous distribution of glaze compounds within the whole sample.
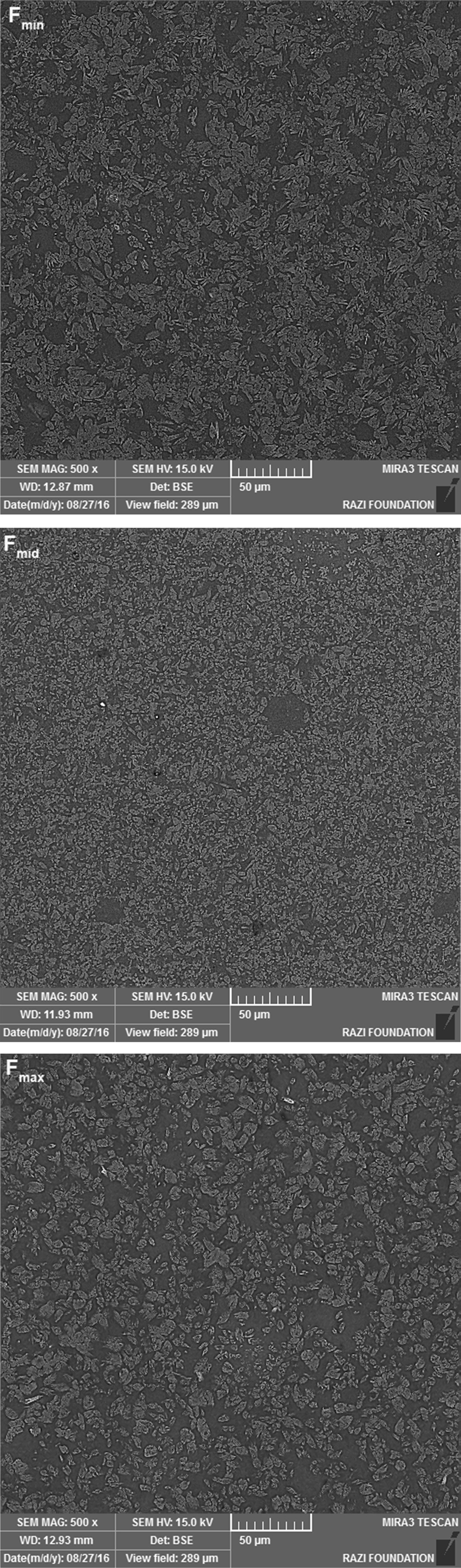
Fig. 5 shows a comparison between microstructure of all zircon-free samples. All SEM images have similar magnification (500X). It can be seen clearly that Fmid sample has a more homogenous and finer microstructure comparing to Fmin and Fmax. This fine and homogenous structure most likely can be due to the lower Tg of this frit resulting in more nucleation and crystallization of wollastonite and diopside phases.
As a result, it can be seen that by proper selection of the composition in Fmid sample, fine crystals uniformly distribute in the matrix leading to an opaque glass ceramic structure. In this regard, determination, definition and considering optimum ratios of Al2O3/B2O3, Al2O3/SR2O, Al2O3/SRO. Al2O3/SiO2, CaO/MgO are key parameters for obtaining desired mixtures.
It can be concluded that whiteness and glossiness behavior of Fmid frit can be due to the formed phases and their distribution mode within the sample. Refraction indices of zircon, diopside and wollastonite phases are 1.93, 1.68 and 1.63, respectively. However, uniform distribution of phases and fine crystalline structure resulted in significant improvement of glaze quality which can be comparable for reference sample containing zircon. The synthesized frit was successfully used as a substitute for zircon containing frits in a tile manufacturing company. The results of this project has been registered under the patent No. 80921 in Iranian Patent registration office.
|
Fig. 1 DTA curves of glaze samples containing tested frits. |
|
Fig. 2 X-ray diffraction patterns of synthesized glazes containing tested frits. |

|
Fig. 3 SEM images of reference sample (Fz). |

|
Fig. 4 SEM images of zircon-free sample (Fmid). |
|
Fig. 5 SEM images of Fmin, Fmid and Fmax samples with same magnification. |
An opaque glaze suitable for application in manufacturing tiles was successfully prepared through synthesis of zircon-free frits containing diopside and wollastonite phases. The properties of this zircon-free frit was very close to the defined standards and commonly used zircon-containing ones. Having optimized amounts of calcium carbonate and dolomite in raw materials resulted in formation of diopside and wollastonite phases which can have as similar opacifing function as zircon. The synthesized frit was successfully tested in tile industry.
The authors would like to thank Mr. Abolfazl Gerveee, Mr.Omid Khanali and Mrs. Zahrasadat Seyed Hoseini for their kindly support on some experimental tests.
- 1. R. Casasola, J.Ma. Rincón, M. Romero, J. Mat. Sci., 47[2] (2012) 553-582
-

- 2. J. R. Taylor, and A. C. Bull, in “Ceramics Glaze Technology”. (Pergamon Press, 1980) p 111
- 3. C. R. Santos, T. L. B. Fontana, E. Uggioni, H. G. Riella, and A. M. Bernardin in Proceedings of the World Congress on Ceramic and Tile Quality (Qualicer), 2004, P 189-194
- 4. Blonski R, Ceram Eng Sci Proc, 15[1] (2008) 249-265.
- 5. C. W. F. Jacobs, J Am Ceram Soc, 37[5] (1954) 216-220.
-

- 6. I. A. Levitskii, Glass Ceram 60[3-4] (2003) 83-86.
-

- 7. R. J. Castilone, D. Sriram, W. M. Carty, R. L. Snyder, J Am Ceram Soc 82[10] (1999) 2819-2824
-

- 8. J. L Amoros, A Escardino, M. J Orts, A Moreno. Br Ceram Trans 93[6] (1994) 224-228.
- 9. M Romero, J. Ma Rincón, A. Acosta, J Eur Ceram Soc, 23 [10] (2003):1629-1635.
-

- 10. M. Gajek, J. Partyka, A. Rapacz-Kmita, K. Gasek, Ceram Int 43 [2] (2017) 1703-1709
-

- 11. N. T. Selli, Ceram Int. 41[6] (2015) 7790-7795
-

- 12. S. Teixeira, A. M. Bernardin, Dyes and Pigments, 80[3] (2009) 292-296
-

- 13. A. Salem, S.H. Jazayeri, E. Rastelli, G. Timellini, J. Ceram. Process. Res. 10[5] (2009) 621-627.
- 14. N. D. Yatsenko and V P. Ratkova., Glass Ceram. 63[7-8] (2006) 265-266.
-

- 15. F. Karam Pourrad, M. Khajeh Aminian, M. Hakimi, J. Color and Science Technology, 8[3] (2014) 179-186
- 16. B. Karasu, K. Pekkan, J. Eur. Ceram. Soc. 29[9] (2009) 1571-1578.
-

- 17. K. Karaveli, B. Karasu1, and H. S. Onal, in Proceedings of the World Congress on Ceramic and Tile Quality (Qualicer), 2008, P 43-49
- 18. J. Aparisi; L.F. Sánchez, J.L. Amorós; A. Escardino; M,J. Orts; S. Mestr, in Proceedings of the World Congress on Ceramic and Tile Quality (Qualicer), 1998, P 65-80
- 19. SACMI, Applied Ceramic Technology, Vol 1, SACMI IMOLA 2002, 444 pages.
 This Article
This Article
-
2019; 20(4): 357-362
Published on Aug 31, 2019
- Received on Feb 11, 2019
- Revised on Jul 13, 2019
- Accepted on Jul 24, 2019
 Services
Services
- Abstract
introduction
experimental procedure
results and discussion
conclusions
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Farzan Karimi
-
Material Engineering Dept., Science and Research Branch, Islamic Azad University, Tehran, Iran
Tel : +98-9127832573
Fax: +98-32570057 - E-mail: farzan.karimi8@gmail.com






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.